CO
-
 Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
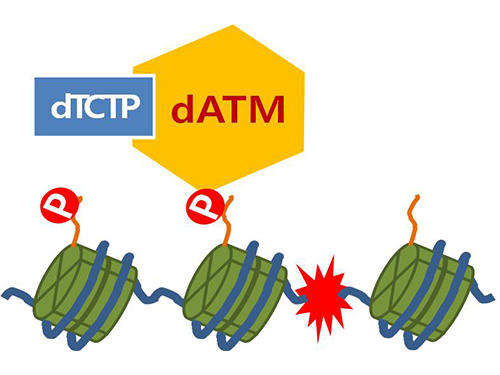
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 16537
Mechanism in regulation of cancer-related key enzyme, ATM, for DNA damage and repair revealed
Professor Kwang-Wook Choi
A research team led by Professor Kwang-Wook Choi and Dr. Seong-Tae Hong from the Department of Biological Sciences at KAIST has successfully investigated the operational mechanism of the protein Ataxia Telangiectasia Mutated (ATM), an essential protein to the function of a crucial key enzyme that repairs the damaged DNA which stores biometric information. The results were published on December 19th Nature Communications online edition.
All organisms, including humans, constantly strive to protect the information within their DNA from damages posed by a number of factors, such as carbonized materials in our daily food intake, radioactive materials such as radon emitting from the cement of buildings or ultraviolet of the sunlight, which could be a trigger for cancer.
In order to keep the DNA information safe, the organisms are always carrying out complex and sophisticated DNA repair work, which involves the crucial DNA damage repair protein ATM. Consequently, a faulty ATM leads to higher risks of cancer.
Until now, academia predicted that the Translationally Controlled Tumor Protein (TCTP) will play an important role in regulating the function of ATM. However, since most of main research regarding TCTP has only been conducted in cultured cells, it was unable to identify exactly what mechanisms TCTP employs to control ATM.
The KAIST research team identified that TCTP can combine with ATM or increase the enzymatic activity of ATM. In addition, Drosophilia, one of the most widely used model organisms for molecular genetics, has been used to identify that TCTP and ATM play a very important role in repairing the DNA damaged by radiation. This information has allowed the researchers to establish TCTP’s essential function in maintaining the DNA information in cell cultures and even in higher organisms, and to provide specific and important clues to the regulation of ATM by TCTP.
Professor Kwang-Wook Choi said, “Our research is a good example that basic research using Drosophilia can make important contributions to understanding the process of diseases, such as cancer, and to developing adequate treatment.”
The research has been funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, and the National Research Foundation of Korea.
Figure 1. When the amount of TCTP protein is reduced, cells of the Drosophila's eye are abnormally deformed by radiation. Scale bars = 200mm
Figure 2. When the amount of TCTP protein is reduced, the chromosomes of Drosophilia are easily broken by radiation. Scale bars = 10 mm.
Figure 3. When gene expressions of TCTP and ATM are reduced, large defects occur in the normal development of the eye. (Left: normal Drosophilia's eye, right: development-deficient eye)
Figure 4. ATM marks the position of the broken DNA, with TCTP helping to facilitate this reaction. DNA (blue line) within the cell nucleus is coiled around the histone protein (green cylinder). When DNA is broken, ATM protein attaches a phosphate group (P). Multiple DNA repair protein recognizes the phosphate as a signal that requires repair and gathers at the site.
2014.01.07 View 16537 -
 Professor Dong-Soo Han received the Prime Minister's award for creative economy in 2013
The 2013 Korea Creative Economy Awards, presented to companies or individuals that contributed to the implementation of the Korean government’s economic growth initiative called “creative economy” with the development of new technology, was held on December 12th at the InterContinental Seoul Coex. Professor Dong-Soo Han from the Department of Computer Science at KAIST received the Prime Minister’s award.
Professor Han was recognized for his research accomplishments on the development of an indoor GPS system and an integrated indoor/outdoor navigation system, as well as for his publication of a book for beginners who are interested in patents. He has applied 50 patents and registered 30 patents in Korea and abroad for the indoor positioning technology and smartphone application services.
His research work was also introduced to the public at the exhibition held after the award ceremony during December 12th-15th at the Coex convention center in Seoul.
2014.01.05 View 9630
Professor Dong-Soo Han received the Prime Minister's award for creative economy in 2013
The 2013 Korea Creative Economy Awards, presented to companies or individuals that contributed to the implementation of the Korean government’s economic growth initiative called “creative economy” with the development of new technology, was held on December 12th at the InterContinental Seoul Coex. Professor Dong-Soo Han from the Department of Computer Science at KAIST received the Prime Minister’s award.
