Sang+Yup+Lee
-
 Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
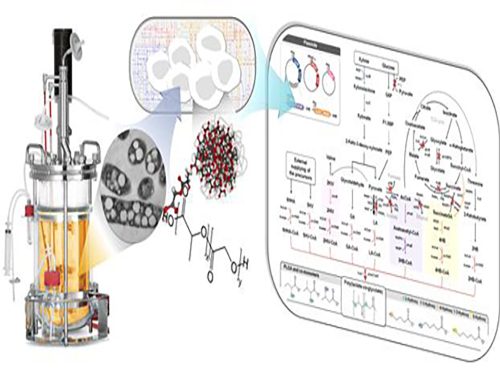
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12300
Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12300 -
 Asia Pacific Biotech News' Special Coverage of Korean Biotechnology
The Asia Pacific Biotech News covered five major biotechnology research projects sponsored by the Korean government in the areas of biofuels, biomedicine, bio-nano healthcare, and biorefinery.
The Asia Pacific Biotech News (APBN), a monthly magazine based in Singapore, which offers comprehensive reports on the fields of pharmaceuticals, healthcare, and biotechnology, recently published a special feature on Korea’s biotechnology research and development (R&D) programs.
The magazine feature selected five research programs sponsored by the Korean government, which are either part of the Global Frontier or the Climate Change Technology Development Projects.
The programs are:
Systems Metabolic Engineering Research: Distinguished Professor Sang Yup Lee
of the Chemical and Biomolecular Engineering Department at the Korea Advanced
Institute of Science and Technology (KAIST) has been leading a research group to
develop biorefining technology using renewable non-food biomass to produce
chemicals, fuels, and materials that were largely drawn from fossil resources
through petrochemical refinery processes. Applying a systems metabolic
engineering approach, the group succeeded in modifying the metabolic pathways of
microorganisms. As a result, they produced, for the first time in the world,
engineered plastic raw materials and gasoline. The team also developed a technique
to produce butanol and succinic acid with a higher titer and yield using metabolically
engineered microorganisms.
Next-generation Biomass Research: Under the leadership of Professor Yong-
Keun Chang of the Chemical and Biomolecular Engineering Department at KAIST,
the research project, which belongs to the Global Frontier Project, develops biofuels
and bioproducts utilizing microalgae typically found in water and other marine
systems.
Convergence Research for Biomedicine: Professor Sung-Hoon Kim of Seoul
National University leads this project that develops targeted new drugs based on
convergence research strategies.
Bionano Healthcare Chip Research: Director Bong-Hyun Chung of the Korea
Research Institute of Bioscience and Biotechnology has integrated information and
communications technology, nanotechnology, and biotechnology to develop a
diagnostic kit that can screen toxic germs, virus, and toxic materials in a prompt
and accurate manner.
Biosynergy Research: Led by Professor Do-Hun Lee of the Bio and Brain
Engineering Department at KAIST, this research project develops new treatments
with a multi-target, multi-component approach in the context of systems biology
through an analysis of synergistic reactions between multi-compounds in traditional
East Asian medicine and human metabolites. In East Asian medicine, treatment and
caring of the human body are considered analogous to the politics of governing a
nation. Based on such system, the research focuses on designing a foundation for
the integration of traditional medicine with modern drug discovery and development.
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning, Republic of Korea, who is responsible for the Global Frontier Program and the Technology to Solve Climate Change, said, “It is great to see that Asia Pacific Biotech News published an extensive coverage of Korea’s several key research programs on biotechnology as its first issue of this year. I am sure that these programs will lead to great outcomes to solve many worldwide pending issues including climate change and healthcare in the aging society.”
Professor Sang Yup Lee, who served as an editor of the feature, said, “At the request of the magazine, we have already published lead articles on our biotechnology research three times in the past in 2002, 2006, and 2011. I am pleased to see continued coverage of Korean biotechnology by the magazine because it recognizes the excellence of our research. Biotechnology has emerged as one of the strong fields that addresses important global issues such as climate change and sustainability.”
2016.02.04 View 13479
Asia Pacific Biotech News' Special Coverage of Korean Biotechnology
The Asia Pacific Biotech News covered five major biotechnology research projects sponsored by the Korean government in the areas of biofuels, biomedicine, bio-nano healthcare, and biorefinery.
The Asia Pacific Biotech News (APBN), a monthly magazine based in Singapore, which offers comprehensive reports on the fields of pharmaceuticals, healthcare, and biotechnology, recently published a special feature on Korea’s biotechnology research and development (R&D) programs.
The magazine feature selected five research programs sponsored by the Korean government, which are either part of the Global Frontier or the Climate Change Technology Development Projects.
The programs are:
Systems Metabolic Engineering Research: Distinguished Professor Sang Yup Lee
of the Chemical and Biomolecular Engineering Department at the Korea Advanced
Institute of Science and Technology (KAIST) has been leading a research group to
develop biorefining technology using renewable non-food biomass to produce
chemicals, fuels, and materials that were largely drawn from fossil resources
through petrochemical refinery processes. Applying a systems metabolic
engineering approach, the group succeeded in modifying the metabolic pathways of
microorganisms. As a result, they produced, for the first time in the world,
engineered plastic raw materials and gasoline. The team also developed a technique
to produce butanol and succinic acid with a higher titer and yield using metabolically
engineered microorganisms.
Next-generation Biomass Research: Under the leadership of Professor Yong-
Keun Chang of the Chemical and Biomolecular Engineering Department at KAIST,
the research project, which belongs to the Global Frontier Project, develops biofuels
and bioproducts utilizing microalgae typically found in water and other marine
systems.
Convergence Research for Biomedicine: Professor Sung-Hoon Kim of Seoul
National University leads this project that develops targeted new drugs based on
convergence research strategies.
Bionano Healthcare Chip Research: Director Bong-Hyun Chung of the Korea
Research Institute of Bioscience and Biotechnology has integrated information and
communications technology, nanotechnology, and biotechnology to develop a
diagnostic kit that can screen toxic germs, virus, and toxic materials in a prompt
and accurate manner.
