research
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
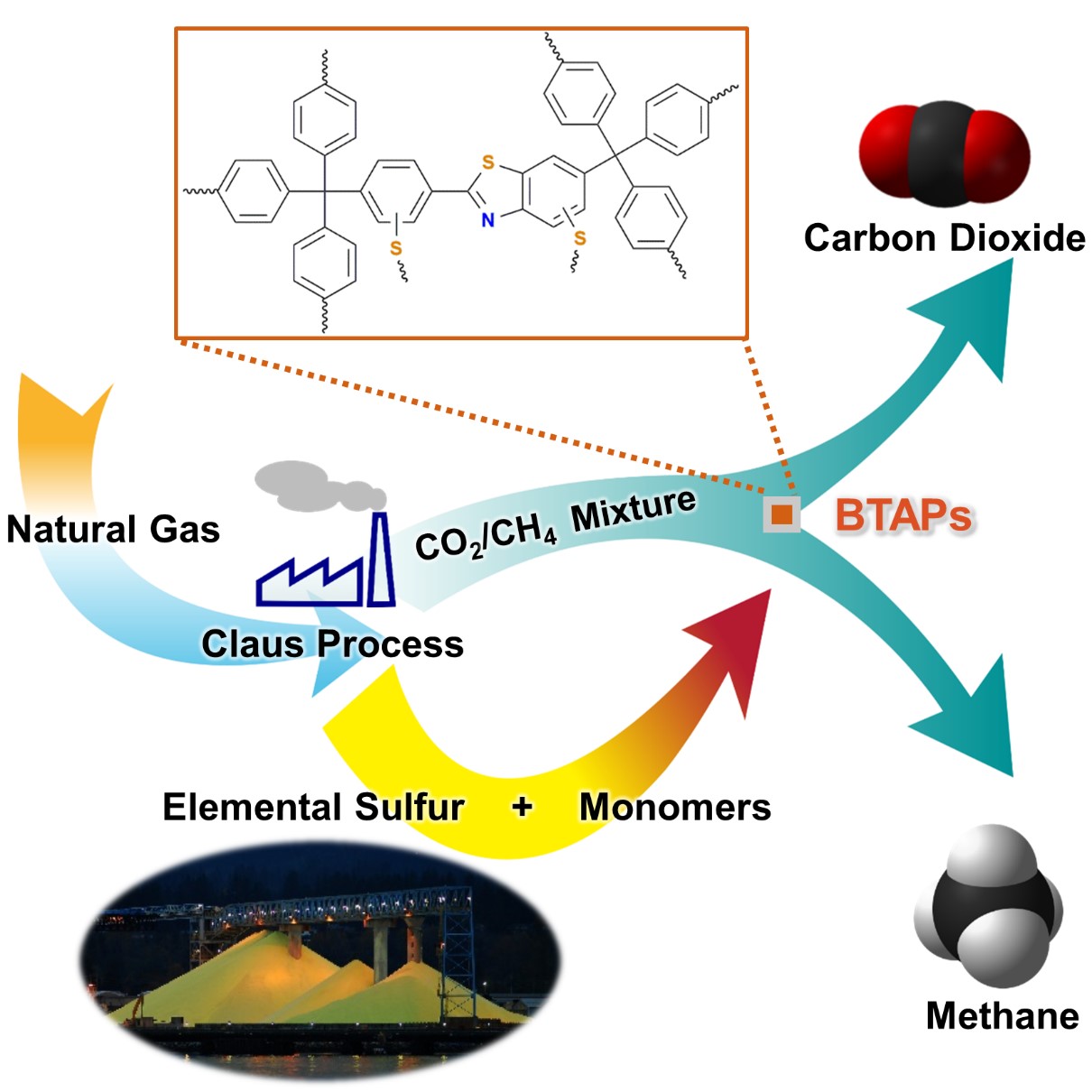
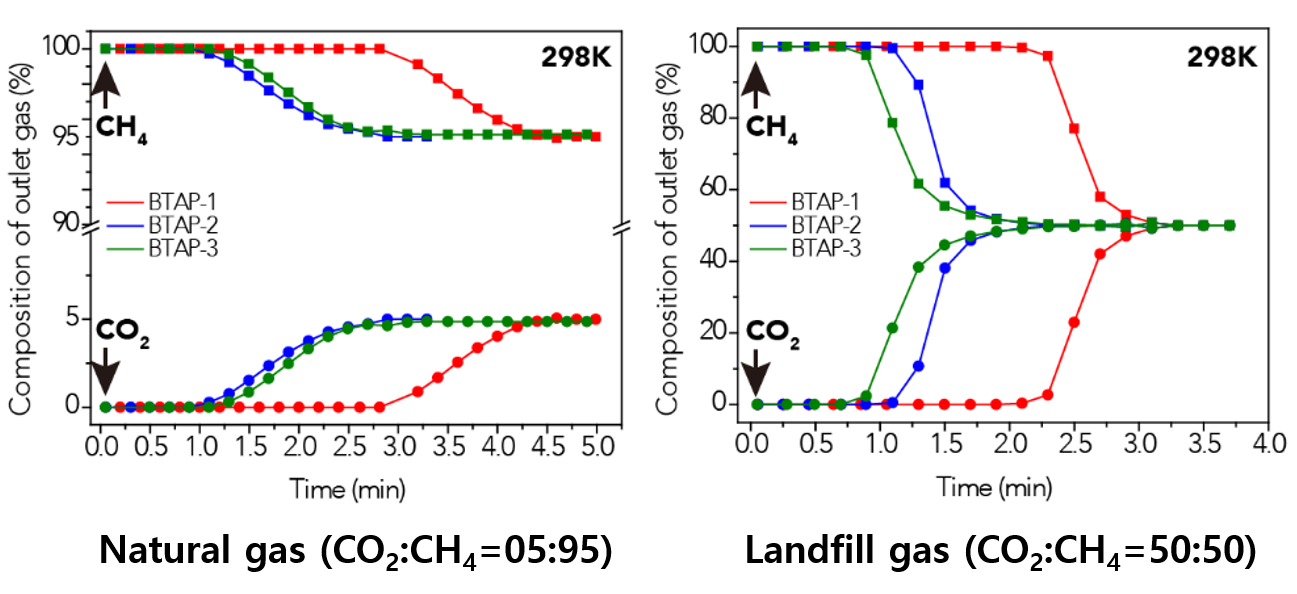
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
-
research New and Highly Efficient Recycling Technology to Turn Used Tires into Raw Materials for Rubber and Nylon
< (From left) Kyungmin Choi (MS-Ph.D. integrated course, Department of Chemistry), Dr. Beomsoon Park, Professor Soon Hyeok Hong, Dr. Kyoungil Cho > Approximately 1.5 billions of tires are discarded globally every year, and this is identified as one of the major causes of serious environmental pollution. The research team at the Department of Chemistry at KAIST has achieved a breakthrough by selectively converting waste tires into high-purity cyclic alkenes, valuable chemical buildin
2025-06-26 -
people Distinguished Professor Sang Yup Lee Wins 2025 Global Metabolic Engineering Award
< Distinguished Professor Sang Yup Lee (Senior Vice President for Research) from the Department of Chemical & Biomolecular Engineering > KAIST announced on the 20th that Professor Sang Yup Lee, who serves as the Vice President for Research and a Distinguished Professor at our university, has been awarded the '2025 Gregory N. Stephanopoulos Award for Metabolic Engineering' by the International Metabolic Engineering Society (IMES). Professor Lee delivered his award lecture at the 16
2025-06-20 -
research KAIST Develops Glare-Free, Heat-Blocking 'Smart Window'... Applicable to Buildings and Vehicles
