FLUID
-
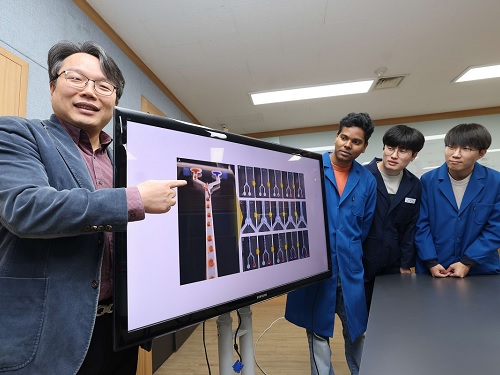 KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 12587
KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 12587 -
 The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 11365
The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 11365 -
 Nanoscale Self-Assembling Salt-Crystal ‘Origami’ Balls Envelop Liquids
Mechanical engineers have devised a ‘crystal capillary origami’ technique where salt crystals spontaneously encapsulate liquid droplets
Researchers have developed a technique whereby they can spontaneously encapsulate microscopic droplets of water and oil emulsion in a tiny sphere made of salt crystals—sort of like a minute, self-constructing origami soccer ball filled with liquid. The process, which they are calling ‘crystal capillary origami,’ could be used in a range of fields from more precise drug delivery to nanoscale medical devices.The technique is described in a paper appearing in the journal Nanoscale on September 21.
Capillary action, or ‘capillarity,’ will be familiar to most people as the way that water or other liquids can move up narrow tubes or other porous materials seemingly in defiance of gravity (for example within the vascular systems of plants, or even more simply, the drawing up of paint between the hairs of a paintbrush). This effect is due to the forces of cohesion (the tendency of a liquid’s molecules to stick together), which results in surface tension, and adhesion (their tendency to stick to the surface of other substances). The strength of the capillarity depends on the chemistry of the liquid, the chemistry of the porous material, and on the other forces acting on them both. For example, a liquid with lower surface tension than water would not be able to hold up a water strider insect.
Less well known is a related phenomenon, elasto-capillarity, that takes advantage of the relationship between capillarity and the elasticity of a very tiny flat sheet of a solid material. In certain circumstances, the capillary forces can overcome the elastic bending resistance of the sheet.
This relationship can be exploited to create ‘capillary origami,’ or three-dimensional structures. When a liquid droplet is placed on the flat sheet, the latter can spontaneously encapsulate the former due to surface tension. Capillary origami can take on other forms including wrinkling, buckling, or self-folding into other shapes. The specific geometrical shape that the 3D capillary origami structure ends up taking is determined by both the chemistry of the flat sheet and that of the liquid, and by carefully designing the shape and size of the sheet.
There is one big problem with these small devices, however. “These conventional self-assembled origami structures cannot be completely spherical and will always have discontinuous boundaries, or what you might call ‘edges,’ as a result of the original two-dimensional shape of the sheet,” said Kwangseok Park, a lead researcher on the project. He added, “These edges could turn out to be future defects with the potential for failure in the face of increased stress.” Non-spherical particles are also known to be more disadvantageous than spherical particles in terms of cellular uptake.
Professor Hyoungsoo Kim from the Department of Mechanical Engineering explained, “This is why researchers have long been on the hunt for substances that could produce a fully spherical capillary origami structure.”
The authors of the study have demonstrated such an origami sphere for the first time. They showed how instead of a flat sheet, the growth of salt-crystals can perform capillary origami action in a similar manner. What they call ‘crystal capillary origami’ spontaneously constructs a smooth spherical shell capsule from these same surface tension effects, but now the spontaneous encapsulation of a liquid is determined by the elasto-capillary conditions of growing crystals.
Here, the term ‘salt’ refers to a compound of one positively charged ion and another negatively charged. Table salt, or sodium chloride, is just one example of a salt. The researchers used four other salts: calcium propionate, sodium salicylate, calcium nitrate tetrahydrate, and sodium bicarbonate to envelop a water-oil emulsion. Normally, a salt such as sodium chloride has a cubical crystal structure, but these four salts form plate-like structures as crystallites or ‘grains’ (the microscopic shape that forms when a crystal first starts to grow) instead. These plates then self-assemble into perfect spheres.
Using scanning electron microscopy and X-ray diffraction analysis, they investigated the mechanism of such formation and concluded that it was ‘Laplace pressure’ that drives the crystallite plates to cover the emulsion surface. Laplace pressure describes the pressure difference between the interior and exterior of a curved surface caused by the surface tension at the interface between the two substances, in this case between the salt water and the oil.
The researchers hope that these self-assembling nanostructures can be used for encapsulation applications in a range of sectors, from the food industry and cosmetics to drug delivery and even tiny medical devices.
-Publication
Kwangseok Park, Hyoungsoo Kim “Crystal capillary origami capsule with self-assembled nanostructure,” Nanoscale, 13(35), 14656-14665 (DOI: 10.1039/d1nr02456f)
-Profile
Professor Hyoungsoo Kim
Fluid and Interface Laboratory
http://fil.kaist.ac.kr
Department of Mechanical Engineering
KAIST
2021.11.04 View 11876
Nanoscale Self-Assembling Salt-Crystal ‘Origami’ Balls Envelop Liquids
Mechanical engineers have devised a ‘crystal capillary origami’ technique where salt crystals spontaneously encapsulate liquid droplets
Researchers have developed a technique whereby they can spontaneously encapsulate microscopic droplets of water and oil emulsion in a tiny sphere made of salt crystals—sort of like a minute, self-constructing origami soccer ball filled with liquid. The process, which they are calling ‘crystal capillary origami,’ could be used in a range of fields from more precise drug delivery to nanoscale medical devices.The technique is described in a paper appearing in the journal Nanoscale on September 21.
