research
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
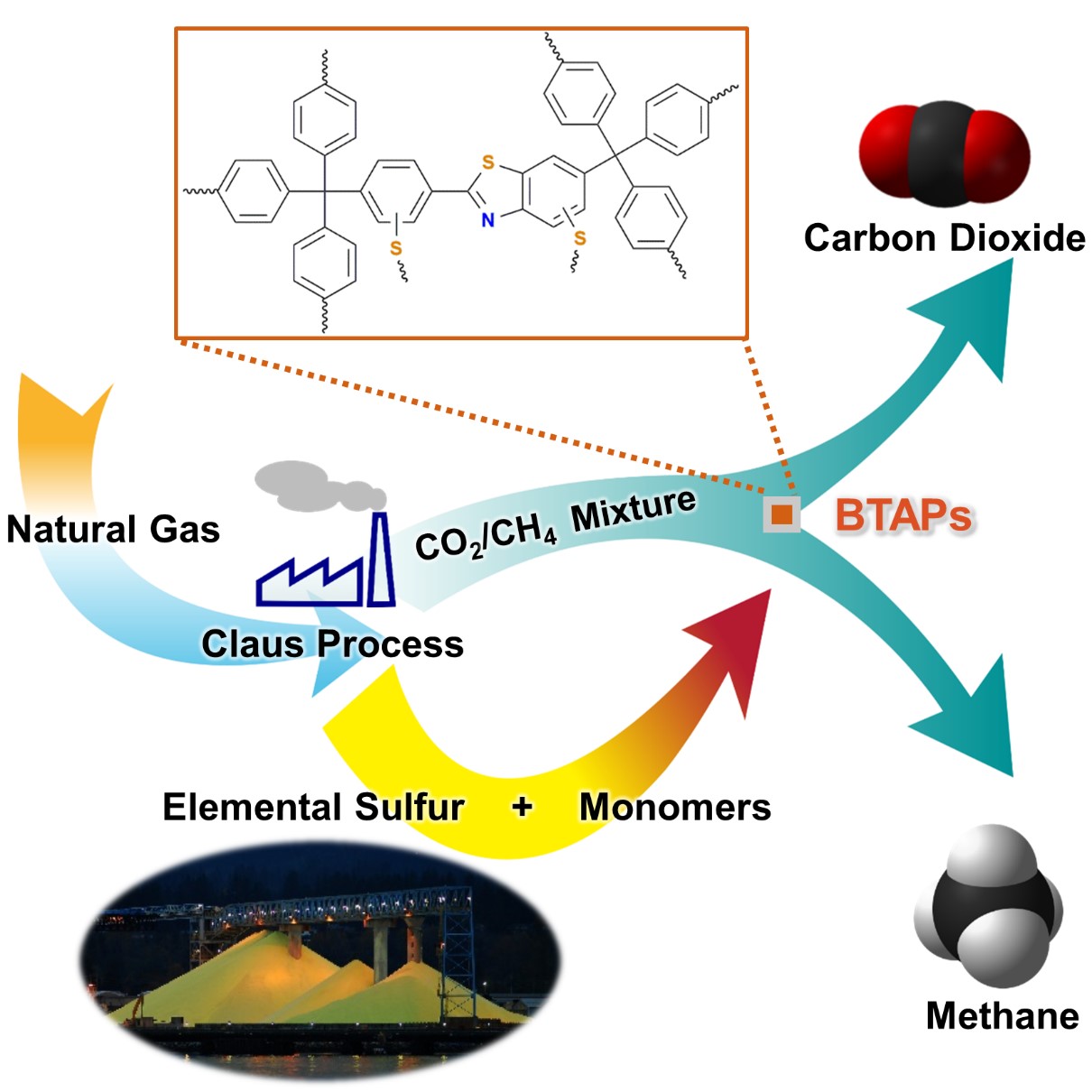
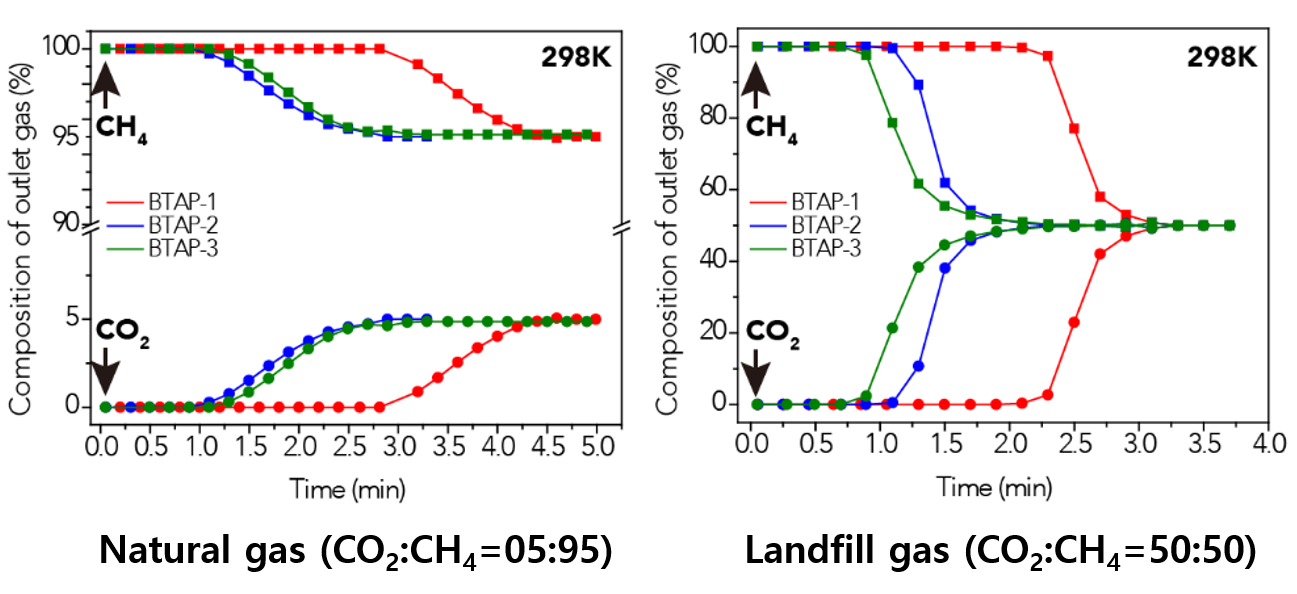
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
-
research KAIST Develops AI That Automatically Designs Optimal Drug Candidates for Cancer-Targeting Mutations
< (From left) Ph.D candidate Wonho Zhung, Ph.D cadidate Joongwon Lee , Prof. Woo Young Kim , Ph.D candidate Jisu Seo > Traditional drug development methods involve identifying a target protin (e.g., a cancer cell receptor) that causes disease, and then searching through countless molecular candidates (potential drugs) that could bind to that protein and block its function. This process is costly, time-consuming, and has a low success rate. KAIST researchers have developed an AI model th
2025-08-12 -
research Anti-Neuroinflammatory Natural Products from Isopod-Related Fungus Now Accessible via Chemical Synthesis
<(From left) Professor Sunkyu Han, Ph.D candidate Yoojin Lee, Ph.D candidate Taewan Kim> "Herpotrichone" is a natural substance that has been evaluated highly for its excellent ability to suppress inflammation in the brain and protect nerve cells, displaying significant potential to be developed as a therapeutic agent for neurodegenerative brain diseases such as Alzheimer's disease and Parkinson's disease. This substance could only be obtained in minute quantities from fungi that are
2025-08-04 -
research KAIST Enables On-Site Disease Diagnosis in Just 3 Minutes... Nanozyme Reaction Selectivity Improved 38-Fold
