Sukbok+Chang
-
 Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 9335
Distinguished Professor Sukbok Chang Donates His Prize Money
The honoree of the 2019 Korea Best Scientist and Technologist Award, Distinguished Professor Sukbok Chang donated his prize money of one hundred million KRW to the Chemistry Department Scholarship Fund and the Lyu Keun-Chul Sports Complex Management Fund during a donation ceremony last week.
Professor Chang won the award last month in recognition of his pioneering achievements and lifetime contributions to the development of carbon-hydrogen activation strategies, especially for carbon-carbon, carbon-nitrogen, and carbon-oxygen formations. Professor Chang, a world renowned chemist, has been recognized for his highly selective catalytic systems, allowing the controlled defunctionalization of bio-derived platform substrates under mild conditions and opening a new avenue for the utilization of biomass-derived platform chemicals.
“All my achievements are the results of my students’ hard work and dedication. I feel very fortunate to have such talented team members. I want to express my sincere gratitude for such a great research environment that we have worked together in so far,” said Professor Chang at the ceremony.
KAIST President Sung-Chul Shin said, “Not only will Professor Chang’s donation make a significant contribution to the Department of Chemistry, but also to the improvement of the Lyu Keun-Chul Sports Complex’s management, which directly links to the health and welfare of the KAIST community.”
Professor Chang currently holds the position of distinguished professor at KAIST and director of the Center for Catalytic Hydrocarbon Functionalizations in the Institute for Basic Science (IBS). He previously received the Kyung-Ahm Academic Award in 2013 and the Korea Toray Science Award in 2018. All these prize money also went to the school.
(END)
2019.08.26 View 9335 -
 New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7994
New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7994 -
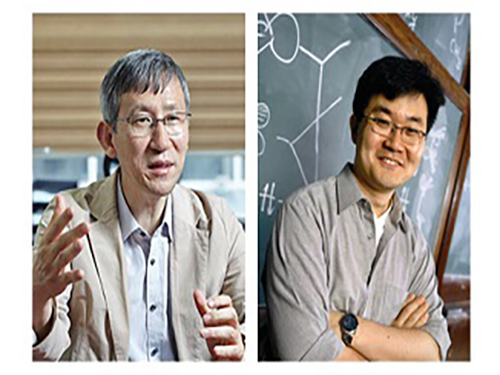 New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6373
New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6373