Capillary action, or ‘capillarity,’ will be familiar to most people as the way that water or other liquids can move up narrow tubes or other porous materials seemingly in defiance of gravity (for example within the vascular systems of plants, or even more simply, the drawing up of paint between the hairs of a paintbrush). This effect is due to the forces of cohesion (the tendency of a liquid’s molecules to stick together), which results in surface tension, and adhesion (their tendency to stick to the surface of other substances). The strength of the capillarity depends on the chemistry of the liquid, the chemistry of the porous material, and on the other forces acting on them both. For example, a liquid with lower surface tension than water would not be able to hold up a water strider insect.
Less well known is a related phenomenon, elasto-capillarity, that takes advantage of the relationship between capillarity and the elasticity of a very tiny flat sheet of a solid material. In certain circumstances, the capillary forces can overcome the elastic bending resistance of the sheet.
This relationship can be exploited to create ‘capillary origami,’ or three-dimensional structures. When a liquid droplet is placed on the flat sheet, the latter can spontaneously encapsulate the former due to surface tension. Capillary origami can take on other forms including wrinkling, buckling, or self-folding into other shapes. The specific geometrical shape that the 3D capillary origami structure ends up taking is determined by both the chemistry of the flat sheet and that of the liquid, and by carefully designing the shape and size of the sheet.
There is one big problem with these small devices, however. “These conventional self-assembled origami structures cannot be completely spherical and will always have discontinuous boundaries, or what you might call ‘edges,’ as a result of the original two-dimensional shape of the sheet,” said Kwangseok Park, a lead researcher on the project. He added, “These edges could turn out to be future defects with the potential for failure in the face of increased stress.” Non-spherical particles are also known to be more disadvantageous than spherical particles in terms of cellular uptake.
Professor Hyoungsoo Kim from the Department of Mechanical Engineering explained, “This is why researchers have long been on the hunt for substances that could produce a fully spherical capillary origami structure.”
The authors of the study have demonstrated such an origami sphere for the first time. They showed how instead of a flat sheet, the growth of salt-crystals can perform capillary origami action in a similar manner. What they call ‘crystal capillary origami’ spontaneously constructs a smooth spherical shell capsule from these same surface tension effects, but now the spontaneous encapsulation of a liquid is determined by the elasto-capillary conditions of growing crystals.
Here, the term ‘salt’ refers to a compound of one positively charged ion and another negatively charged. Table salt, or sodium chloride, is just one example of a salt. The researchers used four other salts: calcium propionate, sodium salicylate, calcium nitrate tetrahydrate, and sodium bicarbonate to envelop a water-oil emulsion. Normally, a salt such as sodium chloride has a cubical crystal structure, but these four salts form plate-like structures as crystallites or ‘grains’ (the microscopic shape that forms when a crystal first starts to grow) instead. These plates then self-assemble into perfect spheres.
Using scanning electron microscopy and X-ray diffraction analysis, they investigated the mechanism of such formation and concluded that it was ‘Laplace pressure’ that drives the crystallite plates to cover the emulsion surface. Laplace pressure describes the pressure difference between the interior and exterior of a curved surface caused by the surface tension at the interface between the two substances, in this case between the salt water and the oil.
The researchers hope that these self-assembling nanostructures can be used for encapsulation applications in a range of sectors, from the food industry and cosmetics to drug delivery and even tiny medical devices.
-Publication
Kwangseok Park, Hyoungsoo Kim “Crystal capillary origami capsule with self-assembled nanostructure,” Nanoscale, 13(35), 14656-14665 (DOI: 10.1039/d1nr02456f)
-Profile
Professor Hyoungsoo Kim
Fluid and Interface Laboratory
http://fil.kaist.ac.kr
Department of Mechanical Engineering
KAIST
2021.11.04 View 11876 -
 Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 13490
Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 13490 -
 Ultrafast, on-Chip PCR Could Speed Up Diagnoses during Pandemics
A rapid point-of-care diagnostic plasmofluidic chip can deliver result in only 8 minutes
Reverse transcription-polymerase chain reaction (RT-PCR) has been the gold standard for diagnosis during the COVID-19 pandemic. However, the PCR portion of the test requires bulky, expensive machines and takes about an hour to complete, making it difficult to quickly diagnose someone at a testing site. Now, researchers at KAIST have developed a plasmofluidic chip that can perform PCR in only about 8 minutes, which could speed up diagnoses during current and future pandemics.
The rapid diagnosis of COVID-19 and other highly contagious viral diseases is important for timely medical care, quarantining and contact tracing. Currently, RT-PCR uses enzymes to reverse transcribe tiny amounts of viral RNA to DNA, and then amplifies the DNA so that it can be detected by a fluorescent probe. It is the most sensitive and reliable diagnostic method.
But because the PCR portion of the test requires 30-40 cycles of heating and cooling in special machines, it takes about an hour to perform, and samples must typically be sent away to a lab, meaning that a patient usually has to wait a day or two to receive their diagnosis.
Professor Ki-Hun Jeong at the Department of Bio and Brain Engineering and his colleagues wanted to develop a plasmofluidic PCR chip that could quickly heat and cool miniscule volumes of liquids, allowing accurate point-of-care diagnoses in a fraction of the time. The research was reported in ACS Nano on May 19.
The researchers devised a postage stamp-sized polydimethylsiloxane chip with a microchamber array for the PCR reactions. When a drop of a sample is added to the chip, a vacuum pulls the liquid into the microchambers, which are positioned above glass nanopillars with gold nanoislands. Any microbubbles, which could interfere with the PCR reaction, diffuse out through an air-permeable wall. When a white LED is turned on beneath the chip, the gold nanoislands on the nanopillars quickly convert light to heat, and then rapidly cool when the light is switched off.
The researchers tested the device on a piece of DNA containing a SARS-CoV-2 gene, accomplishing 40 heating and cooling cycles and fluorescence detection in only 5 minutes, with an additional 3 minutes for sample loading. The amplification efficiency was 91%, whereas a comparable conventional PCR process has an efficiency of 98%. With the reverse transcriptase step added prior to sample loading, the entire testing time with the new method could take 10-13 minutes, as opposed to about an hour for typical RT-PCR testing. The new device could provide many opportunities for rapid point-of-care diagnostics during a pandemic, the researchers say.
