research

< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
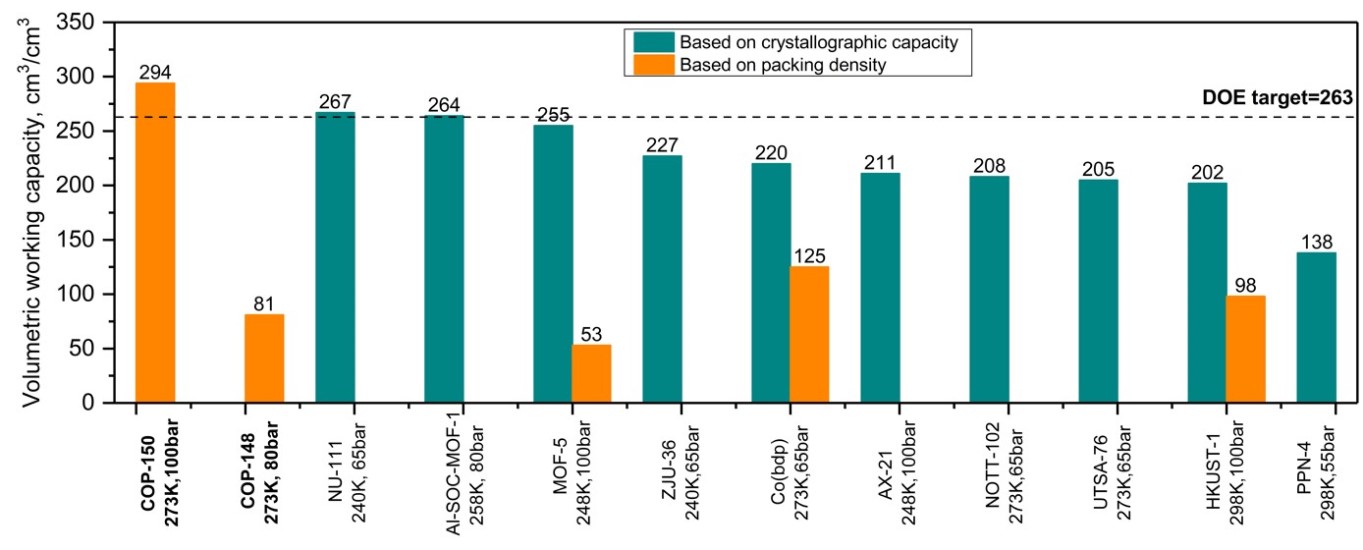
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
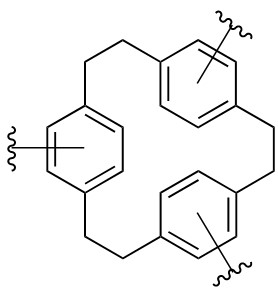
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< 20190809114013_21726_594363160.jpg >
< Suggested chemical structure of COP-150 >

< 20190809114220_53158_561104017.jpg >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< 20190809114507_17336_810323032.jpg >
< Comparison of highest reported volumetric working capacities >
(END)