research
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
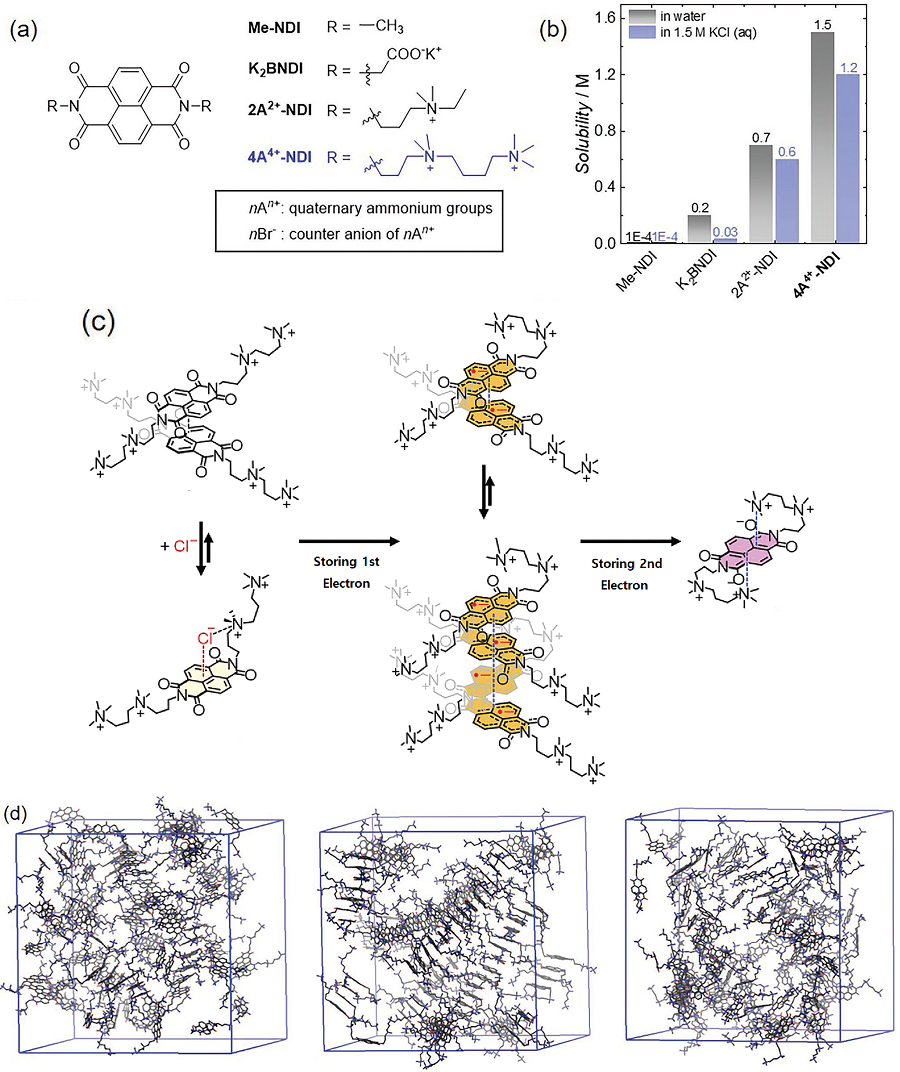
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
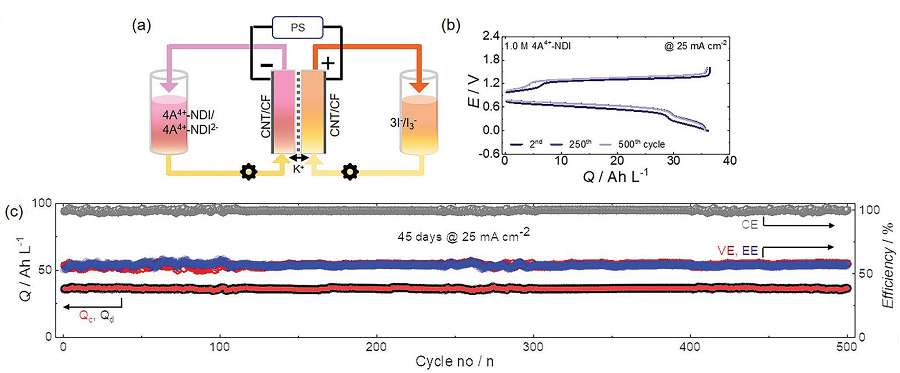
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.