-Publication
Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics
ACS Nano (https://doi.org/10.1021/acsnano.1c02154)
-Professor
Ki-Hun Jeong
Biophotonics Laboratory
https://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineeinrg
KAIST
2021.06.08 View 13903
Ultrafast, on-Chip PCR Could Speed Up Diagnoses during Pandemics
A rapid point-of-care diagnostic plasmofluidic chip can deliver result in only 8 minutes
Reverse transcription-polymerase chain reaction (RT-PCR) has been the gold standard for diagnosis during the COVID-19 pandemic. However, the PCR portion of the test requires bulky, expensive machines and takes about an hour to complete, making it difficult to quickly diagnose someone at a testing site. Now, researchers at KAIST have developed a plasmofluidic chip that can perform PCR in only about 8 minutes, which could speed up diagnoses during current and future pandemics.
The rapid diagnosis of COVID-19 and other highly contagious viral diseases is important for timely medical care, quarantining and contact tracing. Currently, RT-PCR uses enzymes to reverse transcribe tiny amounts of viral RNA to DNA, and then amplifies the DNA so that it can be detected by a fluorescent probe. It is the most sensitive and reliable diagnostic method.
But because the PCR portion of the test requires 30-40 cycles of heating and cooling in special machines, it takes about an hour to perform, and samples must typically be sent away to a lab, meaning that a patient usually has to wait a day or two to receive their diagnosis.
Professor Ki-Hun Jeong at the Department of Bio and Brain Engineering and his colleagues wanted to develop a plasmofluidic PCR chip that could quickly heat and cool miniscule volumes of liquids, allowing accurate point-of-care diagnoses in a fraction of the time. The research was reported in ACS Nano on May 19.
The researchers devised a postage stamp-sized polydimethylsiloxane chip with a microchamber array for the PCR reactions. When a drop of a sample is added to the chip, a vacuum pulls the liquid into the microchambers, which are positioned above glass nanopillars with gold nanoislands. Any microbubbles, which could interfere with the PCR reaction, diffuse out through an air-permeable wall. When a white LED is turned on beneath the chip, the gold nanoislands on the nanopillars quickly convert light to heat, and then rapidly cool when the light is switched off.
The researchers tested the device on a piece of DNA containing a SARS-CoV-2 gene, accomplishing 40 heating and cooling cycles and fluorescence detection in only 5 minutes, with an additional 3 minutes for sample loading. The amplification efficiency was 91%, whereas a comparable conventional PCR process has an efficiency of 98%. With the reverse transcriptase step added prior to sample loading, the entire testing time with the new method could take 10-13 minutes, as opposed to about an hour for typical RT-PCR testing. The new device could provide many opportunities for rapid point-of-care diagnostics during a pandemic, the researchers say.
-Publication
Ultrafast and Real-Time Nanoplasmonic On-Chip Polymerase Chain Reaction for Rapid and Quantitative Molecular Diagnostics
ACS Nano (https://doi.org/10.1021/acsnano.1c02154)
-Professor
Ki-Hun Jeong
Biophotonics Laboratory
https://biophotonics.kaist.ac.kr/
Department of Bio and Brain Engineeinrg
KAIST
2021.06.08 View 13903 -
 Slippery When Wet: Fish and Seaweed Inspire Ships to Reduce Fluid Friction
Faster ships could be on the horizon after KAIST scientists develop a slippery surface inspired by fish and seaweed to reduce the hull's drag through the water.
Long-distance cargo ships lose a significant amount of energy due to fluid friction. Looking to the drag reduction mechanisms employed by aquatic life can provide inspiration on how to improve efficiency.
Fish and seaweed secrete a layer of mucus to create a slippery surface, reducing their friction as they travel through water. A potential way to mimic this is by creating lubricant-infused surfaces covered with cavities. As the cavities are continuously filled with the lubricant, a layer is formed over the surface.
Though this method has previously been shown to work, reducing drag by up to 18%, the underlying physics is not fully understood. KAIST researchers in collaboration with a team of researchers from POSTECH conducted simulations of this process to help explain the effects, and their findings were published in the journal Physics of Fluids on September 15.
The group looked at the average speed of a cargo ship with realistic material properties and simulated how it behaves under various lubrication setups. Specifically, they monitored the effects of the open area of the lubricant-filled cavities, as well as the thickness of the cavity lids.
They found that for larger open areas, the lubricant spreads more than it does with smaller open areas, leading to a slipperier surface. On the other hand, the lid thickness does not have much of an effect on the slip, though a thicker lid does create a thicker lubricant buildup layer.
Professor Emeritus Hyung Jin Sung from the KAIST Department of Mechanical Engineering who led this study said, “Our investigation of the hydrodynamics of a lubricant layer and how it results in drag reduction with a slippery surface in a basic configuration has provided significant insight into the benefits of a lubricant-infused surface.”
Now that they have worked on optimizing the lubricant secretion design, the authors hope it can be implemented in real-life marine vehicles.
“If the present design parameters are adopted, the drag reduction rate will increase significantly,” Professor Sung added.
This work was supported by the National Research Foundation (NRF) of Korea.
Source:
Materials provided by American Institute of Physics.
Publication:
Kim, Seung Joong, et al. (2020). A lubricant-infused slip surface for drag reduction. Physics of Fluids. Available online at https://doi.org/10.1063/5.0018460
Profile:
Hyung Jin Sung
Professor Emeritus
hyungjin@kaist.ac.kr
http://flow.kaist.ac.kr/index.php
Flow Control Lab. (FCL)
Department of Mechanical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.10.12 View 9249
Slippery When Wet: Fish and Seaweed Inspire Ships to Reduce Fluid Friction
Faster ships could be on the horizon after KAIST scientists develop a slippery surface inspired by fish and seaweed to reduce the hull's drag through the water.
Long-distance cargo ships lose a significant amount of energy due to fluid friction. Looking to the drag reduction mechanisms employed by aquatic life can provide inspiration on how to improve efficiency.
Fish and seaweed secrete a layer of mucus to create a slippery surface, reducing their friction as they travel through water. A potential way to mimic this is by creating lubricant-infused surfaces covered with cavities. As the cavities are continuously filled with the lubricant, a layer is formed over the surface.