<(From Left) Professor Jinwoo Lee, Ph.D candidate Seonhye Park and Ph.D candidate Daeeun Choi from Chemical & Biomolecular Engineering> To enable early diagnosis of acute illnesses and effective management of chronic conditions, point-of-care testing (POCT) technology—diagnostics conducted near the patient—is drawing global attention. The key to POCT lies in enzymes that recognize and react precisely with specific substances. However, traditional natural enzymes are expe
2025-07-29 -
research KAIST Designs a New Atomic Catalyst for Air Pollution Reduction
<(From Left)Professor Jong Hun Kim from Inha University, Dr. Gyuho Han and Professor Jeong Young Park from KAIST> Platinum diselenide (PtSe2) is a two-dimensional multilayer material in which each layer is composed of platinum (Pt) and selenium (Se). It is known that its excellent crystallinity and precise control of interlayer interactions allow modulation of various physical and chemical properties. Due to these characteristics, it has been actively researched in multiple fields, incl
2025-07-22 -
research A KAIST Team Engineers a Microbial Platform for Efficient Lutein Production
<(From Left) Ph.D. Candidate Hyunmin Eun, Distinguished Professor Sang Yup Lee, , Dr. Cindy Pricilia Surya Prabowo> The application of systems metabolic engineering strategies, along with the construction of an electron channeling system, has enabled the first gram-per-liter scale production of lutein from Corynebacterium glutamicum, providing a viable alternative to plant-derived lutein production. A research group at KAIST has successfully engineered a microbial strain capable o
2025-07-14