Professor Han was recognized for his research accomplishments on the development of an indoor GPS system and an integrated indoor/outdoor navigation system, as well as for his publication of a book for beginners who are interested in patents. He has applied 50 patents and registered 30 patents in Korea and abroad for the indoor positioning technology and smartphone application services.
His research work was also introduced to the public at the exhibition held after the award ceremony during December 12th-15th at the Coex convention center in Seoul.
2014.01.05 View 9630 -
 KAIST Student Awarded Prize from Energy Saving Contest
Jun-Min Kwon, an undergraduate student in the Department of Chemistry at KAIST, was awarded a prize from the Ministry of Trade, Industry and Energy, Republic of Korea, at the 35th Energy Saving Contest which was held on November 20.
The student club he has been leading was also selected as one of the best groups by the Save Energy Save Earth (SESE), a volunteer organization supported by the Korea Energy Management Corporation and the Ministry of Knowledge Economy, Republic of Korea.
Kwon began promoting energy conservation through a blog and participated in related meetings and workshops as a high school student to improve the understanding on the importance of energy saving and recycling.He also received awards from the Second National Assembly Forum on Climate Change, the Korean National Science Fair, as well as the Samsung Human Tech Paper Award.
2013.12.24 View 15675
KAIST Student Awarded Prize from Energy Saving Contest
Jun-Min Kwon, an undergraduate student in the Department of Chemistry at KAIST, was awarded a prize from the Ministry of Trade, Industry and Energy, Republic of Korea, at the 35th Energy Saving Contest which was held on November 20.
The student club he has been leading was also selected as one of the best groups by the Save Energy Save Earth (SESE), a volunteer organization supported by the Korea Energy Management Corporation and the Ministry of Knowledge Economy, Republic of Korea.
Kwon began promoting energy conservation through a blog and participated in related meetings and workshops as a high school student to improve the understanding on the importance of energy saving and recycling.He also received awards from the Second National Assembly Forum on Climate Change, the Korean National Science Fair, as well as the Samsung Human Tech Paper Award.
2013.12.24 View 15675 -
 Rechargeable Lithium Sulfur Battery for Greater Battery Capacity
Professor Do Kyung Kim from the Department of Material Science and Engineering and Professor Jang Wook Choi from the Graduate School of EEWS have been featured in the lead story of the renowned nanoscience journal Advanced Materials for their research on the lithium sulfur battery. This new type of battery developed by Professor Kim is expected to have a longer life battery life and [higher] energy density than currently commercial batteries.
With ample energy density up to 2100Wh/kg—almost 5.4 times that of lithium ion batteries—lithium sulfur batteries can withstand the sharp decrease in energy capacity resulting from charging and discharging—which has been considered the inherent limitation of the conventional batteries.
Professor Kim and his research team used one-dimensional, vertical alignment of 75nm tick, 15μm long sulfur nanowires to maximize electric conductivity. Then, to prevent loss of battery life, they carbon-coated each nanowire and prohibited direct contact between the sulfur and electrolyte.
The result was one of the most powerful batteries in terms of both energy performance and density. Compared to conventional batteries which suffer from continuous decrease in energy capacity after being discharged, the lithium sulfur battery maintained 99.2% of its initial capacity after being charged and discharged 300 times and up to 70% even after 1000 times.
Professor Kim claims that his new battery is an important step forward towards a high-performance rechargeable battery which is a vital technology for unmanned vehicles, electric automobiles and energy storage. He hopes that his research can solve the problems of battery-capacity loss and contribute to South Korea’s leading position in battery technology. Professor Kim’s research team has filed applications for one domestic and international patent for their research.
2013.12.11 View 15689
Rechargeable Lithium Sulfur Battery for Greater Battery Capacity
Professor Do Kyung Kim from the Department of Material Science and Engineering and Professor Jang Wook Choi from the Graduate School of EEWS have been featured in the lead story of the renowned nanoscience journal Advanced Materials for their research on the lithium sulfur battery. This new type of battery developed by Professor Kim is expected to have a longer life battery life and [higher] energy density than currently commercial batteries.
With ample energy density up to 2100Wh/kg—almost 5.4 times that of lithium ion batteries—lithium sulfur batteries can withstand the sharp decrease in energy capacity resulting from charging and discharging—which has been considered the inherent limitation of the conventional batteries.
Professor Kim and his research team used one-dimensional, vertical alignment of 75nm tick, 15μm long sulfur nanowires to maximize electric conductivity. Then, to prevent loss of battery life, they carbon-coated each nanowire and prohibited direct contact between the sulfur and electrolyte.
The result was one of the most powerful batteries in terms of both energy performance and density. Compared to conventional batteries which suffer from continuous decrease in energy capacity after being discharged, the lithium sulfur battery maintained 99.2% of its initial capacity after being charged and discharged 300 times and up to 70% even after 1000 times.