Biosynergy Research: Led by Professor Do-Hun Lee of the Bio and Brain
Engineering Department at KAIST, this research project develops new treatments
with a multi-target, multi-component approach in the context of systems biology
through an analysis of synergistic reactions between multi-compounds in traditional
East Asian medicine and human metabolites. In East Asian medicine, treatment and
caring of the human body are considered analogous to the politics of governing a
nation. Based on such system, the research focuses on designing a foundation for
the integration of traditional medicine with modern drug discovery and development.
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning, Republic of Korea, who is responsible for the Global Frontier Program and the Technology to Solve Climate Change, said, “It is great to see that Asia Pacific Biotech News published an extensive coverage of Korea’s several key research programs on biotechnology as its first issue of this year. I am sure that these programs will lead to great outcomes to solve many worldwide pending issues including climate change and healthcare in the aging society.”
Professor Sang Yup Lee, who served as an editor of the feature, said, “At the request of the magazine, we have already published lead articles on our biotechnology research three times in the past in 2002, 2006, and 2011. I am pleased to see continued coverage of Korean biotechnology by the magazine because it recognizes the excellence of our research. Biotechnology has emerged as one of the strong fields that addresses important global issues such as climate change and sustainability.”
2016.02.04 View 13479 -
 IdeasLab Presents Biotechnology Solutions for Aging Populations at 2016 Davos Forum
KAIST researchers will discuss how biological sciences and health technologies can address challenges and opportunities posed by aging populations in an era of increasing longevity.
Many countries around the world today are experiencing the rapid growth of aging populations, with a decline in fertility rate and longer life expectancy.
At this year's Annual Meeting of the World Economic Forum (a.k.a. Davos Forum) on January 20-23, 2016 in Davos-Klosters, Switzerland, four researchers in the field of biological sciences and biotechnology at the Korea Advanced Institute of Science and Technology (KAIST) will discuss the implications of an aging population and explore possible solutions to provide better health care services to the elderly.
KAIST will host an IdeasLab twice on the theme "Biotechnology Solutions for Ageing Populations" on January 21st and 23rd, respectively.
Professor Byung-Kwan Cho of the Biological Sciences Department will give a presentation on "Rejuvenation via the Microbiome," explaining how microorganisms in the human gut play an important role in preventing aging, or even rejuvenating it.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will talk about "Traditional Medicine Reimagined through Modern Systems Biology." Professor Lee will introduce his research results published in Nature Biotechnology (March 6, 2015) and some more new results. He discovered the mechanisms of traditional oriental medicine's (TOM) efficacy by applying systems biology to study structural similarities between natural and nontoxic multi-compounds in the medicine and human metabolites. He will discuss TOM's multi-target approach, which is based on the synergistic combinations of multi-compounds to treat symptoms of a disease, can contribute to the development of new drugs, cosmetics, and nutrients.
Professor Youn-Kyung Lim of the Industrial Design Department will speak about a mobile and the Internet of Things-based health care service called "Dr. M" in her presentation on "Advanced Mobile Healthcare Systems."
Professor Daesoo Kim of the Biological Sciences Department will share his research on human's happiness and greed in the context of nueroscience and behavioral and biological sciences in a talk entitled "A Neural Switch for Being Happy with Less on a Crowded Planet."
KAIST has hosted IdeasLabs several times at the Summer Davos Forum in China, but this is the first time it will participate in the Davos Forum in January.
Professor Lee said, "Just like climate change, the issue of how to address aging populations has become a major global issue. We will share some exciting research results and hope to have in depth discussion on this issue with the leaders attending the Davos Forum. KAIST will engage actively in finding solutions that benefit not only Korea but also the international community."
2016.01.19 View 11775
IdeasLab Presents Biotechnology Solutions for Aging Populations at 2016 Davos Forum
KAIST researchers will discuss how biological sciences and health technologies can address challenges and opportunities posed by aging populations in an era of increasing longevity.
Many countries around the world today are experiencing the rapid growth of aging populations, with a decline in fertility rate and longer life expectancy.
At this year's Annual Meeting of the World Economic Forum (a.k.a. Davos Forum) on January 20-23, 2016 in Davos-Klosters, Switzerland, four researchers in the field of biological sciences and biotechnology at the Korea Advanced Institute of Science and Technology (KAIST) will discuss the implications of an aging population and explore possible solutions to provide better health care services to the elderly.
KAIST will host an IdeasLab twice on the theme "Biotechnology Solutions for Ageing Populations" on January 21st and 23rd, respectively.
Professor Byung-Kwan Cho of the Biological Sciences Department will give a presentation on "Rejuvenation via the Microbiome," explaining how microorganisms in the human gut play an important role in preventing aging, or even rejuvenating it.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will talk about "Traditional Medicine Reimagined through Modern Systems Biology." Professor Lee will introduce his research results published in Nature Biotechnology (March 6, 2015) and some more new results. He discovered the mechanisms of traditional oriental medicine's (TOM) efficacy by applying systems biology to study structural similarities between natural and nontoxic multi-compounds in the medicine and human metabolites. He will discuss TOM's multi-target approach, which is based on the synergistic combinations of multi-compounds to treat symptoms of a disease, can contribute to the development of new drugs, cosmetics, and nutrients.
Professor Youn-Kyung Lim of the Industrial Design Department will speak about a mobile and the Internet of Things-based health care service called "Dr. M" in her presentation on "Advanced Mobile Healthcare Systems."
Professor Daesoo Kim of the Biological Sciences Department will share his research on human's happiness and greed in the context of nueroscience and behavioral and biological sciences in a talk entitled "A Neural Switch for Being Happy with Less on a Crowded Planet."
KAIST has hosted IdeasLabs several times at the Summer Davos Forum in China, but this is the first time it will participate in the Davos Forum in January.
