Drug+Discovery
-
 KAIST Changes the Paradigm of Drug Discovery with World's First Atomic Editing
In pioneering drug development, the new technology that enables the easy and rapid editing of key atoms responsible for drug efficacy has been regarded as a fundamental and "dream" technology, revolutionizing the process of discovering potential drug candidates. KAIST researchers have become the first in the world to successfully develop single-atom editing technology that maximizes drug efficacy.
On October 8th, KAIST (represented by President Kwang-Hyung Lee) announced that Professor Yoonsu Park’s research team from the Department of Chemistry successfully developed technology that enables the easy editing and correction of oxygen atoms in furan compounds into nitrogen atoms, directly converting them into pyrrole frameworks, which are widely used in pharmaceuticals.
< Image. Conceptual image illustrating the main idea of the research >
This research was published in the prestigious scientific journal Science on October 3rd under the title "Photocatalytic Furan-to-Pyrrole Conversion."
Many drugs have complex chemical structures, but their efficacy is often determined by a single critical atom. Atoms like oxygen and nitrogen play a central role in enhancing the pharmacological effects of these drugs, particularly against viruses.
This phenomenon, where the introduction of specific atoms into a drug molecule dramatically affects its efficacy, is known as the "Single Atom Effect." In leading-edge drug development, discovering atoms that maximize drug efficacy is key.
However, evaluating the Single Atom Effect has traditionally required multi-step, costly synthesis processes, as it has been difficult to selectively edit single atoms within stable ring structures containing oxygen or nitrogen.
Professor Park’s team overcame this challenge by introducing a photocatalyst that uses light energy. They developed a photocatalyst that acts as a “molecular scissor,” freely cutting and attaching five-membered rings, enabling single-atom editing at room temperature and atmospheric pressure—a world first.
The team discovered a new reaction mechanism in which the excited molecular scissor removes oxygen from furan via single-electron oxidation and then sequentially adds a nitrogen atom.
Donghyeon Kim and Jaehyun You, the study's first authors and candidates in KAIST’s integrated master's and doctoral program in the Department of Chemistry, explained that this technique offers high versatility by utilizing light energy to replace harsh conditions. They further noted that the technology enables selective editing, even when applied to complex natural products or pharmaceuticals. Professor Yoonsu Park, who led the research, remarked, "This breakthrough, which allows for the selective editing of five-membered organic ring structures, will open new doors for building libraries of drug candidates, a key challenge in pharmaceuticals. I hope this foundational technology will be used to revolutionize the drug development process."
The significance of this research was highlighted in the Perspective section of Science, a feature where a peer scientist of prominence outside of the project group provides commentary on an impactful research.
This research was supported by the National Research Foundation of Korea’s Creative Research Program, the Cross-Generation Collaborative Lab Project at KAIST, and the POSCO Science Fellowship of the POSCO TJ Park Foundation.
2024.10.11 View 6510
KAIST Changes the Paradigm of Drug Discovery with World's First Atomic Editing
In pioneering drug development, the new technology that enables the easy and rapid editing of key atoms responsible for drug efficacy has been regarded as a fundamental and "dream" technology, revolutionizing the process of discovering potential drug candidates. KAIST researchers have become the first in the world to successfully develop single-atom editing technology that maximizes drug efficacy.
On October 8th, KAIST (represented by President Kwang-Hyung Lee) announced that Professor Yoonsu Park’s research team from the Department of Chemistry successfully developed technology that enables the easy editing and correction of oxygen atoms in furan compounds into nitrogen atoms, directly converting them into pyrrole frameworks, which are widely used in pharmaceuticals.
< Image. Conceptual image illustrating the main idea of the research >
This research was published in the prestigious scientific journal Science on October 3rd under the title "Photocatalytic Furan-to-Pyrrole Conversion."
Many drugs have complex chemical structures, but their efficacy is often determined by a single critical atom. Atoms like oxygen and nitrogen play a central role in enhancing the pharmacological effects of these drugs, particularly against viruses.