Professor Kim claims that his new battery is an important step forward towards a high-performance rechargeable battery which is a vital technology for unmanned vehicles, electric automobiles and energy storage. He hopes that his research can solve the problems of battery-capacity loss and contribute to South Korea’s leading position in battery technology. Professor Kim’s research team has filed applications for one domestic and international patent for their research.
2013.12.11 View 15689 -
 Graduate Student at KAIST Awarded Best Prize at the 9th Inside Edge
Sun-Jin Choi, a Ph. D. candidate in the Department of Materials Science and Engineering at KAIST, under the guidance of Professor Il-Doo Kim, won the best prize at the 9th Inside Edge Contest hosted by Samsung Electro-Mechanics.
Choi was awarded prize money totaling fifteen million won at the award ceremony held on November 22 at the Mirae Hall at the headquarters of Samsung Electro-Mechanics in Suwon.
Choi’s research, titled “Exhaled Breath Sensor Arrays for the Non-invasive and Real-time Diagnosis of Diabetes by Detection of Acetone,” was recognized for its creativity and uniqueness.The Inside Edge is an international thesis competition which was started in 2005 to encourage and support creative research and potential technological development among young scientists and engineers.
Sun-Jin Choi (left) and Professor Il-Doo Kim (right).
2013.12.11 View 11588
Graduate Student at KAIST Awarded Best Prize at the 9th Inside Edge
Sun-Jin Choi, a Ph. D. candidate in the Department of Materials Science and Engineering at KAIST, under the guidance of Professor Il-Doo Kim, won the best prize at the 9th Inside Edge Contest hosted by Samsung Electro-Mechanics.
Choi was awarded prize money totaling fifteen million won at the award ceremony held on November 22 at the Mirae Hall at the headquarters of Samsung Electro-Mechanics in Suwon.
Choi’s research, titled “Exhaled Breath Sensor Arrays for the Non-invasive and Real-time Diagnosis of Diabetes by Detection of Acetone,” was recognized for its creativity and uniqueness.The Inside Edge is an international thesis competition which was started in 2005 to encourage and support creative research and potential technological development among young scientists and engineers.
Sun-Jin Choi (left) and Professor Il-Doo Kim (right).
2013.12.11 View 11588 -
 First International Conference on Science and Technology for Society
KAIST co-organized the 2013 International Conference on Science and Technology for Society which was held on November 28 at the Grace Hall in Seoul EL-Tower. More than 300 people, including members of the Global Social Technology Advisory Board, domestic social technology experts, private companies, government officials, private citizens, and students joined the conference to discuss the roles and responsibilities of science and technology for society.
R&D policies and technologies for solving social issues were introduced, and discussions were held on desirable directions for technological development.
The first speaker, Yasushi Watanabe, Director of RISTEX (Research Institute of Science and Technology for Society) in Japan, introduced the importance of science and technology for society under the title “Change of R&D Paradigm for Society.”
Robert Wimmer, GrAT (Center for Appropriate Technology), Vienna University of Technology in Austria, presented “Need-oriented Design & Solutions for Development.” Kiyoaki Murakami, MRI, Japan, presented “Introduction of Platinum Vision” and Robert Ries, University of Florida, U.S.A., presented “Evaluating the Social Impacts of the Built Environment Using Life Cycle Assessment.”
Case studies on social enterprises and presentations on R&D for solving social problems were introduced by ICISTS (International Conference for the Integration of Science, Technology and Society), which is a student group at KAIST, National Research Foundation of Korea (NRF), Korea Institute of Machinery and Materials (KIMM), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Korea Institute of Industrial Technology (KITECH), Electronics and Telecommunication Research Institute (ETRI), and Korea Research Institute of Chemical Technology (KRICT).The conference was hosted by the Ministry of Science, ICT, and Future Planning and co-organized by NRF, KIMM, KRIBB, KITECH, ETRI and KRICT.
2013.12.11 View 14437
First International Conference on Science and Technology for Society
KAIST co-organized the 2013 International Conference on Science and Technology for Society which was held on November 28 at the Grace Hall in Seoul EL-Tower. More than 300 people, including members of the Global Social Technology Advisory Board, domestic social technology experts, private companies, government officials, private citizens, and students joined the conference to discuss the roles and responsibilities of science and technology for society.
R&D policies and technologies for solving social issues were introduced, and discussions were held on desirable directions for technological development.
The first speaker, Yasushi Watanabe, Director of RISTEX (Research Institute of Science and Technology for Society) in Japan, introduced the importance of science and technology for society under the title “Change of R&D Paradigm for Society.”
Robert Wimmer, GrAT (Center for Appropriate Technology), Vienna University of Technology in Austria, presented “Need-oriented Design & Solutions for Development.” Kiyoaki Murakami, MRI, Japan, presented “Introduction of Platinum Vision” and Robert Ries, University of Florida, U.S.A., presented “Evaluating the Social Impacts of the Built Environment Using Life Cycle Assessment.”