Professor Lee said, "Just like climate change, the issue of how to address aging populations has become a major global issue. We will share some exciting research results and hope to have in depth discussion on this issue with the leaders attending the Davos Forum. KAIST will engage actively in finding solutions that benefit not only Korea but also the international community."
2016.01.19 View 11775 -
 HUBO to Present at the 2016 World Economic Forum
KAIST researchers will lead an IdeasLab on biotechnology for an aging society while HUBO, the winner of the 2015 DARPA Robotics Challenge, will interact with the forum participants, offering an experience of state-of-the-art robotics technology.
Representatives from KAIST will attend the 2016 Annual Meeting of the World Economic Forum to run an IdeasLab and showcase its humanoid robot.
With over 2,500 leaders from business, government, international organizations, civil society, academia, media, and the arts expected to participate, the 2016 Annual Meeting will take place on January 20-23, 2016 in Davos-Klosters, Switzerland. Under the theme of “Mastering the Fourth Industrial Revolution,” global leaders will discuss the period of digital transformation that will have profound effects on economies, societies, and human behavior.
President Sung-Mo Kang will join the Global University Leaders Forum (GULF), a high-level academic meeting to foster collaboration among experts on issues of global concern for the future of higher education and the role of science in society. He will discuss how the emerging revolution in technology will affect the way universities operate and serve society. KAIST is the only Korean university participating in GULF, which is composed of prestigious universities invited from around the world.
Four KAIST professors, including Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department, will lead an IdeasLab on “Biotechnology for an Aging Society.”
Professor Lee said, “In recent decades, much attention has been paid to the potential effect of the growth of an aging population and problems posed by it. At our IdeasLab, we will introduce some of our research breakthroughs in biotechnology to address the challenges of an aging society.”
In particular, he will present his latest research in systems biotechnology and metabolic engineering. His research has explained the mechanisms of how traditional Oriental medicine works in our bodies by identifying structural similarities between effective compounds in traditional medicine and human metabolites, and has proposed more effective treatments by employing such compounds.
KAIST will also display its networked mobile medical service system, “Dr. M.” Built upon a ubiquitous and mobile Internet, such as the Internet of Things, wearable electronics, and smart homes and vehicles, Dr. M will provide patients with a more affordable and accessible healthcare service.
In addition, Professor Jun-Ho Oh of the Mechanical Engineering Department will showcase his humanoid robot, “HUBO,” during the Annual Meeting. His research team won the International Humanoid Robotics Challenge hosted by the United States Defense Advanced Research Projects Agency (DARPA), which was held in Pomona, California, on June 5-6, 2015. With 24 international teams participating in the finals, HUBO completed all eight tasks in 44 minutes and 28 seconds, 6 minutes earlier than the runner-up, and almost 11 minutes earlier than the third-place team. Team KAIST walked away with the grand prize of USD 2 million.
Professor Oh said, “Robotics technology will grow exponentially in this century, becoming a real driving force to expedite the Fourth Industrial Revolution. I hope HUBO will offer an opportunity to learn about the current advances in robotics technology.”
President Kang pointed out, “KAIST has participated in the Annual Meeting of the World Economic Forum since 2011 and has engaged with a broad spectrum of global leaders through numerous presentations and demonstrations of our excellence in education and research. Next year, we will choreograph our first robotics exhibition on HUBO and present high-tech research results in biotechnology, which, I believe, epitomizes how science and technology breakthroughs in the Fourth Industrial Revolution will shape our future in an unprecedented way.”
2015.11.18 View 13094
HUBO to Present at the 2016 World Economic Forum
KAIST researchers will lead an IdeasLab on biotechnology for an aging society while HUBO, the winner of the 2015 DARPA Robotics Challenge, will interact with the forum participants, offering an experience of state-of-the-art robotics technology.
Representatives from KAIST will attend the 2016 Annual Meeting of the World Economic Forum to run an IdeasLab and showcase its humanoid robot.
With over 2,500 leaders from business, government, international organizations, civil society, academia, media, and the arts expected to participate, the 2016 Annual Meeting will take place on January 20-23, 2016 in Davos-Klosters, Switzerland. Under the theme of “Mastering the Fourth Industrial Revolution,” global leaders will discuss the period of digital transformation that will have profound effects on economies, societies, and human behavior.
President Sung-Mo Kang will join the Global University Leaders Forum (GULF), a high-level academic meeting to foster collaboration among experts on issues of global concern for the future of higher education and the role of science in society. He will discuss how the emerging revolution in technology will affect the way universities operate and serve society. KAIST is the only Korean university participating in GULF, which is composed of prestigious universities invited from around the world.
Four KAIST professors, including Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department, will lead an IdeasLab on “Biotechnology for an Aging Society.”
Professor Lee said, “In recent decades, much attention has been paid to the potential effect of the growth of an aging population and problems posed by it. At our IdeasLab, we will introduce some of our research breakthroughs in biotechnology to address the challenges of an aging society.”
In particular, he will present his latest research in systems biotechnology and metabolic engineering. His research has explained the mechanisms of how traditional Oriental medicine works in our bodies by identifying structural similarities between effective compounds in traditional medicine and human metabolites, and has proposed more effective treatments by employing such compounds.
KAIST will also display its networked mobile medical service system, “Dr. M.” Built upon a ubiquitous and mobile Internet, such as the Internet of Things, wearable electronics, and smart homes and vehicles, Dr. M will provide patients with a more affordable and accessible healthcare service.
In addition, Professor Jun-Ho Oh of the Mechanical Engineering Department will showcase his humanoid robot, “HUBO,” during the Annual Meeting. His research team won the International Humanoid Robotics Challenge hosted by the United States Defense Advanced Research Projects Agency (DARPA), which was held in Pomona, California, on June 5-6, 2015. With 24 international teams participating in the finals, HUBO completed all eight tasks in 44 minutes and 28 seconds, 6 minutes earlier than the runner-up, and almost 11 minutes earlier than the third-place team. Team KAIST walked away with the grand prize of USD 2 million.