This phenomenon, where the introduction of specific atoms into a drug molecule dramatically affects its efficacy, is known as the "Single Atom Effect." In leading-edge drug development, discovering atoms that maximize drug efficacy is key.
However, evaluating the Single Atom Effect has traditionally required multi-step, costly synthesis processes, as it has been difficult to selectively edit single atoms within stable ring structures containing oxygen or nitrogen.
Professor Park’s team overcame this challenge by introducing a photocatalyst that uses light energy. They developed a photocatalyst that acts as a “molecular scissor,” freely cutting and attaching five-membered rings, enabling single-atom editing at room temperature and atmospheric pressure—a world first.
The team discovered a new reaction mechanism in which the excited molecular scissor removes oxygen from furan via single-electron oxidation and then sequentially adds a nitrogen atom.
Donghyeon Kim and Jaehyun You, the study's first authors and candidates in KAIST’s integrated master's and doctoral program in the Department of Chemistry, explained that this technique offers high versatility by utilizing light energy to replace harsh conditions. They further noted that the technology enables selective editing, even when applied to complex natural products or pharmaceuticals. Professor Yoonsu Park, who led the research, remarked, "This breakthrough, which allows for the selective editing of five-membered organic ring structures, will open new doors for building libraries of drug candidates, a key challenge in pharmaceuticals. I hope this foundational technology will be used to revolutionize the drug development process."
The significance of this research was highlighted in the Perspective section of Science, a feature where a peer scientist of prominence outside of the project group provides commentary on an impactful research.
This research was supported by the National Research Foundation of Korea’s Creative Research Program, the Cross-Generation Collaborative Lab Project at KAIST, and the POSCO Science Fellowship of the POSCO TJ Park Foundation.
2024.10.11 View 6510 -
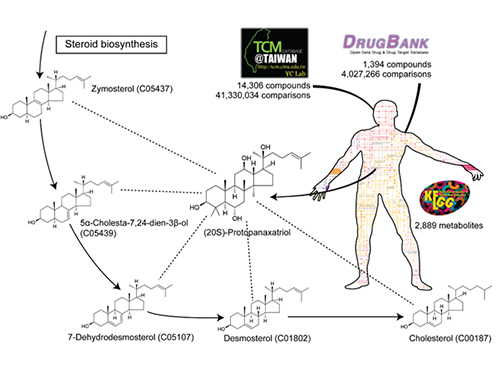 System Approach Using Metabolite Structural Similarity Toward TOM Suggested
A Korean research team at KAIST suggests that a system approach using metabolite structural similarity helps to elucidate the mechanisms of action of traditional oriental medicine.
Traditional oriental medicine (TOM) has been practiced in Asian countries for centuries, and is gaining increasing popularity around the world. Despite its efficacy in various symptoms, TOM has been practiced without precise knowledge of its mechanisms of action. Use of TOM largely comes from empirical knowledge practiced over a long period of time. The fact that some of the compounds found in TOM have led to successful modern drugs such as artemisinin for malaria and taxol (Paclitaxel) for cancer has spurred modernization of TOM.
A research team led by Sang-Yup Lee at KAIST has focused on structural similarities between compounds in TOM and human metabolites to help explain TOM’s mechanisms of action. This systems approach using structural similarities assumes that compounds which are structurally similar to metabolites could affect relevant metabolic pathways and reactions by biosynthesizing structurally similar metabolites.
Structural similarity analysis has helped to identify mechanisms of action of TOM. This is described in a recent study entitled “A systems approach to traditional oriental medicine,” published online in Nature Biotechnology on March 6, 2015. In this study, the research team conducted structural comparisons of all the structurally known compounds in TOM and human metabolites on a large-scale. As a control, structures of all available approved drugs were also compared against human metabolites. This structural analysis provides two important results. First, the identification of metabolites structurally similar to TOM compounds helped to narrow down the candidate target pathways and reactions for the effects from TOM compounds. Second, it suggested that a greater fraction of all the structurally known TOM compounds appeared to be more similar to human metabolites than the approved drugs. This second finding indicates that TOM has a great potential to interact with diverse metabolic pathways with strong efficacy. This finding, in fact, shows that TOM compounds might be advantageous for the multitargeting required to cure complex diseases. “Once we have narrowed down candidate target pathways and reactions using this structural similarity approach, additional in silico tools will be necessary to characterize the mechanisms of action of many TOM compounds at a molecular level,” said Hyun Uk Kim, a research professor at KAIST.