Though this method has previously been shown to work, reducing drag by up to 18%, the underlying physics is not fully understood. KAIST researchers in collaboration with a team of researchers from POSTECH conducted simulations of this process to help explain the effects, and their findings were published in the journal Physics of Fluids on September 15.
The group looked at the average speed of a cargo ship with realistic material properties and simulated how it behaves under various lubrication setups. Specifically, they monitored the effects of the open area of the lubricant-filled cavities, as well as the thickness of the cavity lids.
They found that for larger open areas, the lubricant spreads more than it does with smaller open areas, leading to a slipperier surface. On the other hand, the lid thickness does not have much of an effect on the slip, though a thicker lid does create a thicker lubricant buildup layer.
Professor Emeritus Hyung Jin Sung from the KAIST Department of Mechanical Engineering who led this study said, “Our investigation of the hydrodynamics of a lubricant layer and how it results in drag reduction with a slippery surface in a basic configuration has provided significant insight into the benefits of a lubricant-infused surface.”
Now that they have worked on optimizing the lubricant secretion design, the authors hope it can be implemented in real-life marine vehicles.
“If the present design parameters are adopted, the drag reduction rate will increase significantly,” Professor Sung added.
This work was supported by the National Research Foundation (NRF) of Korea.
Source:
Materials provided by American Institute of Physics.
Publication:
Kim, Seung Joong, et al. (2020). A lubricant-infused slip surface for drag reduction. Physics of Fluids. Available online at https://doi.org/10.1063/5.0018460
Profile:
Hyung Jin Sung
Professor Emeritus
hyungjin@kaist.ac.kr
http://flow.kaist.ac.kr/index.php
Flow Control Lab. (FCL)
Department of Mechanical Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.10.12 View 9249 -
 Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-Publication
Park, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-Profile
Professor Seongjun Park
Bio and Neural Interfaces Laboratory
Department of Bio and Brain Engineering
KAIST
2020.07.13 View 9867
Hydrogel-Based Flexible Brain-Machine Interface
The interface is easy to insert into the body when dry, but behaves ‘stealthily’ inside the brain when wet
Professor Seongjun Park’s research team and collaborators revealed a newly developed hydrogel-based flexible brain-machine interface. To study the structure of the brain or to identify and treat neurological diseases, it is crucial to develop an interface that can stimulate the brain and detect its signals in real time. However, existing neural interfaces are mechanically and chemically different from real brain tissue. This causes foreign body response and forms an insulating layer (glial scar) around the interface, which shortens its lifespan.
To solve this problem, the research team developed a ‘brain-mimicking interface’ by inserting a custom-made multifunctional fiber bundle into the hydrogel body. The device is composed not only of an optical fiber that controls specific nerve cells with light in order to perform optogenetic procedures, but it also has an electrode bundle to read brain signals and a microfluidic channel to deliver drugs to the brain.
The interface is easy to insert into the body when dry, as hydrogels become solid. But once in the body, the hydrogel will quickly absorb body fluids and resemble the properties of its surrounding tissues, thereby minimizing foreign body response.
The research team applied the device on animal models, and showed that it was possible to detect neural signals for up to six months, which is far beyond what had been previously recorded. It was also possible to conduct long-term optogenetic and behavioral experiments on freely moving mice with a significant reduction in foreign body responses such as glial and immunological activation compared to existing devices.
“This research is significant in that it was the first to utilize a hydrogel as part of a multifunctional neural interface probe, which increased its lifespan dramatically,” said Professor Park. “With our discovery, we look forward to advancements in research on neurological disorders like Alzheimer’s or Parkinson’s disease that require long-term observation.”
The research was published in Nature Communications on June 8, 2021. (Title: Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity) The study was conducted jointly with an MIT research team composed of Professor Polina Anikeeva, Professor Xuanhe Zhao, and Dr. Hyunwoo Yook.
This research was supported by the National Research Foundation (NRF) grant for emerging research, Korea Medical Device Development Fund, KK-JRC Smart Project, KAIST Global Initiative Program, and Post-AI Project.
-Publication
Park, S., Yuk, H., Zhao, R. et al. Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nat Commun 12, 3435 (2021). https://doi.org/10.1038/s41467-021-23802-9
-Profile
Professor Seongjun Park
Bio and Neural Interfaces Laboratory
Department of Bio and Brain Engineering
KAIST
2020.07.13 View 9867 -
 Professor Sung Yong Kim Elected as the Chair of PICES MONITOR
< Professor Sung Yong Kim >
Professor Sung Yong Kim from the Department of Mechanical Engineering was elected as the chair of the Technical Committee on Monitoring (MONITOR) of the North Pacific Marine Science Organization (PICES).
PICES is an intergovernmental marine science organization that was established in 1992 through a collaboration between six North Pacific nations including South Korea, Russia, the United States, Japan, China, and Canada to exchange and discuss research on the Pacific waters. Its headquarters is located in Canada and the organization consists of seven affiliated maritime science and marine technology committees.
Professor Kim was elected as the chair of the technical committee that focuses on monitoring and will be part of the Science Board as an ex-officio member. His term will last three years from November 2019.
Professor Kim was recognized for his academic excellence, expertise, and leadership among oceanographers both domestically and internationally.
Professor Kim will also participate as an academia civilian committee member of the Maritime and Fisheries Science and Technology Committee under the Korean Ministry of Oceans and Fisheries for two years from December 18, 2019.
He stated, “I will give my full efforts to broaden Korean oceanography research by participating in maritime leadership positions at home and abroad, and help South Korea become a maritime powerhouse.”
(END)
2019.12.22 View 12455
Professor Sung Yong Kim Elected as the Chair of PICES MONITOR
< Professor Sung Yong Kim >
Professor Sung Yong Kim from the Department of Mechanical Engineering was elected as the chair of the Technical Committee on Monitoring (MONITOR) of the North Pacific Marine Science Organization (PICES).
