Nature
-
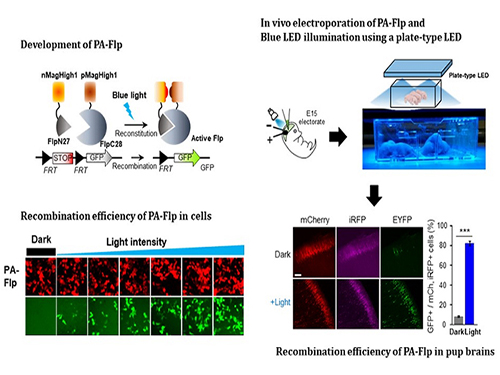 Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7512
Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7512 -
 Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 6578
Technology to Control Near-Field Thermal Radiation
(from left clockwise: Professor Seung Seob Lee, Professor Bong Jae Lee, PhD Mikyung Lim and PhD candidate Jaeman Song)
A KAIST research team succeeded in measuring and controlling the near-field thermal radiation between metallo-dielectric (MD) multilayer structures.
Their thermal radiation control technology can be applied to next-generation semiconductor packaging, thermophotovoltaic cells and thermal management systems. It also has the potential to be applied to a sustainable energy source for IoT sensors.
In the nanoscale gaps, thermal radiation between objects increases greatly with closer distances. The amount of heat transfer in this scale was found to be from 1,000 to 10,000 times greater than the blackbody radiation heat transfer, which was once considered the theoretical maximum for the rate of thermal radiation. This phenomenon is called near-field thermal radiation. With recent developments in nanotechnology, research into near-field thermal radiation between various materials has been actively carried out.
Surface polariton coupling generated from nanostructures has been of particular interest because it enhances the amount of near-field thermal radiation between two objects, and allows the spectral control of near-field thermal radiation. This advantage has motivated much of the recent theoretical research on the application of near-field thermal radiation using nanostructures, such as thin films, multilayer nanostructures, and nanowires. Nevertheless, thus far, most of the studies have focused on measuring near-field thermal radiation between isotropic materials.
A joint team led by Professor Bong Jae Lee and Professor Seung Seob Lee from the Department of Mechanical Engineering succeeded in measuring near-field thermal radiation according to the vacuum distance between MD multilayer nanostructures by using a custom MEMS (Micro-Electro-Mechanical Systems)-device-integrated platform with three-axis nanopositioner.
MD multilayer nanostructures refer to structures in which metal and dielectric layers with regular thickness alternate. The MD single-layer pair is referred to as a unit cell, and the ratio of the thickness occupied by the metal layer in the unit cell is called the fill factor.
By measuring the near-field thermal radiation with a varying number of unit cells and the fill factor of the multilayer nanostructures, the team demonstrated that the surface plasmon polariton coupling enhances near-field thermal radiation greatly, and allows spectral control over the heat transfer.
Professor B. J. Lee said, “The isotropic materials that have so far been studied experimentally had limited spectral control over the near-field thermal radiation. Our near-field thermal radiation control technology using multilayer nanostructures is expected to become the first step toward developing various near-field thermal radiation applications.”
This research, led by PhD Mikyung Lim and PhD candidate Jaeman Song, was published in Nature Communications on October 16.
Figure 1. Experimental setup for measuring near-field thermal radiation between MD multilayers
Figure 2. Investigation of manipulated near-field heat flux by modifying the surface conditions with MD multilayers
2019.01.04 View 6578 -
 Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9003
Ultrathin Digital Camera Inspired by Xenos Peckii Eyes
(Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering)
The visual system of Xenos peckii, an endoparasite of paper wasps, demonstrates distinct benefits for high sensitivity and high resolution, differing from the compound eyes of most insects. Taking their unique features, a KAIST team developed an ultrathin digital camera that emulates the unique eyes of Xenos peckii.
The ultrathin digital camera offers a wide field of view and high resolution in a slimmer body compared to existing imaging systems. It is expected to support various applications, such as monitoring equipment, medical imaging devices, and mobile imaging systems.