TOM’s multicomponent, multitarget approach wherein multiple components show synergistic effects to treat symptoms is highly distinctive. The researchers investigated previously observed effects recorded since 2000 of a set of TOM compounds with known mechanisms of action. TOM compounds’ synergistic combinations largely consist of a major compound providing the intended efficacy to the target site and supporting compounds which maximize the efficacy of the major compound. In fact, such combination designs appear to mirror the Kun-Shin-Choa-Sa design principle of TOM.
That principle, Kun-Shin-Choa-Sa (君臣佐使 or Jun-Chen-Zuo-Shi in Chinese) literally means “king-minister-assistant-ambassador.” In ancient East Asian medicine, treating human diseases and taking good care of the human body are analogous to the politics of governing a nation. Just as good governance requires that a king be supported by ministers, assistants and/or ambassadors, treating diseases or good care of the body required the combined use of herbal medicines designed based on the concept of Kun-Shin-Choa-Sa. Here, the Kun (king or the major component) indicates the major medicine (or herb) conveying the major drug efficacy, and is supported by three different types of medicines: the Shin (minister or the complementary component) for enhancing and/or complementing the efficacy of the Kun, Choa (assistant or the neutralizing component) for reducing any side effects caused by the Kun and reducing the minor symptoms accompanying major symptom, and Sa (ambassador or the delivery/retaining component) which facilitated the delivery of the Kun to the target site, and retaining the Kun for prolonged availability in the cells.
The synergistic combinations of TOM compounds reported in the literature showed four different types of synergisms: complementary action (similar to Kun-Shin), neutralizing action (similar to Kun-Choa), facilitating action or pharmacokinetic potentiation (both similar to Kun-Choa or Kun-Sa). Additional structural analyses for these compounds with synergism show that they appeared to affect metabolism of amino acids, co-factors and vitamins as major targets.
Professor Sang Yup Lee remarks, “This study lays a foundation for the integration of traditional oriental medicine with modern drug discovery and development. The systems approach taken in this analysis will be used to elucidate mechanisms of action of unknown TOM compounds which will then be subjected to rigorous validation through clinical and in silico experiments.”
Sources: Kim, H.U. et al. “A systems approach to traditional oriental medicine.” Nature Biotechnology. 33: 264-268 (2015).
This work was supported by the Bio-Synergy Research Project (2012M3A9C4048759) of the Ministry of Science, ICT and Future Planning through the National Research Foundation. This work was also supported by the Novo Nordisk Foundation.
The picture below presents the structural similarity analysis of comparing compounds in traditional oriental medicine and those in all available approved drugs against the structures of human metabolites.
2015.03.09 View 11846
System Approach Using Metabolite Structural Similarity Toward TOM Suggested
A Korean research team at KAIST suggests that a system approach using metabolite structural similarity helps to elucidate the mechanisms of action of traditional oriental medicine.
Traditional oriental medicine (TOM) has been practiced in Asian countries for centuries, and is gaining increasing popularity around the world. Despite its efficacy in various symptoms, TOM has been practiced without precise knowledge of its mechanisms of action. Use of TOM largely comes from empirical knowledge practiced over a long period of time. The fact that some of the compounds found in TOM have led to successful modern drugs such as artemisinin for malaria and taxol (Paclitaxel) for cancer has spurred modernization of TOM.
A research team led by Sang-Yup Lee at KAIST has focused on structural similarities between compounds in TOM and human metabolites to help explain TOM’s mechanisms of action. This systems approach using structural similarities assumes that compounds which are structurally similar to metabolites could affect relevant metabolic pathways and reactions by biosynthesizing structurally similar metabolites.