Case studies on social enterprises and presentations on R&D for solving social problems were introduced by ICISTS (International Conference for the Integration of Science, Technology and Society), which is a student group at KAIST, National Research Foundation of Korea (NRF), Korea Institute of Machinery and Materials (KIMM), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Korea Institute of Industrial Technology (KITECH), Electronics and Telecommunication Research Institute (ETRI), and Korea Research Institute of Chemical Technology (KRICT).The conference was hosted by the Ministry of Science, ICT, and Future Planning and co-organized by NRF, KIMM, KRIBB, KITECH, ETRI and KRICT.
2013.12.11 View 14437 -
 Wearable computer follows suit of smart phones
KAIST hosts “Wearable Computer Competition” in KI Building, Daejeon Campus, on the 7th-8th of November
“Computer that controls smart phones with the movement of facial muscles” and 12 other wearable computers to be presented
As technology transitions to “Wearable Computers,” KAIST is hosting its 9th “Wearable Computer Competition.” The competition will take place over two days, 7th-8th of November, in KI building, on the main Daejeon Campus.
The “wearable computer” is designed to enable users to use the computer whilst moving by limiting its weight and size so that it can be worn as a part of the body and clothing. Wearable computers have been considered the future of information technology (IT) ever since smart phones and other miniaturized IT devices made an appearance.
The “Wearable Computer Competition” has been held since 2005 under the leadership of Professor Hoi-Jun Yoo from the KAIST Department of Electrical Engineering. It is the only competition in the nation where undergraduate students use their unique ideas and newest technology to produce computers that seem to be existed only in sci-fi movies and comic books.
A total of 15 teams out of 70 made the competition and went through a rigorous selection process based on written applications and interviews to enter the final. The teams at the final received USD 1,400 and IT devices including smart phones to produce a wearable computer.
KAIST increased the number of finalists from the last year"s 10 to 15 this year as the wearable computer industry is extending, and there is growing interest in the computer around the world after the launch of Google Glass and Samsung Galaxy Gear.
This year’s entries included a product for quadriplegic patients to control smart phones with the movement of facial muscles, which attracted public interest. The product in the form of a headband can be worn by quadriplegic patients or someone with limited hand movement. The user can activate the product by clenching their molars and move the mouse on the smart phones with the movement of muscles in their face.
Furthermore, a wearable band shaped device that can control smart phones with simple hand movements is also attracting interest. Broad hand movements of the user allows him/her to receive calls and take photos, and handshakes between users control sharing of files. Body communication can be used to protect private information without a password or locking the device.
In addition, gloves and shoes that can sense the user’s movement to play an instrument without the instrument being present; a cane for the blind that converts visual information to tactile; a belt that protects children from sexual crimes; and a game where the user can be Super Mario to play and other practical products are presented.
The chairman of the competition, Professor Yoo said, “As you can see from the launch of Samsung Galaxy Gear, wearable computers will follow smart phones as the leader of IT devices in the next generation.” He continued, “This competition and workshop is an opportunity to increase public interest in wearable computers and serves as a communication platform for experts to view the present and the future of wearable computers.”
The “Wearable Computer Workshop” will be held this year as well. The workshop under the theme of “the present and the future of wearable computers” invited Professor Kyu-Ho Park, Vice President of KAIST, as a keynote speaker to talk on “ubiquitous, fashionable computers.” Moreover, Samsung’s Dong-Jun Geum and the Electronics and Telecommunications Research Institute’s Hyeon-Tae Jeong will lecture on the “trend and direction of progress of wearable devices” and the “technological trend and prospect of industry of wearable computers,” respectively.
To participate in the competition or the workshop, please visit the website (http://www.ufcom.org)
for further information.
2013.11.28 View 11984
Wearable computer follows suit of smart phones
KAIST hosts “Wearable Computer Competition” in KI Building, Daejeon Campus, on the 7th-8th of November
“Computer that controls smart phones with the movement of facial muscles” and 12 other wearable computers to be presented
As technology transitions to “Wearable Computers,” KAIST is hosting its 9th “Wearable Computer Competition.” The competition will take place over two days, 7th-8th of November, in KI building, on the main Daejeon Campus.
The “wearable computer” is designed to enable users to use the computer whilst moving by limiting its weight and size so that it can be worn as a part of the body and clothing. Wearable computers have been considered the future of information technology (IT) ever since smart phones and other miniaturized IT devices made an appearance.
The “Wearable Computer Competition” has been held since 2005 under the leadership of Professor Hoi-Jun Yoo from the KAIST Department of Electrical Engineering. It is the only competition in the nation where undergraduate students use their unique ideas and newest technology to produce computers that seem to be existed only in sci-fi movies and comic books.