Professor Ki-Hun Jeong from the Department of Bio and Brain Engineering and his team are known for mimicking biological visual organs. The team’s past research includes an LED lens based on the abdominal segments of fireflies and biologically inspired anti-reflective structures.
Recently, the demand for ultrathin digital cameras has increased, due to the miniaturization of electronic and optical devices. However, most camera modules use multiple lenses along the optical axis to compensate for optical aberrations, resulting in a larger volume as well as a thicker total track length of digital cameras. Resolution and sensitivity would be compromised if these modules were to be simply reduced in size and thickness.
To address this issue, the team have developed micro-optical components, inspired from the visual system of Xenos peckii, and combined them with a CMOS (complementary metal oxide semiconductor) image sensor to achieve an ultrathin digital camera.
This new camera, measuring less than 2mm in thickness, emulates the eyes of Xenos peckii by using dozens of microprism arrays and microlens arrays. A microprism and microlens pair form a channel and the light-absorbing medium between the channels reduces optical crosstalk. Each channel captures the partial image at slightly different orientation, and the retrieved partial images are combined into a single image, thereby ensuring a wide field of view and high resolution.
Professor Jeong said, “We have proposed a novel method of fabricating an ultrathin camera. As the first insect-inspired, ultrathin camera that integrates a microcamera on a conventional CMOS image sensor array, our study will have a significant impact in optics and related fields.”
This research, led by PhD candidates Dongmin Keum and Kyung-Won Jang, was published in Light: Science & Applications on October 24, 2018.
Figure 1. Natural Xenos peckii eye and the biological inspiration for the ultrathin digital camera (Light: Science & Applications 2018)
Figure 2. Optical images captured by the bioinspired ultrathin digital camera (Light: Science & Applications 2018)
2018.12.31 View 9003 -
 From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 8368
From Concept to Reality: Changing Color of Light Using a Spatiotemporal Boundary
(from left: Professor Bumki Min, PhD candidate Jaehyeon Son and PhD Kanghee Lee)
A KAIST team developed an optical technique to change the color (frequency) of light using a spatiotemporal boundary. The research focuses on realizing a spatiotemporal boundary with a much higher degree of freedom than the results of previous studies by fabricating a thin metal structure on a semiconductor surface. Such a spatiotemporal boundary is expected to be applicable to an ultra-thin film type optical device capable of changing the color of light.
The optical frequency conversion device plays a key role in precision measurement and communication technology, and the device has been developed mainly based on optical nonlinearity.
If the intensity of light is very strong, the optical medium responds nonlinearly so the nonlinear optical phenomena, such as frequency doubling or frequency mixing, can be observed. Such optical nonlinear phenomena are realized usually by the interaction between a high-intensity laser and a nonlinear medium.
As an alternative method frequency conversion is observed by temporally modifying the optical properties of the medium through which light travels using an external stimulus. Since frequency conversion in this way can be observed even in weak light, such a technique could be particularly useful in communication technology.
However, rapid optical property modification of the medium by an external stimulus and subsequent light frequency conversion techniques have been researched only in the pertubative regime, and it has been difficult to realize these theoretical results in practical applications.
To realize such a conceptual idea, Professor Bumki Min from the Department of Mechanical Engineering and his team collaborated with Professor Wonju Jeon from the Department of Mechanical Engineering and Professor Fabian Rotermund from the Department of Physics. They developed an artificial optical material (metamaterial) by arranging a metal microstructure that mimics an atomic structure and succeeded in creating a spatiotemporal boundary by changing the optical property of the artificial material abruptly.
While previous studies only slightly modified the refractive index of the medium, this study provided a spatiotemporal boundary as a platform for freely designing and changing the spectral properties of the medium. Using this, the research team developed a device that can control the frequency of light to a large degree.
The research team said a spatiotemporal boundary, which was only conceptually considered in previous research and realized in the pertubative regime, was developed as a step that can be realized and applied.
Professor Min said, “The frequency conversion of light becomes designable and predictable, so our research could be applied in many optical applications. This research will present a new direction for time-variant media research projects in the field of optics.”