Structural similarity analysis has helped to identify mechanisms of action of TOM. This is described in a recent study entitled “A systems approach to traditional oriental medicine,” published online in Nature Biotechnology on March 6, 2015. In this study, the research team conducted structural comparisons of all the structurally known compounds in TOM and human metabolites on a large-scale. As a control, structures of all available approved drugs were also compared against human metabolites. This structural analysis provides two important results. First, the identification of metabolites structurally similar to TOM compounds helped to narrow down the candidate target pathways and reactions for the effects from TOM compounds. Second, it suggested that a greater fraction of all the structurally known TOM compounds appeared to be more similar to human metabolites than the approved drugs. This second finding indicates that TOM has a great potential to interact with diverse metabolic pathways with strong efficacy. This finding, in fact, shows that TOM compounds might be advantageous for the multitargeting required to cure complex diseases. “Once we have narrowed down candidate target pathways and reactions using this structural similarity approach, additional in silico tools will be necessary to characterize the mechanisms of action of many TOM compounds at a molecular level,” said Hyun Uk Kim, a research professor at KAIST.
TOM’s multicomponent, multitarget approach wherein multiple components show synergistic effects to treat symptoms is highly distinctive. The researchers investigated previously observed effects recorded since 2000 of a set of TOM compounds with known mechanisms of action. TOM compounds’ synergistic combinations largely consist of a major compound providing the intended efficacy to the target site and supporting compounds which maximize the efficacy of the major compound. In fact, such combination designs appear to mirror the Kun-Shin-Choa-Sa design principle of TOM.
That principle, Kun-Shin-Choa-Sa (君臣佐使 or Jun-Chen-Zuo-Shi in Chinese) literally means “king-minister-assistant-ambassador.” In ancient East Asian medicine, treating human diseases and taking good care of the human body are analogous to the politics of governing a nation. Just as good governance requires that a king be supported by ministers, assistants and/or ambassadors, treating diseases or good care of the body required the combined use of herbal medicines designed based on the concept of Kun-Shin-Choa-Sa. Here, the Kun (king or the major component) indicates the major medicine (or herb) conveying the major drug efficacy, and is supported by three different types of medicines: the Shin (minister or the complementary component) for enhancing and/or complementing the efficacy of the Kun, Choa (assistant or the neutralizing component) for reducing any side effects caused by the Kun and reducing the minor symptoms accompanying major symptom, and Sa (ambassador or the delivery/retaining component) which facilitated the delivery of the Kun to the target site, and retaining the Kun for prolonged availability in the cells.
The synergistic combinations of TOM compounds reported in the literature showed four different types of synergisms: complementary action (similar to Kun-Shin), neutralizing action (similar to Kun-Choa), facilitating action or pharmacokinetic potentiation (both similar to Kun-Choa or Kun-Sa). Additional structural analyses for these compounds with synergism show that they appeared to affect metabolism of amino acids, co-factors and vitamins as major targets.
Professor Sang Yup Lee remarks, “This study lays a foundation for the integration of traditional oriental medicine with modern drug discovery and development. The systems approach taken in this analysis will be used to elucidate mechanisms of action of unknown TOM compounds which will then be subjected to rigorous validation through clinical and in silico experiments.”
Sources: Kim, H.U. et al. “A systems approach to traditional oriental medicine.” Nature Biotechnology. 33: 264-268 (2015).
This work was supported by the Bio-Synergy Research Project (2012M3A9C4048759) of the Ministry of Science, ICT and Future Planning through the National Research Foundation. This work was also supported by the Novo Nordisk Foundation.
The picture below presents the structural similarity analysis of comparing compounds in traditional oriental medicine and those in all available approved drugs against the structures of human metabolites.
2015.03.09 View 11846 -
 The hereditary factor of autism revealed
Korean researchers have successfully investigated the causes and hereditary factors for autistic behavior and proposed a new treatment method with fewer side effects.