A total of 15 teams out of 70 made the competition and went through a rigorous selection process based on written applications and interviews to enter the final. The teams at the final received USD 1,400 and IT devices including smart phones to produce a wearable computer.
KAIST increased the number of finalists from the last year"s 10 to 15 this year as the wearable computer industry is extending, and there is growing interest in the computer around the world after the launch of Google Glass and Samsung Galaxy Gear.
This year’s entries included a product for quadriplegic patients to control smart phones with the movement of facial muscles, which attracted public interest. The product in the form of a headband can be worn by quadriplegic patients or someone with limited hand movement. The user can activate the product by clenching their molars and move the mouse on the smart phones with the movement of muscles in their face.
Furthermore, a wearable band shaped device that can control smart phones with simple hand movements is also attracting interest. Broad hand movements of the user allows him/her to receive calls and take photos, and handshakes between users control sharing of files. Body communication can be used to protect private information without a password or locking the device.
In addition, gloves and shoes that can sense the user’s movement to play an instrument without the instrument being present; a cane for the blind that converts visual information to tactile; a belt that protects children from sexual crimes; and a game where the user can be Super Mario to play and other practical products are presented.
The chairman of the competition, Professor Yoo said, “As you can see from the launch of Samsung Galaxy Gear, wearable computers will follow smart phones as the leader of IT devices in the next generation.” He continued, “This competition and workshop is an opportunity to increase public interest in wearable computers and serves as a communication platform for experts to view the present and the future of wearable computers.”
The “Wearable Computer Workshop” will be held this year as well. The workshop under the theme of “the present and the future of wearable computers” invited Professor Kyu-Ho Park, Vice President of KAIST, as a keynote speaker to talk on “ubiquitous, fashionable computers.” Moreover, Samsung’s Dong-Jun Geum and the Electronics and Telecommunications Research Institute’s Hyeon-Tae Jeong will lecture on the “trend and direction of progress of wearable devices” and the “technological trend and prospect of industry of wearable computers,” respectively.
To participate in the competition or the workshop, please visit the website (http://www.ufcom.org)
for further information.
2013.11.28 View 11984 -
 UN biological weapons expert gives lecture at KAIST
KAIST’s student organization, the ICISTS Organizing Committee, invited United Nations Security Council expert Terence Taylor to deliver a speech under the topic of ‘Terrorists and Scientists: Biological Weapons and its impact on Global Society’. The lecture took place on November 19 on the Daejeon campus.
Taylor shared his experiences as a biochemical weapons expert at Iraq and discussed the fast-approaching future of the world with biochemical weapons.
Terence Taylor is a former British military officer, who served various governmental and non-governmental organizations around the world, including UK and U.S. agencies, as well as the UN. His current work involves the non-proliferation and disarmament of nuclear or biological weapons, toxic substances and other weapons of mass destruction.
ICISTS Organizing Committee is a student organization run by of KAIST students. Since 2005, it has actively held one of the largest student conferences in Asia, ICISTS-KAIST, at KAIST every year. "ICISTS" stands for “International Conference for the Integration of Science, Technology, and Society”, which conveys its vision in achieving a harmony between science and society.
UN Security Council expert Terence Taylor
2013.11.28 View 11058
UN biological weapons expert gives lecture at KAIST
KAIST’s student organization, the ICISTS Organizing Committee, invited United Nations Security Council expert Terence Taylor to deliver a speech under the topic of ‘Terrorists and Scientists: Biological Weapons and its impact on Global Society’. The lecture took place on November 19 on the Daejeon campus.
Taylor shared his experiences as a biochemical weapons expert at Iraq and discussed the fast-approaching future of the world with biochemical weapons.
Terence Taylor is a former British military officer, who served various governmental and non-governmental organizations around the world, including UK and U.S. agencies, as well as the UN. His current work involves the non-proliferation and disarmament of nuclear or biological weapons, toxic substances and other weapons of mass destruction.
ICISTS Organizing Committee is a student organization run by of KAIST students. Since 2005, it has actively held one of the largest student conferences in Asia, ICISTS-KAIST, at KAIST every year. "ICISTS" stands for “International Conference for the Integration of Science, Technology, and Society”, which conveys its vision in achieving a harmony between science and society.
UN Security Council expert Terence Taylor
2013.11.28 View 11058 -
 Cambridge University Press and HISTAC to Publish Science and Civilization in Korea
The KAIST Research Institute for the History of Science, Technology and Civilization of Korea (HISTAC) and Cambridge University Press have agreed to publish a 10-volume collection entitled “Science and Civilization in Korea” in collaboration with the Needham Research Institute.
HISTAC was found in December 2012 with the support of the Academy of Korean Studies and the Korean Studies Promotion Service with the aim of publishing a collection composed of 30 Korean books and 7 English books on Korean science and civilization.