Professor Oh said, “Robotics technology will grow exponentially in this century, becoming a real driving force to expedite the Fourth Industrial Revolution. I hope HUBO will offer an opportunity to learn about the current advances in robotics technology.”
President Kang pointed out, “KAIST has participated in the Annual Meeting of the World Economic Forum since 2011 and has engaged with a broad spectrum of global leaders through numerous presentations and demonstrations of our excellence in education and research. Next year, we will choreograph our first robotics exhibition on HUBO and present high-tech research results in biotechnology, which, I believe, epitomizes how science and technology breakthroughs in the Fourth Industrial Revolution will shape our future in an unprecedented way.”
2015.11.18 View 13094 -
 KAIST and Hanwha Chemical Agree on Research Collaboration
KAIST signed a memorandum of understanding (MOU) with Hanwha Chemical Co., Ltd., a Korean chemical and auto manufacturer, on November 2, 2015 to establish a research center on campus.
The research center, which will be named “KAIST-Hanwha Chemical Future Technology Research Center,” will implement joint research projects for five years beginning from 2016 to develop innovative, green technologies that will help the Korean chemical industry boost its global competitiveness and to nurture top researchers and engineers in chemical engineering.
The research center will lead the development of next-generation petrochemical materials and manufacturing technology and the establishment of pure high-refining processes which are more energy-efficient and environmentally friendly. KAIST and Hanwha will strive to secure new technologies that have the greatest commercialization potential in the global market. They will also establish a scholarship fund for 15 KAIST doctoral students in the Department of Chemical and Biomolecular Engineering.
Many professors from the Chemical and Biomolecular Engineering Department including Distinguished Professor Sang Yup Lee, who was listed in the Top 20 Translational Researchers of 2014 by Nature Biotechnology this year, and Professor Hyunjoo Lee who received the Woman Scholar award at the 2015 World Chemistry Conference, will work at the research center.
Professor Lee, the head of the research center, said, “Collaborating with Hanwha will give us a strong basis for our efforts to carry out original research and train the best researchers in the field.”
Chang-Bum Kim, the Chief Executive Officer (CEO) of Hanwha Chemical, said,
“We hope our collaborations with KAIST will go beyond the typical industry and university cooperation. The two organizations will indeed jointly operate the research center, and this will become a new model for industry and university cooperation. We expect that the research center will play a crucial role in the development of new products and technologies to grow the Korean chemical industry.”
In the photo, President Steve Kang of KAIST (fourth from left) and CEO Chang-Bum Kim of Hanwha Chemical (fifth from left) hold the MOU together.
2015.11.01 View 12214
KAIST and Hanwha Chemical Agree on Research Collaboration
KAIST signed a memorandum of understanding (MOU) with Hanwha Chemical Co., Ltd., a Korean chemical and auto manufacturer, on November 2, 2015 to establish a research center on campus.
The research center, which will be named “KAIST-Hanwha Chemical Future Technology Research Center,” will implement joint research projects for five years beginning from 2016 to develop innovative, green technologies that will help the Korean chemical industry boost its global competitiveness and to nurture top researchers and engineers in chemical engineering.
The research center will lead the development of next-generation petrochemical materials and manufacturing technology and the establishment of pure high-refining processes which are more energy-efficient and environmentally friendly. KAIST and Hanwha will strive to secure new technologies that have the greatest commercialization potential in the global market. They will also establish a scholarship fund for 15 KAIST doctoral students in the Department of Chemical and Biomolecular Engineering.
Many professors from the Chemical and Biomolecular Engineering Department including Distinguished Professor Sang Yup Lee, who was listed in the Top 20 Translational Researchers of 2014 by Nature Biotechnology this year, and Professor Hyunjoo Lee who received the Woman Scholar award at the 2015 World Chemistry Conference, will work at the research center.
Professor Lee, the head of the research center, said, “Collaborating with Hanwha will give us a strong basis for our efforts to carry out original research and train the best researchers in the field.”
Chang-Bum Kim, the Chief Executive Officer (CEO) of Hanwha Chemical, said,
“We hope our collaborations with KAIST will go beyond the typical industry and university cooperation. The two organizations will indeed jointly operate the research center, and this will become a new model for industry and university cooperation. We expect that the research center will play a crucial role in the development of new products and technologies to grow the Korean chemical industry.”
In the photo, President Steve Kang of KAIST (fourth from left) and CEO Chang-Bum Kim of Hanwha Chemical (fifth from left) hold the MOU together.
2015.11.01 View 12214 -
 Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
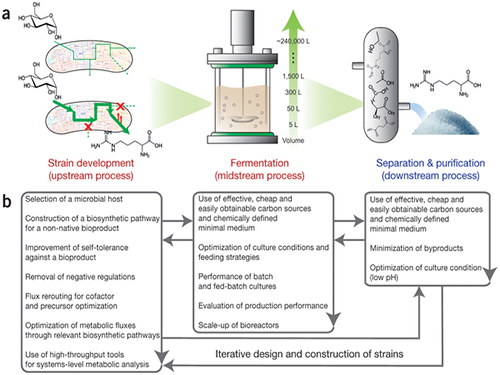
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11321
Establishment of System Metabolic Engineering Strategies
Although conventional petrochemical processes have generated chemicals and materials which have been useful to mankind, they have also triggered a variety of environmental problems including climate change and relied too much on nonrenewable natural resources. To ameliorate this, researchers have actively pursued the development of industrial microbial strains around the globe in order to overproduce industrially useful chemicals and materials from microbes using renewable biomass. This discipline is called metabolic engineering.
Thanks to advances in genetic engineering and our knowledge of cellular metabolism, conventional metabolic engineering efforts have succeeded to a certain extent in developing microbial strains that overproduce bioproducts at an industrial level. However, many metabolic engineering projects launched in academic labs do not reach commercial markets due to a failure to fully integrate industrial bioprocesses.