PICES is an intergovernmental marine science organization that was established in 1992 through a collaboration between six North Pacific nations including South Korea, Russia, the United States, Japan, China, and Canada to exchange and discuss research on the Pacific waters. Its headquarters is located in Canada and the organization consists of seven affiliated maritime science and marine technology committees.
Professor Kim was elected as the chair of the technical committee that focuses on monitoring and will be part of the Science Board as an ex-officio member. His term will last three years from November 2019.
Professor Kim was recognized for his academic excellence, expertise, and leadership among oceanographers both domestically and internationally.
Professor Kim will also participate as an academia civilian committee member of the Maritime and Fisheries Science and Technology Committee under the Korean Ministry of Oceans and Fisheries for two years from December 18, 2019.
He stated, “I will give my full efforts to broaden Korean oceanography research by participating in maritime leadership positions at home and abroad, and help South Korea become a maritime powerhouse.”
(END)
2019.12.22 View 12455 -
 Manipulating Brain Cells by Smartphone
Researchers have developed a soft neural implant that can be wirelessly controlled using a smartphone. It is the first wireless neural device capable of indefinitely delivering multiple drugs and multiple colour lights, which neuroscientists believe can speed up efforts to uncover brain diseases such as Parkinson’s, Alzheimer’s, addiction, depression, and pain.
A team under Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST and his collaborators have invented a device that can control neural circuits using a tiny brain implant controlled by a smartphone. The device, using Lego-like replaceable drug cartridges and powerful, low-energy Bluetooth, can target specific neurons of interest using drugs and light for prolonged periods. This study was published in Nature Biomedical Engineering.
“This novel device is the fruit of advanced electronics design and powerful micro and nanoscale engineering,” explained Professor Jeong. “We are interested in further developing this technology to make a brain implant for clinical applications.”
This technology significantly overshadows the conventional methods used by neuroscientists, which usually involve rigid metal tubes and optical fibers to deliver drugs and light. Apart from limiting the subject’s movement due to bulky equipment, their relatively rigid structure causes lesions in soft brain tissue over time, therefore making them not suitable for long-term implantation. Although some efforts have been made to partly mitigate adverse tissue response by incorporating soft probes and wireless platforms, the previous solutions were limited by their inability to deliver drugs for long periods of time as well as their bulky and complex control setups.
To achieve chronic wireless drug delivery, scientists had to solve the critical challenge of the exhaustion and evaporation of drugs. To combat this, the researchers invented a neural device with a replaceable drug cartridge, which could allow neuroscientists to study the same brain circuits for several months without worrying about running out of drugs.
These ‘plug-n-play’ drug cartridges were assembled into a brain implant for mice with a soft and ultrathin probe (with the thickness of a human hair), which consisted of microfluidic channels and tiny LEDs (smaller than a grain of salt), for unlimited drug doses and light delivery.
Controlled with an elegant and simple user interface on a smartphone, neuroscientists can easily trigger any specific combination or precise sequencing of light and drug delivery in any implanted target animal without the need to be physically inside the laboratory. Using these wireless neural devices, researchers can also easily setup fully automated animal studies where the behaviour of one animal could affect other animals by triggering light and/or drug delivery.
“The wireless neural device enables chronic chemical and optical neuromodulation that has never been achieved before,” said lead author Raza Qazi, a researcher with KAIST and the University of Colorado Boulder.
This work was supported by grants from the National Research Foundation of Korea, US National Institute of Health, National Institute on Drug Abuse, and Mallinckrodt Professorship.
(A neural implant with replaceable drug cartridges and Bluetooth low-energy can target specific neurons .)
(Micro LED controlling using smartphone application)
2019.08.07 View 35356
Manipulating Brain Cells by Smartphone
Researchers have developed a soft neural implant that can be wirelessly controlled using a smartphone. It is the first wireless neural device capable of indefinitely delivering multiple drugs and multiple colour lights, which neuroscientists believe can speed up efforts to uncover brain diseases such as Parkinson’s, Alzheimer’s, addiction, depression, and pain.
A team under Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST and his collaborators have invented a device that can control neural circuits using a tiny brain implant controlled by a smartphone. The device, using Lego-like replaceable drug cartridges and powerful, low-energy Bluetooth, can target specific neurons of interest using drugs and light for prolonged periods. This study was published in Nature Biomedical Engineering.
“This novel device is the fruit of advanced electronics design and powerful micro and nanoscale engineering,” explained Professor Jeong. “We are interested in further developing this technology to make a brain implant for clinical applications.”
This technology significantly overshadows the conventional methods used by neuroscientists, which usually involve rigid metal tubes and optical fibers to deliver drugs and light. Apart from limiting the subject’s movement due to bulky equipment, their relatively rigid structure causes lesions in soft brain tissue over time, therefore making them not suitable for long-term implantation. Although some efforts have been made to partly mitigate adverse tissue response by incorporating soft probes and wireless platforms, the previous solutions were limited by their inability to deliver drugs for long periods of time as well as their bulky and complex control setups.
To achieve chronic wireless drug delivery, scientists had to solve the critical challenge of the exhaustion and evaporation of drugs. To combat this, the researchers invented a neural device with a replaceable drug cartridge, which could allow neuroscientists to study the same brain circuits for several months without worrying about running out of drugs.
These ‘plug-n-play’ drug cartridges were assembled into a brain implant for mice with a soft and ultrathin probe (with the thickness of a human hair), which consisted of microfluidic channels and tiny LEDs (smaller than a grain of salt), for unlimited drug doses and light delivery.
Controlled with an elegant and simple user interface on a smartphone, neuroscientists can easily trigger any specific combination or precise sequencing of light and drug delivery in any implanted target animal without the need to be physically inside the laboratory. Using these wireless neural devices, researchers can also easily setup fully automated animal studies where the behaviour of one animal could affect other animals by triggering light and/or drug delivery.
“The wireless neural device enables chronic chemical and optical neuromodulation that has never been achieved before,” said lead author Raza Qazi, a researcher with KAIST and the University of Colorado Boulder.