By November 2013, the HISTAC research team submitted a research paper composed of 11 Korean and 1 English book. It has now exceeded its initial goal of publishing 7 English books by signing the recent agreement with the Cambridge University Press.
“Science and Civilization in Korea” is the second collection of non-western science to be published by the Cambridge University Press since 1954 following “Science and Civilization in China” by Joseph Needham who is well-known for his momentous achievements in history of science in East Asia. This collection will highlight the achievements of Korea in science and civilization of Korea, much of which has been under-valued compared to those of China and Japan.[ It now has the significance similar to the Western science and civilization].
HISTAC appointed Professor Hong-Gi Yoon from the University of Auckland as the translator and invited Professor Christopher Cullen from Cambridge University and Professor Morris Low from the University of Queensland as co-editors. Professor Cullen was an editor of “Science and Civilization in China” and is now the director of the Needham Research Institute and Professor Low is an expert in modern science of East Asia.
The series includes:
- History of Science and Technology in Korea
- Technology, Everyday Life, and Korean Civilization
- History and Cultural Studies of Geomancy in Korea
- Patients, Doctors and the State: History of Korean Medical and Pharmaceutical Culture
- History of Astronomy in Korea
- Mathematics and the History of Korean Civilization
- The West and Korea in the History of Science and Technology, 1600-1950
- Imperialism, Colonialism, Post-colonialism and Technological Science in Korea
- Development of Science and Technology Under the Korean Authoritarian Regime
- Dynamics of Technological Development in Korean Industrialization
The HISTAC team believes that the publication will illuminate the nation’s triumphs in science and technology and expects that the publication will serve as valuable research resources for the study of the history of East Asian scientific civilization which has mainly focused on China and Japan. Further, by adopting various case studies of scientific achievements of South Korea and developing countries, they hope to propose a new model for studying history of science and civilization.
2013.11.28 View 10699
Cambridge University Press and HISTAC to Publish Science and Civilization in Korea
The KAIST Research Institute for the History of Science, Technology and Civilization of Korea (HISTAC) and Cambridge University Press have agreed to publish a 10-volume collection entitled “Science and Civilization in Korea” in collaboration with the Needham Research Institute.
HISTAC was found in December 2012 with the support of the Academy of Korean Studies and the Korean Studies Promotion Service with the aim of publishing a collection composed of 30 Korean books and 7 English books on Korean science and civilization.
By November 2013, the HISTAC research team submitted a research paper composed of 11 Korean and 1 English book. It has now exceeded its initial goal of publishing 7 English books by signing the recent agreement with the Cambridge University Press.
“Science and Civilization in Korea” is the second collection of non-western science to be published by the Cambridge University Press since 1954 following “Science and Civilization in China” by Joseph Needham who is well-known for his momentous achievements in history of science in East Asia. This collection will highlight the achievements of Korea in science and civilization of Korea, much of which has been under-valued compared to those of China and Japan.[ It now has the significance similar to the Western science and civilization].
HISTAC appointed Professor Hong-Gi Yoon from the University of Auckland as the translator and invited Professor Christopher Cullen from Cambridge University and Professor Morris Low from the University of Queensland as co-editors. Professor Cullen was an editor of “Science and Civilization in China” and is now the director of the Needham Research Institute and Professor Low is an expert in modern science of East Asia.
The series includes:
- History of Science and Technology in Korea
- Technology, Everyday Life, and Korean Civilization
- History and Cultural Studies of Geomancy in Korea
- Patients, Doctors and the State: History of Korean Medical and Pharmaceutical Culture
- History of Astronomy in Korea
- Mathematics and the History of Korean Civilization
- The West and Korea in the History of Science and Technology, 1600-1950
- Imperialism, Colonialism, Post-colonialism and Technological Science in Korea
- Development of Science and Technology Under the Korean Authoritarian Regime
- Dynamics of Technological Development in Korean Industrialization
The HISTAC team believes that the publication will illuminate the nation’s triumphs in science and technology and expects that the publication will serve as valuable research resources for the study of the history of East Asian scientific civilization which has mainly focused on China and Japan. Further, by adopting various case studies of scientific achievements of South Korea and developing countries, they hope to propose a new model for studying history of science and civilization.
2013.11.28 View 10699 -
 Green Technology for Data Centers: Ultra-low Power 100 Gbps Ethernet Integrated Circuit Developed
A new integrated circuit (IC), consuming only 0.75W of electricity, will reduce the power usage of data chips installed at data centers by one-third.
Each day, billions of people surf the Internet for information, entertainment, and educational content. The Internet contains an immeasurable amount of information and knowledge generated every minute all around the world that is readily available to everyone with a click of a computer mouse. The real magic of the Internet, however, lies in data centers, where hundreds of billions of data are stored and distributed to designated users around the clock.