In response to this, Distinguished Professor Sang Yup Lee and Dr. Hyun Uk Kim, both from the Department of Chemical and Biomolecular Engineering at KAIST, have recently suggested ten general strategies of systems metabolic engineering to successfully develop industrial microbial strains. Systems metabolic engineering differs from conventional metabolic engineering by incorporating traditional metabolic engineering approaches along with tools of other fields, such as systems biology, synthetic biology, and molecular evolution.
The ten strategies of systems metabolic engineering have been featured in Nature Biotechnology released online in October 2015, which is entitled "Systems strategies for developing industrial microbial strains."
The strategies cover economic, state-of-the-art biological techniques and traditional bioprocess aspects. Specifically, they consist of: 1) project design including economic evaluation of a target bioproduct; 2) selection of host strains to be used for overproduction of a bioproduct; 3) metabolic pathway reconstruction for bioproducts that are not naturally produced in the selected host strains; 4) increasing tolerance of a host strain against the bioproduct; 5) removing negative regulatory circuits in the microbial host limiting overproduction of a bioproduct; 6) rerouting intracellular fluxes to optimize cofactor and precursor availability necessary for the bioproduct formation; 7) diagnosing and optimizing metabolic fluxes towards product formation; 8) diagnosis and optimization of microbial culture conditions including carbon sources; 9) system-wide gene manipulation to further increase the host strain's production performance using high-throughput genome-scale engineering and computational tools; and 10) scale-up fermentation of the developed strain and diagnosis for the reproducibility of the strain's production performance.
These ten strategies were articulated with successful examples of the production of L-arginine using Corynebacterium glutamicum, 1,4-butanediol using Escherichia coli, and L-lysine and bio-nylon using C. glutamicum.
Professor Sang Yup Lee said, "At the moment, the chance of commercializing microbial strains developed in academic labs is very low. The strategies of systems metabolic engineering outlined in this analysis can serve as guidelines when developing industrial microbial strains. We hope that these strategies contribute to improving opportunities to commercialize microbial strains developed in academic labs with drastically reduced costs and efforts, and that a large fraction of petroleum-based processes will be replaced with sustainable bioprocesses."
Lee S. Y. & Kim, H. U. Systems Strategies for Developing Industrial Microbial Strains. Nature Biotechnology (2015).
This work was supported by the Technology Development Program to Solve Climate Change on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556) and by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) from the Ministry of Science, ICT and Future Planning (MSIP), Korea, and through the National Research Foundation (NRF) of Korea. This work was also supported by the Novo Nordisk Foundation.
Picture: Concept of the Systems Metabolic Engineering Framework
(a) Three major bioprocess stages (b) Considerations in systems metabolic engineering to optimize the whole bioprocess. List of considerations for the strain development and fermentation contribute to improving microbial strain's production performance (red), whereas those for the separation and purification help in reducing overall operation costs by facilitating the downstream process (blue). Some of the considerations can be repeated in the course of systems metabolic engineering.
2015.10.19 View 11321 -
 Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
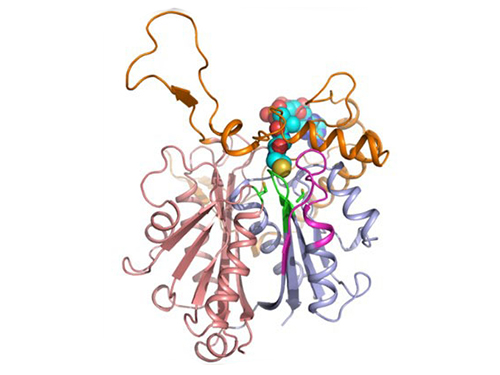
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11215
Discovery of Redox-Switch of KEenzyme Involved in N-Butanol Biosynthesis
Research teams at KAIST and Kyungpook National University (KNU) have succeeded in uncovering the redox-switch of thiolase, a key enzyme for n-butanol production in Clostridium acetobutylicum, one of the best known butanol-producing bacteria.
Biological n-butanol production was first reported by Louis Pasteur in 1861, and the bioprocess was industrialized usingClostridium acetobutylicum. The fermentation process by Clostridium strains has been known to be the most efficient one for n-butanol production. Due to growing world-wide issues such as energy security and climate change, the biological production of n-butanol has been receiving much renewed interest. This is because n-butanol possesses much better fuel characteristics compared to ethanol, such as higher energy content (29.2 MJ/L vs 19.6 MJ/L), less corrosiveness, less hygroscopy, and the ease with which it can be blended with gasoline and diesel.
In the paper published in Nature Communications, a broad-scope, online-only, and open access journal issued by the Nature Publishing Group (NPG), on September 22, 2015, Professor Kyung-Jin Kim at the School of Life Sciences, KNU, and Distinguished Professor Sang Yup Lee at the Department of Chemical and Biomolecular Engineering, KAIST, have proved that the redox-switch of thiolase plays a role in a regulation of metabolic flux in C. acetobutylicum by using in silico modeling and simulation tools.
The research team has redesigned thiolase with enhanced activity on the basis of the 3D structure of the wild-type enzyme. To reinforce a metabolic flux toward butanol production, the metabolic network of C. acetobutylicum strain was engineered with the redesigned enzyme. The combination of the discovery of 3D enzyme structure and systems metabolic engineering approaches resulted in increased n-butanol production in C. acetobutylicum, which allows the production of this important industrial chemical to be cost competitive.
Professors Kim and Lee said, "We have reported the 3D structure of C. acetobutylicum thiolase-a key enzyme involved in n-butanol biosynthesis, for the first time. Further study will be done to produce butanol more economically on the basis of the 3D structure of C. acetobutylicum thiolase."
This work was published online in Nature Communications on September 22, 2015.
Reference: Kim et al. "Redox-switch regulatory mechanism of thiolase from Clostridium acetobutylicum," Nature Communications
This research was supported by the Technology Development Program to Solve Climate Changes from the Ministry of Education, Science and Technology (MEST), Korea, the National Research Foundation of Korea, and the Advanced Biomass Center through the Global Frontier Research Program of the MEST, Korea.