This work was supported by grants from the National Research Foundation of Korea, US National Institute of Health, National Institute on Drug Abuse, and Mallinckrodt Professorship.
(A neural implant with replaceable drug cartridges and Bluetooth low-energy can target specific neurons .)
(Micro LED controlling using smartphone application)
2019.08.07 View 35356 -
 Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 34493
Newly Identified Meningeal Lymphatic Vessels Answers the Key Questions on Brain Clearance
(Figure: Schematic images of location and features of meningeal lymphatic vessels and their changes associated with ageing.)
Just see what happens when your neighborhood’s waste disposal system is out of service. Not only do the piles of trash stink but they can indeed hinder the area’s normal functioning. That is also the case when the brain’s waste management is on the blink.
The buildup of toxic proteins in the brain causes a massive damage to the nerves, leading to cognitive dysfunction and increased probability of developing neurodegenerative disorders such as Alzheimer's disease. Though the brain drains its waste via the cerebrospinal fluid (CSF), little has been understood about an accurate route for the brain’s cleansing mechanism.
Medical scientists led by Professor Gou Young Koh at the Graduate School of Medical Science and Engineering have reported the basal side of the skull as the major route, so called “hotspot” for CSF drainage.
They found that basal meningeal lymphatic vessels (mLVs) function as the main plumbing pipes for CSF. They confirmed macromolecules in the CSF mainly runs through the basal mLVs. Notably, the team also revealed that the brain’s major drainage system, specifically basal mLVs are impaired with aging. Their findings have been reported in the journal Nature on July 24.
Throughout our body, excess fluids and waste products are removed from tissues via lymphatic vessels. It was only recently discovered that the brain also has a lymphatic drainage system. mLVs are supposed to carry waste from the brain tissue fluid and the CSF down the deep cervical lymph nodes for disposal. Still scientist are left with one perplexing question — where is the main exit for the CSF? Though mLVs in the upper part of the skull (dorsal meningeal lymphatic vessels) were reported as the brain’s clearance pathways in 2014, no substantial drainage mechanism was observed in that section.
“As a hidden exit for CSF, we looked into the mLVs trapped within complex structures at the base of the skull,” says Dr. Ji Hoon Ahn, the first author of this study. The researchers used several techniques to characterize the basal mLVs in detail. They used a genetically engineered lymphatic-reporter mouse model to visualize mLVs under a fluorescence microscope. By performing a careful examination of the mice skull, they found distinctive features of basal mLVs that make them suitable for CSF uptake and drainage. Just like typical functional lymphatic vessels, basal mLVs are found to have abundant lymphatic vessel branches with finger-like protrusions. Additionally, valves inside the basal mLVs allow the flow to go in one direction. In particular, they found that the basal mLVs are closely located to the CSF. Dr. Hyunsoo Cho, the first author of this study explains, “All up, it seemed a solid case that basal mLVs are the brain’s main clearance pathways.
The researchers verified such specialized morphologic characteristics of basal mLVs indeed facilitate the CSF uptake and drainage. Using CSF contrast-enhanced magnetic resonance imaging in a rat model, they found that CSF is drained preferentially through the basal mLVs. They also utilized a lymphatic-reporter mouse model and discovered that fluorescence-tagged tracer injected into the brain itself or the CSF is cleared mainly through the basal mLVs. Jun-Hee Kim, the first author of this study notes, “We literally saw that the brain clearance mechanism utilizing basal outflow route to exit the skull.
It has long been suggested that CSF turnover and drainage declines with ageing. However, alteration of mLVs associated with ageing is poorly understood. In this study, the researchers observed changes of mLVs in young (3-month-old) and aged (24~27-months-old) mice. They found that the structure of the basal mLVs and their lymphatic valves in aged mice become severely flawed, thus hampering CSF clearance. The corresponding author of this study, Dr. Koh says, “By characterizing the precise route for fluids leaving the brain, this study improves our understanding on how waste is cleared from the brain. Our findings also provide further insights into the role of impaired CSF clearance in the development of age-related neurodegenerative diseases.”
Many current therapies for Alzheimer’s disease target abnormally accumulated proteins, such as beta-amyloid. By mapping out a precise route for the brain’s waste clearance system, this study may be able to help find ways to improve the brain’s cleansing function. Such breakthrough might become quite a sensational strategy for eliminating the buildup of aging-related toxic proteins. “It definitely warrants more extensive investigation of mLVs in patients with age-related neurodegenerative disease such as Alzheimer’s disease prior to clinical investigation,” adds Professor Koh.
2019.07.25 View 34493 -
 Flexible User Interface Distribution for Ubiquitous Multi-Device Interaction
< Research Group of Professor Insik Shin (center) >
KAIST researchers have developed mobile software platform technology that allows a mobile application (app) to be executed simultaneously and more dynamically on multiple smart devices. Its high flexibility and broad applicability can help accelerate a shift from the current single-device paradigm to a multiple one, which enables users to utilize mobile apps in ways previously unthinkable.
Recent trends in mobile and IoT technologies in this era of 5G high-speed wireless communication have been hallmarked by the emergence of new display hardware and smart devices such as dual screens, foldable screens, smart watches, smart TVs, and smart cars. However, the current mobile app ecosystem is still confined to the conventional single-device paradigm in which users can employ only one screen on one device at a time. Due to this limitation, the real potential of multi-device environments has not been fully explored.
A KAIST research team led by Professor Insik Shin from the School of Computing, in collaboration with Professor Steve Ko’s group from the State University of New York at Buffalo, has developed mobile software platform technology named FLUID that can flexibly distribute the user interfaces (UIs) of an app to a number of other devices in real time without needing any modifications. The proposed technology provides single-device virtualization, and ensures that the interactions between the distributed UI elements across multiple devices remain intact.
This flexible multimodal interaction can be realized in diverse ubiquitous user experiences (UX), such as using live video steaming and chatting apps including YouTube, LiveMe, and AfreecaTV. FLUID can ensure that the video is not obscured by the chat window by distributing and displaying them separately on different devices respectively, which lets users enjoy the chat function while watching the video at the same time.