Today, almost every business or organization either has its own data centers or outsources data center services to a third party. These centers house highly specialized equipment responsible for the support of computers, networks, data storage, and business security. Accordingly, the operational cost of data centers is tremendous because they consume a large amount of electricity.
Data centers can consume up to 100 times more energy than a standard office building. Data center energy consumption doubled from 2000 to 2006, reaching more than 60 billion kilowatt hours per year. If the current usage and technology trends continue, the energy consumption of data centers in the US will reach 8% of the country’s total electric power consumption by 2020.
A research team at the Korea Advanced Institute of Science and Technology (KAIST) and Terasquare, Inc. (
http://www.terasquare.co.kr
), a spin-off company of the university,
developed an extremely low-powered integrated circuit for Ethernet that consumes less than 0.75W of electricity but is able to send and receive data at the high speed of 100 gigabits per second (Gbps). The research team, headed by Hyeon-Min Bae, assistant professor of electrical engineering at KAIST, claims that the new microchip uses only one-third of the electricity consumed by the currently installed chips at data centers, thereby helping the centers to save energy.
Integrated circuits are embedded on communication modules that are inserted into a line card. Data centers have numerous line cards to build a network including routers and switches. Currently, 8W ICs are the most common in the market, and they consume a lot of energy and require the largest modules (112 cm
2
of CFP), decreasing the port density of line cards and, thus, limiting the amount of data transmission.
The ultra-low-power-circuit, 100-gigabit, full-transceiver CDR, is the world’s first solution that can be loaded to the smallest communication modules (20 cm
2
of CFP4 or 16 cm
2
of
QSFP28), the next-generation chips for data centers. Compared with other chip producers, the 100 Gbps CDR is a greener version of the technology that improves the energy efficiency of data centers while maintaining the high speed of data transmission.
Professor Hyeon-Min Bae said, “When we demonstrate our chip in September of this year at one of the leading companies that manufacture optical communication components and systems, they said that our product is two years ahead of those of our competitors. We plan to produce the chip from 2014 and expect that it will lead the 100 Gbps Ethernet IC market, which is expected to grow to USD 1 billion by 2017.”
The commercial model of the IC was first introduced at the 39
th
European Conference and Exhibition on Optical Communication (ECOC), the largest optical communication forum for new results and developments in Europe, held from September 22-26 at ExCeL London, an international exhibition and convention center.
Professor Bae added, “We received positive responses to our ultra-low-power 100-Gbps Ethernet IC at the ECOC. The chip will be used not only for a particular industry but also for many of next-generation, super-high-speed information communications technologies, such as high-speed USB, high-definition multimedia interface (HDMI), and TV interface.”
Before joining KAIST, Hyeon-Min Bae worked for many years at Finisar as a researcher who designed and developed the world’s first super-high-speed circuit, the 100 Gbps Ethernet IC.
2013.11.25 View 11071
Green Technology for Data Centers: Ultra-low Power 100 Gbps Ethernet Integrated Circuit Developed
A new integrated circuit (IC), consuming only 0.75W of electricity, will reduce the power usage of data chips installed at data centers by one-third.
Each day, billions of people surf the Internet for information, entertainment, and educational content. The Internet contains an immeasurable amount of information and knowledge generated every minute all around the world that is readily available to everyone with a click of a computer mouse. The real magic of the Internet, however, lies in data centers, where hundreds of billions of data are stored and distributed to designated users around the clock.
Today, almost every business or organization either has its own data centers or outsources data center services to a third party. These centers house highly specialized equipment responsible for the support of computers, networks, data storage, and business security. Accordingly, the operational cost of data centers is tremendous because they consume a large amount of electricity.
Data centers can consume up to 100 times more energy than a standard office building. Data center energy consumption doubled from 2000 to 2006, reaching more than 60 billion kilowatt hours per year. If the current usage and technology trends continue, the energy consumption of data centers in the US will reach 8% of the country’s total electric power consumption by 2020.
A research team at the Korea Advanced Institute of Science and Technology (KAIST) and Terasquare, Inc. (
http://www.terasquare.co.kr
), a spin-off company of the university,
developed an extremely low-powered integrated circuit for Ethernet that consumes less than 0.75W of electricity but is able to send and receive data at the high speed of 100 gigabits per second (Gbps). The research team, headed by Hyeon-Min Bae, assistant professor of electrical engineering at KAIST, claims that the new microchip uses only one-third of the electricity consumed by the currently installed chips at data centers, thereby helping the centers to save energy.
Integrated circuits are embedded on communication modules that are inserted into a line card. Data centers have numerous line cards to build a network including routers and switches. Currently, 8W ICs are the most common in the market, and they consume a lot of energy and require the largest modules (112 cm
2
of CFP), decreasing the port density of line cards and, thus, limiting the amount of data transmission.