For further information, contact Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea (leesy@kaist.ac.kr, +82-42-350-3930); and Dr. Kyung-Jin Kim, Professor, KNU, Daegu, Korea (kkim@knu.ac.kr, +82-53-950-6088).
Figure 1: A redox-switch of thiolase involves in butanol biosynthesis in Clostridium acetobutylicum. Thiolase condenses two acetyl-CoA molecules for initiating four carbon flux towards butanol.
Figure 2: Thiolase catalyzes the condensation reaction of acetyl-CoA to acetoacetyl-CoA. Two catalytic cysteine residues at 88th and 378th are oxidized and formed an intermolecular disulfide bond in an oxidized status, which results in inactivation of the enzyme for n-butanol biosynthesis. The intermolecular disulfide bond is broken enabling the n-butanol biosynthesis, when the environment status is reduced.
2015.09.23 View 11215 -
 KAIST Participates in the World Economic Forum's Annual Meeting of the New Champions 2015 in China
KAIST’s president and its professors actively engage in discussions of major issues on higher education, technology innovation, and industry-university collaboration with global leaders from across all sectors.
President Steve Kang of KAIST participated in the Annual Meeting of the New Champions 2015 (a.k.a., Summer Davos Forum) hosted by the World Economic Forum (WEF). With the theme of “Charting a New Course for Growth,” the Summer Davos Forum took place on September 9-11, 2015 in, Dalian, China.
Currently, KAIST is a member of the Global University Leaders Forum (GULF) of WEF, a gathering of the presidents of the top 25 universities in the world, including Harvard University, Massachusetts Institute of Technology, University of Tokyo, University of Oxford, Peking University, and National University of Singapore. GULF allows university leaders an opportunity to have high-level dialogues on higher education and research and explore prospects for cooperative ventures.
President Kang led the discussion of the GULF session at the Summer Davos Forum, which was held on September 10, 2015, with 25 university leaders as well as two business leaders from Chinese companies: Huawei Technologies Co. Ltd., and Sanofi China. The participants shared candid perspectives on industry-university collaboration, particularly the need for such partnerships in Asia.
In addition, KAIST hosted the fourth IdeasLab session, entitled “Bio versus Nano Materials, on September 9, 2015. At the session, four KAIST professors held an in-depth debate and discussion with the audience on whether the next industrial revolution would be driven by advances in biomaterials or nanomaterials.
The topics under discussion were:
- New materials that mimic biology by Professor Hea Shin Lee
- Bio-based materials that replace petroleum-based materials by Professor Sang Yup Lee
- New materials designed at sub-nano scale by Professor Hee Tae Jung
- A hydrogen economy with nanomaterials by Professor Eun Ae Cho
Since its establishment in 2007, the Summer Davos Forum has become the biggest business and political gathering in Asia, held annually either in Dalian or Tianjin, China. The Forum has attracted more than 1,500 participants primarily from emerging nations such as China, India, Russia, Mexico, and Brazil, and has offered an open platform to address issues important to the region and the global community.
2015.09.14 View 9034
KAIST Participates in the World Economic Forum's Annual Meeting of the New Champions 2015 in China
KAIST’s president and its professors actively engage in discussions of major issues on higher education, technology innovation, and industry-university collaboration with global leaders from across all sectors.
President Steve Kang of KAIST participated in the Annual Meeting of the New Champions 2015 (a.k.a., Summer Davos Forum) hosted by the World Economic Forum (WEF). With the theme of “Charting a New Course for Growth,” the Summer Davos Forum took place on September 9-11, 2015 in, Dalian, China.
Currently, KAIST is a member of the Global University Leaders Forum (GULF) of WEF, a gathering of the presidents of the top 25 universities in the world, including Harvard University, Massachusetts Institute of Technology, University of Tokyo, University of Oxford, Peking University, and National University of Singapore. GULF allows university leaders an opportunity to have high-level dialogues on higher education and research and explore prospects for cooperative ventures.
President Kang led the discussion of the GULF session at the Summer Davos Forum, which was held on September 10, 2015, with 25 university leaders as well as two business leaders from Chinese companies: Huawei Technologies Co. Ltd., and Sanofi China. The participants shared candid perspectives on industry-university collaboration, particularly the need for such partnerships in Asia.
In addition, KAIST hosted the fourth IdeasLab session, entitled “Bio versus Nano Materials, on September 9, 2015. At the session, four KAIST professors held an in-depth debate and discussion with the audience on whether the next industrial revolution would be driven by advances in biomaterials or nanomaterials.
The topics under discussion were:
- New materials that mimic biology by Professor Hea Shin Lee
- Bio-based materials that replace petroleum-based materials by Professor Sang Yup Lee
- New materials designed at sub-nano scale by Professor Hee Tae Jung
- A hydrogen economy with nanomaterials by Professor Eun Ae Cho
Since its establishment in 2007, the Summer Davos Forum has become the biggest business and political gathering in Asia, held annually either in Dalian or Tianjin, China. The Forum has attracted more than 1,500 participants primarily from emerging nations such as China, India, Russia, Mexico, and Brazil, and has offered an open platform to address issues important to the region and the global community.
2015.09.14 View 9034 -
 Nature Biotechnology Nominates Sang Yup Lee of KAIST for Top 20 Translational Researchers of 2014
Nature Biotechnology, recognized as the most prestigious journal in the field of biotechnology, has released today its list of the Top 20 Translational Researchers of 2014. Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST (Korea Advanced Institute of Science and Technology) ranked seventh in the list. He is the only Asian researcher listed.
The journal, in partnership with IP Checkups, a patent analytics firm, presents an annual ranking of researchers based on their paper and patent output. The list includes, among others, each researcher’s most-cited patent in the past five years and their H index, a measurement to evaluate the impact of a researcher’s published work utilizing citation analysis. (More details can be found at http://www.nature.com/bioent/2015/150801/full/bioe.2015.9.html.)