In addition, the UI for the destination input on a navigation app can be migrated into the passenger’s device with the help of FLUID, so that the destination can be easily and safely entered by the passenger while the driver is at the wheel.
FLUID can also support 5G multi-view apps – the latest service that allows sports or games to be viewed from various angles on a single device. With FLUID, the user can watch the event simultaneously from different viewpoints on multiple devices without switching between viewpoints on a single screen.
PhD candidate Sangeun Oh, who is the first author, and his team implemented the prototype of FLUID on the leading open-source mobile operating system, Android, and confirmed that it can successfully deliver the new UX to 20 existing legacy apps.
“This new technology can be applied to next-generation products from South Korean companies such as LG’s dual screen phone and Samsung’s foldable phone and is expected to embolden their competitiveness by giving them a head-start in the global market.” said Professor Shin.
This study will be presented at the 25th Annual International Conference on Mobile Computing and Networking (ACM MobiCom 2019) October 21 through 25 in Los Cabos, Mexico. The research was supported by the National Science Foundation (NSF) (CNS-1350883 (CAREER) and CNS-1618531).
Figure 1. Live video streaming and chatting app scenario
Figure 2. Navigation app scenario
Figure 3. 5G multi-view app scenario
Publication: Sangeun Oh, Ahyeon Kim, Sunjae Lee, Kilho Lee, Dae R. Jeong, Steven Y. Ko, and Insik Shin. 2019. FLUID: Flexible User Interface Distribution for Ubiquitous Multi-device Interaction. To be published in Proceedings of the 25th Annual International Conference on Mobile Computing and Networking (ACM MobiCom 2019). ACM, New York, NY, USA. Article Number and DOI Name TBD.
Video Material:
https://youtu.be/lGO4GwH4enA
Profile: Prof. Insik Shin, MS, PhD
ishin@kaist.ac.kr
https://cps.kaist.ac.kr/~ishin
Professor
Cyber-Physical Systems (CPS) Lab
School of Computing
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr Daejeon 34141, Korea
Profile: Sangeun Oh, PhD Candidate
ohsang1213@kaist.ac.kr
https://cps.kaist.ac.kr/
PhD Candidate
Cyber-Physical Systems (CPS) Lab
School of Computing
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr Daejeon 34141, Korea
Profile: Prof. Steve Ko, PhD
stevko@buffalo.edu
https://nsr.cse.buffalo.edu/?page_id=272
Associate Professor
Networked Systems Research Group
Department of Computer Science and Engineering
State University of New York at Buffalo
http://www.buffalo.edu/ Buffalo 14260, USA
(END)
2019.07.20 View 42628
Flexible User Interface Distribution for Ubiquitous Multi-Device Interaction
< Research Group of Professor Insik Shin (center) >
KAIST researchers have developed mobile software platform technology that allows a mobile application (app) to be executed simultaneously and more dynamically on multiple smart devices. Its high flexibility and broad applicability can help accelerate a shift from the current single-device paradigm to a multiple one, which enables users to utilize mobile apps in ways previously unthinkable.
Recent trends in mobile and IoT technologies in this era of 5G high-speed wireless communication have been hallmarked by the emergence of new display hardware and smart devices such as dual screens, foldable screens, smart watches, smart TVs, and smart cars. However, the current mobile app ecosystem is still confined to the conventional single-device paradigm in which users can employ only one screen on one device at a time. Due to this limitation, the real potential of multi-device environments has not been fully explored.
A KAIST research team led by Professor Insik Shin from the School of Computing, in collaboration with Professor Steve Ko’s group from the State University of New York at Buffalo, has developed mobile software platform technology named FLUID that can flexibly distribute the user interfaces (UIs) of an app to a number of other devices in real time without needing any modifications. The proposed technology provides single-device virtualization, and ensures that the interactions between the distributed UI elements across multiple devices remain intact.
This flexible multimodal interaction can be realized in diverse ubiquitous user experiences (UX), such as using live video steaming and chatting apps including YouTube, LiveMe, and AfreecaTV. FLUID can ensure that the video is not obscured by the chat window by distributing and displaying them separately on different devices respectively, which lets users enjoy the chat function while watching the video at the same time.
In addition, the UI for the destination input on a navigation app can be migrated into the passenger’s device with the help of FLUID, so that the destination can be easily and safely entered by the passenger while the driver is at the wheel.
FLUID can also support 5G multi-view apps – the latest service that allows sports or games to be viewed from various angles on a single device. With FLUID, the user can watch the event simultaneously from different viewpoints on multiple devices without switching between viewpoints on a single screen.
PhD candidate Sangeun Oh, who is the first author, and his team implemented the prototype of FLUID on the leading open-source mobile operating system, Android, and confirmed that it can successfully deliver the new UX to 20 existing legacy apps.
“This new technology can be applied to next-generation products from South Korean companies such as LG’s dual screen phone and Samsung’s foldable phone and is expected to embolden their competitiveness by giving them a head-start in the global market.” said Professor Shin.
This study will be presented at the 25th Annual International Conference on Mobile Computing and Networking (ACM MobiCom 2019) October 21 through 25 in Los Cabos, Mexico. The research was supported by the National Science Foundation (NSF) (CNS-1350883 (CAREER) and CNS-1618531).
Figure 1. Live video streaming and chatting app scenario
Figure 2. Navigation app scenario
Figure 3. 5G multi-view app scenario
Publication: Sangeun Oh, Ahyeon Kim, Sunjae Lee, Kilho Lee, Dae R. Jeong, Steven Y. Ko, and Insik Shin. 2019. FLUID: Flexible User Interface Distribution for Ubiquitous Multi-device Interaction. To be published in Proceedings of the 25th Annual International Conference on Mobile Computing and Networking (ACM MobiCom 2019). ACM, New York, NY, USA. Article Number and DOI Name TBD.