The ultra-low-power-circuit, 100-gigabit, full-transceiver CDR, is the world’s first solution that can be loaded to the smallest communication modules (20 cm
2
of CFP4 or 16 cm
2
of
QSFP28), the next-generation chips for data centers. Compared with other chip producers, the 100 Gbps CDR is a greener version of the technology that improves the energy efficiency of data centers while maintaining the high speed of data transmission.
Professor Hyeon-Min Bae said, “When we demonstrate our chip in September of this year at one of the leading companies that manufacture optical communication components and systems, they said that our product is two years ahead of those of our competitors. We plan to produce the chip from 2014 and expect that it will lead the 100 Gbps Ethernet IC market, which is expected to grow to USD 1 billion by 2017.”
The commercial model of the IC was first introduced at the 39
th
European Conference and Exhibition on Optical Communication (ECOC), the largest optical communication forum for new results and developments in Europe, held from September 22-26 at ExCeL London, an international exhibition and convention center.
Professor Bae added, “We received positive responses to our ultra-low-power 100-Gbps Ethernet IC at the ECOC. The chip will be used not only for a particular industry but also for many of next-generation, super-high-speed information communications technologies, such as high-speed USB, high-definition multimedia interface (HDMI), and TV interface.”
Before joining KAIST, Hyeon-Min Bae worked for many years at Finisar as a researcher who designed and developed the world’s first super-high-speed circuit, the 100 Gbps Ethernet IC.
2013.11.25 View 11071 -
 Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 14078
Technology Developed for Flexible, Foldable & Rechargeable Battery
Flexible, Foldable & Rechargeable Battery
The research group of professors Jang-Wook Choi & Jung-Yong Lee from the Graduate School of EEWS and Taek-Soo Kim from the Department of Mechanical Engineering at KAIST has developed technology for flexible and foldable batteries which are rechargeable using solar energy. The research result was published in the online issue of Nano Letters on November 5.
Trial versions of flexible and wearable electronics are being developed and introduced in the market such as Galaxy Gear, Apple’s i-Watch, and Google Glass. Research is being conducted to make the batteries softer and more wearable and to compete in the fast-growing market for flexible electronics.
This new technology is expected to be applied to the development of wearable computers as well as winter outdoor clothing since it is flexible and light. The research group expects that the new technology can be applied to current battery production lines without additional investment.
Professor Choi said, “It can be used as a core-source technology in the rechargeable battery industry in the future. Various wearable mobile electronic products can be developed through cooperation and collaboration within the industry.”
2013.11.21 View 14078 -
 One Day Idea Hackathon, GoGeeks 2013
GoGeeks Creation Group, founded by KAIST alumni, is hosting the GoGeeks One Day Idea Hackathon 2013 on November 23 at the Microsoft (MS) Building in Seoul. The contest was organized to support creative ideas proposed by college students and help them to be applied in real life.
The first GoGeeks contest was held last year by a group of KAIST undergraduate students, which was influenced by Duke University’s Elevator Pitch Competition, an entrepreneurial competition started in 1999.
Applications for the contest should be submitted on www.gogeeks.co.kr by November 20, and the first 30 groups registered will get to compete in the contest.
Each group will make one-minute presentations after six hours of planning on the theme of “Color Your Society.” Then five-minute presentations will follow by the top ten groups selected from the first stage. The top five groups will be each awarded the prize of 2 million Korean won.
The five selected teams will work on their proposed projects during the winter vacation with support from MS Korea and the Center for Science-based Entrepreneurship at KAIST.
Fundraising for the event is going on at http://tumblbug.com/gogeeks2013.
2013.11.21 View 8445
One Day Idea Hackathon, GoGeeks 2013
GoGeeks Creation Group, founded by KAIST alumni, is hosting the GoGeeks One Day Idea Hackathon 2013 on November 23 at the Microsoft (MS) Building in Seoul. The contest was organized to support creative ideas proposed by college students and help them to be applied in real life.
The first GoGeeks contest was held last year by a group of KAIST undergraduate students, which was influenced by Duke University’s Elevator Pitch Competition, an entrepreneurial competition started in 1999.
Applications for the contest should be submitted on www.gogeeks.co.kr by November 20, and the first 30 groups registered will get to compete in the contest.
Each group will make one-minute presentations after six hours of planning on the theme of “Color Your Society.” Then five-minute presentations will follow by the top ten groups selected from the first stage. The top five groups will be each awarded the prize of 2 million Korean won.
The five selected teams will work on their proposed projects during the winter vacation with support from MS Korea and the Center for Science-based Entrepreneurship at KAIST.
Fundraising for the event is going on at http://tumblbug.com/gogeeks2013.
2013.11.21 View 8445