American institutions made up the majority of the list, with 18 universities and research institutes, and the remainder was filled by KAIST in Korea and the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Australia.
Globally known as a leading researcher in systems metabolic engineering, Professor Lee has published more than 500 journal papers and 580 patents. He has received many awards, including the Citation Classic Award, Elmer Gaden Award, Merck Metabolic Engineering Award, ACS Marvin Johnson Award, SIMB Charles Thom Award, POSCO TJ Park Prize, Amgen Biochemical Engineering Award, and the Ho Am Prize in Engineering.
2015.08.27 View 11150
Nature Biotechnology Nominates Sang Yup Lee of KAIST for Top 20 Translational Researchers of 2014
Nature Biotechnology, recognized as the most prestigious journal in the field of biotechnology, has released today its list of the Top 20 Translational Researchers of 2014. Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST (Korea Advanced Institute of Science and Technology) ranked seventh in the list. He is the only Asian researcher listed.
The journal, in partnership with IP Checkups, a patent analytics firm, presents an annual ranking of researchers based on their paper and patent output. The list includes, among others, each researcher’s most-cited patent in the past five years and their H index, a measurement to evaluate the impact of a researcher’s published work utilizing citation analysis. (More details can be found at http://www.nature.com/bioent/2015/150801/full/bioe.2015.9.html.)
American institutions made up the majority of the list, with 18 universities and research institutes, and the remainder was filled by KAIST in Korea and the Commonwealth Scientific and Industrial Research Organization (CSIRO) in Australia.
Globally known as a leading researcher in systems metabolic engineering, Professor Lee has published more than 500 journal papers and 580 patents. He has received many awards, including the Citation Classic Award, Elmer Gaden Award, Merck Metabolic Engineering Award, ACS Marvin Johnson Award, SIMB Charles Thom Award, POSCO TJ Park Prize, Amgen Biochemical Engineering Award, and the Ho Am Prize in Engineering.
2015.08.27 View 11150 -
 'Engineered Bacterium Produces 1,3-Diaminopropane'
A research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported, for the first time, the production of 1,3-diaminopropane via fermentation of an engineered bacterium.
1,3-Diaminopropane is a three carbon diamine, which has a wide range of industrial applications including epoxy resin and cross-linking agents, as well as precursors for pharmaceuticals, agrochemicals, and organic chemicals. It can also be polymerized with dicarboxylic acids to make polyamides (nylons) for use as engineering plastics, medical materials, and adhesives.
Traditionally, 1,3-diaminopropane is derived from petroleum-based processes. In effort to address critical problems such as the depletion of petroleum and environmental issues inherent to the petroleum-based processes, the research team has developed an Escherichia coli (E. coli) strain capable of producing 1,3-diaminopropane. Using this technology, 1,3-diaminopropane can now be produced from renewable biomass instead of petroleum.
E. coli as found in nature is unable to produce 1,3-diaminopropane. Metabolic engineering, a technology to transform microorganisms into highly efficient microbial cell factories capable of producing chemical compounds of interest, was utilized to engineer the E. coli strain. First, naturally existing metabolic pathways for the biosynthesis of 1,3-diaminopropane were introduced into a virtual cell in silico to determine the most efficient metabolic pathway for the 1,3-diaminopropane production. The metabolic pathway selected was then introduced into an E. coli strain and successfully produced 1,3-diaminopropane for the first time in the world.
The research team applied metabolic engineering additionally, and the production titer of 1,3-diaminopropane increased about 21 fold. The Fed-batch fermentation of the engineered E. coli strain produced 13 grams per liter of 1,3-diaminoproapne. With this technology, 1,3-diaminopropane can be produced using renewable biomass, and it will be the starting point for replacing the current petroleum-based processes with bio-based processes.
Professor Lee said, “Our study suggested a possibility to produce 1,3-diaminopropane based on biorefinery. Further study will be done to increase the titer and productivity of 1,3-diaminopropane.”
This work was published online in Scientific Reports on August 11, 2015.
Reference: Chae, T.U. et al. "Metabolic engineering of Escherichia coli for the production of 1,3-diaminopropane, a three carbon diamine," Scientific Reports:
http://www.nature.com/articles/srep13040
This research was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF) of Korea.
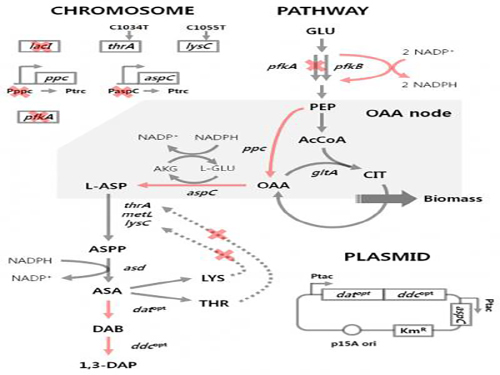
Figure 1: Metabolic engineering strategies for 1,3-diaminopropane production using C4 pathway
Figure 2: Fed-batch fermentation profiles of two final engineered E. coli strains
2015.08.12 View 11469
'Engineered Bacterium Produces 1,3-Diaminopropane'
A research team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported, for the first time, the production of 1,3-diaminopropane via fermentation of an engineered bacterium.
1,3-Diaminopropane is a three carbon diamine, which has a wide range of industrial applications including epoxy resin and cross-linking agents, as well as precursors for pharmaceuticals, agrochemicals, and organic chemicals. It can also be polymerized with dicarboxylic acids to make polyamides (nylons) for use as engineering plastics, medical materials, and adhesives.
Traditionally, 1,3-diaminopropane is derived from petroleum-based processes. In effort to address critical problems such as the depletion of petroleum and environmental issues inherent to the petroleum-based processes, the research team has developed an Escherichia coli (E. coli) strain capable of producing 1,3-diaminopropane. Using this technology, 1,3-diaminopropane can now be produced from renewable biomass instead of petroleum.