• Professor Hong Chul Moon of the Department of Chemical and Biomolecular Engineering develops RECM, a next-generation smart window technology, expecting cooling energy savings and effective indoor thermal management. • When using the developed RECM, a significantly superior temperature reduction effect is observed compared to conventional windows. • With a 'pedestrian-friendly smart window' design that eliminates glare by suppressing external reflections, it is expected to be a
2025-06-20 -
research Simultaneous Analysis of 21 Chemical Reactions... AI to Transform New Drug Development
< Photo 1. (From left) Professor Hyunwoo Kim and students Donghun Kim and Gyeongseon Choi in the Integrated M.S./Ph.D. program of the Department of Chemistry > Thalidomide, a drug once used to alleviate morning sickness in pregnant women, exhibits distinct properties due to its optical isomers* in the body: one isomer has a sedative effect, while the other causes severe side effects like birth defects. As this example illustrates, precise organic synthesis techniques, which selectivel
2025-06-16 -
research KAIST develops technology for selective RNA modification in living cells and animals
· A team led by Professor Won Do Heo from the Department of Biological Sciences, KAIST, has developed a pioneering technology that selectively acetylates specific RNA molecules in living cells and tissues. · The platform uses RNA-targeting CRISPR tools in combination with RNA-modifying enzymes to chemically modify only the intended RNA. · The method opens new possibilities for gene therapy by enabling precise control of disease-related RNA without affecting the rest of the
2025-06-10