Video Material:
https://youtu.be/lGO4GwH4enA
Profile: Prof. Insik Shin, MS, PhD
ishin@kaist.ac.kr
https://cps.kaist.ac.kr/~ishin
Professor
Cyber-Physical Systems (CPS) Lab
School of Computing
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr Daejeon 34141, Korea
Profile: Sangeun Oh, PhD Candidate
ohsang1213@kaist.ac.kr
https://cps.kaist.ac.kr/
PhD Candidate
Cyber-Physical Systems (CPS) Lab
School of Computing
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr Daejeon 34141, Korea
Profile: Prof. Steve Ko, PhD
stevko@buffalo.edu
https://nsr.cse.buffalo.edu/?page_id=272
Associate Professor
Networked Systems Research Group
Department of Computer Science and Engineering
State University of New York at Buffalo
http://www.buffalo.edu/ Buffalo 14260, USA
(END)
2019.07.20 View 42628 -
 Micropatch Made of DNA
Researchers reported the fabrication of microstructure arrays of DNA materials using topographic control. This method provides a platform for forming multiscale hierarchical orientations of soft and biomaterials using a process of simple shearing and controlled evaporation on a patterned substrate. This approach enables the potential of patterning applications using DNA or other anisotropic biomaterials.
DNA is one of the most abundant biomaterials found in all living organisms in nature. It has unique characteristics of fine feature size and liquid crystalline phase, enabling to create various kinds of microstructure DNA arrays. Based on these characteristics, DNA has been used as a building block for “origami” and textile art at the nanometer scale.
A KAIST research team led by Professors Dong Ki Yoon and Hyungsoo Kim fabricated a DNA-based micropatch using the “coffee ring effect” and its multi-angle control technology, which was published online in Nature Communications on June 7.
The research team used cheap DNA material extracted from salmon to realize the micropatch structure with well-aligned knit or ice cream cone shapes. When the DNA material in an aqueous solution is rubbed between two solid substrates while water is evaporating, DNA chains are unidirectionally aligned to make a thin film such as in LCD display devices. The DNA chains can make more complex microstructures such as knit or a texture with ice cream cone shapes when the same procedure is carried out in topographical patterns like microposts (Figure 1). This can be applied to make metamaterials by mixing with functionalized gold nanorods to show plasmonic color.
Plasmon resonance is a phenomenon in which electrons vibrate uniformly on the surface of a substrate made of metal, reacting only to light that matches a specific energy to enhance the clarity and expression of colors. For this, the most important factor is the orientation in which the gold nanorods align. That is, when the rods are aligned side by side in one direction, the optical and electrical characteristics are maximized. The research team focused on this point and made the DNA micropatch as a frame to orient the gold nanorods in a unique shape and fabricated a plasmonic color film (Figure 2).
Professor Yoon said this study is meaningful in that it deals with the evaporation phenomenon, which has not been studied much in the field of polymers and biopolymers in terms of basic science. He explained, “This will also help maximize the efficiency of polymeric materials that can be orientated in coating, 2D, and 3D printing applications. Furthermore, DNA that exists infinitely in nature can be expected to have industrial application value as a new material since it can easily form complexes with other materials as described in this study.”
(Figure 1. The DNA micropatch using topographic control. (a) The experimental scheme.
(b) Enlarged image of (e). (c-e) Different micropatches made of DNA using different shearing directions.)
(Figure 2. The knit-like structures made of DNA-gold nanorod complex. (a,b) Optical
and polarized optical microscopy images. (c-f) Plasmonic colors reflected from aligned DNA-gold nanorod complex depending on the sample rotation.)
2019.07.01 View 36547
Micropatch Made of DNA
Researchers reported the fabrication of microstructure arrays of DNA materials using topographic control. This method provides a platform for forming multiscale hierarchical orientations of soft and biomaterials using a process of simple shearing and controlled evaporation on a patterned substrate. This approach enables the potential of patterning applications using DNA or other anisotropic biomaterials.
DNA is one of the most abundant biomaterials found in all living organisms in nature. It has unique characteristics of fine feature size and liquid crystalline phase, enabling to create various kinds of microstructure DNA arrays. Based on these characteristics, DNA has been used as a building block for “origami” and textile art at the nanometer scale.
A KAIST research team led by Professors Dong Ki Yoon and Hyungsoo Kim fabricated a DNA-based micropatch using the “coffee ring effect” and its multi-angle control technology, which was published online in Nature Communications on June 7.
The research team used cheap DNA material extracted from salmon to realize the micropatch structure with well-aligned knit or ice cream cone shapes. When the DNA material in an aqueous solution is rubbed between two solid substrates while water is evaporating, DNA chains are unidirectionally aligned to make a thin film such as in LCD display devices. The DNA chains can make more complex microstructures such as knit or a texture with ice cream cone shapes when the same procedure is carried out in topographical patterns like microposts (Figure 1). This can be applied to make metamaterials by mixing with functionalized gold nanorods to show plasmonic color.
Plasmon resonance is a phenomenon in which electrons vibrate uniformly on the surface of a substrate made of metal, reacting only to light that matches a specific energy to enhance the clarity and expression of colors. For this, the most important factor is the orientation in which the gold nanorods align. That is, when the rods are aligned side by side in one direction, the optical and electrical characteristics are maximized. The research team focused on this point and made the DNA micropatch as a frame to orient the gold nanorods in a unique shape and fabricated a plasmonic color film (Figure 2).
Professor Yoon said this study is meaningful in that it deals with the evaporation phenomenon, which has not been studied much in the field of polymers and biopolymers in terms of basic science. He explained, “This will also help maximize the efficiency of polymeric materials that can be orientated in coating, 2D, and 3D printing applications. Furthermore, DNA that exists infinitely in nature can be expected to have industrial application value as a new material since it can easily form complexes with other materials as described in this study.”
(Figure 1. The DNA micropatch using topographic control. (a) The experimental scheme.
(b) Enlarged image of (e). (c-e) Different micropatches made of DNA using different shearing directions.)
(Figure 2. The knit-like structures made of DNA-gold nanorod complex. (a,b) Optical
and polarized optical microscopy images. (c-f) Plasmonic colors reflected from aligned DNA-gold nanorod complex depending on the sample rotation.)
2019.07.01 View 36547