This research was jointly supported by the Ministry of Education, Science and Technology and the National Research Foundation as part of the Leading Researcher and Science Research Center Program
The research findings were publishing in the June edition of Nature magazine and will also be introduced in the July edition of Nature Reviews Drug Discovery, under the title ‘Autistic-like social behavior in Shank2-mutant mice improved by restoring NMDA receptor function’.
The research team found that lack of Shank2 genes in mice, which are responsible for the production of synapse proteins, caused autistic-like behavior. The results strongly suggested that the Shank2 gene was linked to autistic behavior and that Shank2 deficiency induced autistic behaviors.
Autism is a neural development disorder characterized by impaired social interaction, repetitive behavior, mental retardation, anxiety and hyperactivity. Around 100 million people worldwide display symptoms of autistic behavior. Recent studies conducted by the University of Washington revealed that 1 out of 3 young adults who display autistic behavior do not fit into the workplace or get accepted to college, a much higher rate than any other disorder. However, an effective cure has not yet been developed and current treatments are limited to reducing repetitive behavior.
The research team confirmed autistic-like social behavior in mice without the Shank2 genes and that the mice had decreased levels of neurotransmission in the NMDA receptor. The mice also showed damaged synaptic plasticity* in the hippocampus**.
* Plasticity: ability of the connectionbetween two neurons to change in strength in response to transmission of information
**Hippocampus: part of the brain responsible for short-term and long-term memory as well as spatial navigation.
The research team also found out that, to restore the function of the NMDA receptor, the passive stimulation of certain receptors, such as the mGLuR5, yielded better treatment results than the direct stimulation of the NMDA. This greatly reduces the side effects associated with the direct stimulation of receptors, resulting in a more effective treatment method.
This research successfully investigated the function of the Shank2 gene in the nerve tissue and showed how the reduced function of the NMDA receptor, due to the lack of the gene, resulted in autistic behavior. It also provided new possibilities for the treatment of autistic behavior and impaired social interaction
2012.06.24 View 15004
The hereditary factor of autism revealed
Korean researchers have successfully investigated the causes and hereditary factors for autistic behavior and proposed a new treatment method with fewer side effects.
This research was jointly supported by the Ministry of Education, Science and Technology and the National Research Foundation as part of the Leading Researcher and Science Research Center Program
The research findings were publishing in the June edition of Nature magazine and will also be introduced in the July edition of Nature Reviews Drug Discovery, under the title ‘Autistic-like social behavior in Shank2-mutant mice improved by restoring NMDA receptor function’.
The research team found that lack of Shank2 genes in mice, which are responsible for the production of synapse proteins, caused autistic-like behavior. The results strongly suggested that the Shank2 gene was linked to autistic behavior and that Shank2 deficiency induced autistic behaviors.
Autism is a neural development disorder characterized by impaired social interaction, repetitive behavior, mental retardation, anxiety and hyperactivity. Around 100 million people worldwide display symptoms of autistic behavior. Recent studies conducted by the University of Washington revealed that 1 out of 3 young adults who display autistic behavior do not fit into the workplace or get accepted to college, a much higher rate than any other disorder. However, an effective cure has not yet been developed and current treatments are limited to reducing repetitive behavior.
The research team confirmed autistic-like social behavior in mice without the Shank2 genes and that the mice had decreased levels of neurotransmission in the NMDA receptor. The mice also showed damaged synaptic plasticity* in the hippocampus**.
* Plasticity: ability of the connectionbetween two neurons to change in strength in response to transmission of information
**Hippocampus: part of the brain responsible for short-term and long-term memory as well as spatial navigation.
The research team also found out that, to restore the function of the NMDA receptor, the passive stimulation of certain receptors, such as the mGLuR5, yielded better treatment results than the direct stimulation of the NMDA. This greatly reduces the side effects associated with the direct stimulation of receptors, resulting in a more effective treatment method.
This research successfully investigated the function of the Shank2 gene in the nerve tissue and showed how the reduced function of the NMDA receptor, due to the lack of the gene, resulted in autistic behavior. It also provided new possibilities for the treatment of autistic behavior and impaired social interaction
2012.06.24 View 15004