E. coli as found in nature is unable to produce 1,3-diaminopropane. Metabolic engineering, a technology to transform microorganisms into highly efficient microbial cell factories capable of producing chemical compounds of interest, was utilized to engineer the E. coli strain. First, naturally existing metabolic pathways for the biosynthesis of 1,3-diaminopropane were introduced into a virtual cell in silico to determine the most efficient metabolic pathway for the 1,3-diaminopropane production. The metabolic pathway selected was then introduced into an E. coli strain and successfully produced 1,3-diaminopropane for the first time in the world.
The research team applied metabolic engineering additionally, and the production titer of 1,3-diaminopropane increased about 21 fold. The Fed-batch fermentation of the engineered E. coli strain produced 13 grams per liter of 1,3-diaminoproapne. With this technology, 1,3-diaminopropane can be produced using renewable biomass, and it will be the starting point for replacing the current petroleum-based processes with bio-based processes.
Professor Lee said, “Our study suggested a possibility to produce 1,3-diaminopropane based on biorefinery. Further study will be done to increase the titer and productivity of 1,3-diaminopropane.”
This work was published online in Scientific Reports on August 11, 2015.
Reference: Chae, T.U. et al. "Metabolic engineering of Escherichia coli for the production of 1,3-diaminopropane, a three carbon diamine," Scientific Reports:
http://www.nature.com/articles/srep13040
This research was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries from Ministry of Science, ICT and Future Planning (MSIP) through the National Research Foundation (NRF) of Korea.
Figure 1: Metabolic engineering strategies for 1,3-diaminopropane production using C4 pathway
Figure 2: Fed-batch fermentation profiles of two final engineered E. coli strains
2015.08.12 View 11469 -
 Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 8948
Professor Sang-Yup Lee Receives the Order of Service Merit Red Stripes from the Korean Government
The government of the Republic of Korea named Professor Sang-Yup Lee of the Department of Chemical and Bio-molecular Engineering at KAIST as the fiftieth recipient of the Order of Service Merit Red Stripes on May 19, 2015.
This medal is awarded to government employees, officials, and teachers in recognition of their contributions to public services including education.
Professor Lee is regarded as a leading scientist in the field of metabolic engineering, genomics, proteomics, metabolomics, and bioinformatics on microorganism producing various primary and secondary metabolites. He contributed significantly to the advancement of bio-based engineering research in Korea.
In addition, his research in microorganism metabolic engineering propelled him to the front of his field, making him the world’s founder of systems metabolic engineering, inventing numerous technologies in strain development.
Professor Lee has received many patent rights in bioprocess engineering. While at KAIST, he applied for 585 patents and registered 227 patents. In particular, he has applied for 135 patents and registered 99 patents in the past five years, successfully turning research results into commercial applications.
Professor Lee said, “I’m glad to contribute to the development of Korean science and technology as a researcher and teacher. I would like to share this honor with my students, master’s and doctoral students in particular, because without their support, it wouldn’t have been possible to pull off the highest level of research results recognized by this medal.”
2015.05.21 View 8948 -
 Professor Sangyong Jon Appointed Fellow of AIMBE
Professor Sangyong Jon of the Department of Biological Sciences at KAIST has been appointed a member of the American Institute for Medical and Biological Engineering (AIMBE) fellowship.
Established in 1991, AIMBE is a non-profit organization based in Washington, D.C., representing 50,000 individuals and the top 2% of medical and biological engineers. AIMBE provides policy advice and advocacy for medical and biological engineering for the benefit of humanity. It has had about 1,500 fellows over the past 25 years. Among the members, only 110 are non-American nationalities.
Following the appointment of Dr. Hae-Bang Lee, the former senior researcher at the Korean Research Institute of Chemical Technology, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, Professor Jon is the third Korean to become an AIMBE fellow. He had an induction ceremony for the appointment of his fellowship at the AIMBE’s Annual Event held on March 15-17, 2015 in Washington, D.C.
An authority on nanomedicine, Professor Jon has developed many original technologies including multi-functional Theranostics nano particles for the diagnosis and treatment of diseases. He received the Most Cited Paper Award from Theranostics, an academic journal specialized in nanomedicine, last February.
Additionally, Professor Jon is a leading researcher in the field of translational medicine, using a multi-disciplinary, highly collaborative, “Bench to Bedside” approach for disease treatment and prevention. He created a biotechnology venture company and transferred research developments to the industry in Korea.
2015.03.12 View 13257
Professor Sangyong Jon Appointed Fellow of AIMBE
Professor Sangyong Jon of the Department of Biological Sciences at KAIST has been appointed a member of the American Institute for Medical and Biological Engineering (AIMBE) fellowship.
Established in 1991, AIMBE is a non-profit organization based in Washington, D.C., representing 50,000 individuals and the top 2% of medical and biological engineers. AIMBE provides policy advice and advocacy for medical and biological engineering for the benefit of humanity. It has had about 1,500 fellows over the past 25 years. Among the members, only 110 are non-American nationalities.
Following the appointment of Dr. Hae-Bang Lee, the former senior researcher at the Korean Research Institute of Chemical Technology, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, Professor Jon is the third Korean to become an AIMBE fellow. He had an induction ceremony for the appointment of his fellowship at the AIMBE’s Annual Event held on March 15-17, 2015 in Washington, D.C.
An authority on nanomedicine, Professor Jon has developed many original technologies including multi-functional Theranostics nano particles for the diagnosis and treatment of diseases. He received the Most Cited Paper Award from Theranostics, an academic journal specialized in nanomedicine, last February.
Additionally, Professor Jon is a leading researcher in the field of translational medicine, using a multi-disciplinary, highly collaborative, “Bench to Bedside” approach for disease treatment and prevention. He created a biotechnology venture company and transferred research developments to the industry in Korea.
2015.03.12 View 13257