This research, led by PhD Kanghee Lee and PhD candidate Jaehyeon Son, was published online in Nature Photonics on October 8, 2018.
This work was supported by the National Research Foundation of Korea (NRF) through the government of Korea. The work was also supported by the Center for Advanced Meta-Materials (CAMM) funded by the Korea Government (MSIP) as the Global Frontier Project (NRF-2014M3A6B3063709).
Figure 1. The frequency conversion process of light using a spatiotemporal boundary.
Figure 2. The complex amplitude of light at the converted frequency with the variation of a spatiotemporal boundary.
2018.11.29 View 8368 -
 Lens-free OLEDs with Efficiency comparable to that of Inorganic LEDs
(from left: Professor Seunghyup Yoo and PhD candidate Jinouk Song)
The use of organic light-emitting diodes (OLEDs) has extended to various applications, but their efficiency is still lagging behind inorganic light-emitting diodes. In this research, a KAIST team provided a systematic way to yield OLEDs with an external quantum efficiency (EQE) greater than 50% with an external scattering medium.
Having properties suitable for thin and flexible devices, OLEDs are popular light sources for displays, such as mobile devices and high quality TVs. In recent years, numerous efforts have been made to apply OLEDs in lighting as well as light sources for vehicles.
For such applications, high efficiency is of the upmost importance for the successful deployment of light sources. Thanks to continuous research and the development of OLEDs, their efficiency is steadily on the rise, and a level equivalent to inorganic LEDs has been demonstrated in some reports.
However, these highly efficient OLEDs were often achieved with a macroscopic lens or complex internal nanostructures, which undermines the key advantages of OLEDs as an affordable planar light sources and tends to hinder their stable operation, thus putting a limitation to their commercialization.
Among various methods proven effective for OLED light extraction, a team led by Professor Seunghyup Yoo at the School of Electrical Engineering focused on the external scattering-based approach, as it can maintain planar geometry and compatibility with flexibility. It is also able to be fabricated on a large scale at a low cost and causes no interference with electrical properties of OLEDs.
Conventionally, research on enhancing OLED light extraction using light scattering has been conducted empirically in many cases. This time, the team developed comprehensive and analytical methodology to theoretically predict structures that maximize efficiency.
Considering OLEDs with the external scattering layers as a whole rather than two separate entities, the researchers combined the mathematical description of the scattering phenomena with the optical model for light emission within an OLED to rapidly predict the characteristics of many devices with various structures. Based on this approach, the team theoretically predicted the optimal combination of scattering layers and OLED architectures that can lead to the maximum efficiency.
Following this theoretical prediction, the team experimentally produced the optimal light scattering film and incorporated it to OLEDs with orange emitters having a high degree of horizontal dipole orientation. As a result, the team successfully realized OLEDs exhibiting EQE of 56% and power efficiency of 221 lm/W. This is one of the highest efficiencies ever realized for an OLED unit device without the help of a macroscopic lens or internal light extraction structures.
Professor Yoo said, “There are various technologies developed for improving OLED light extraction efficiency; nevertheless, most of them have not reached a level of practical use. This research mainly provides a systematic way to attain an EQE of 50% or higher in OLEDs while keeping in mind the constraints for commercialization. The approach shown here can readily be applied to lighting devices or sensors of wearable devices.”.
This research, co-led by Professor Jang-Joo Kim from Seoul National University and Professor Yun-Hi Kim from Gyeongsang National University, was published in Nature Communications on August 10, 2018. (J. Song et al. Nature Communications, 9, 3207. DOI: 10.1038/s41467-018-05671-x)
Figure 1.Photographs of OLEDs with SiO₂ -embedded scattering layers according to scatterance
2018.10.26 View 9087
Lens-free OLEDs with Efficiency comparable to that of Inorganic LEDs
(from left: Professor Seunghyup Yoo and PhD candidate Jinouk Song)
The use of organic light-emitting diodes (OLEDs) has extended to various applications, but their efficiency is still lagging behind inorganic light-emitting diodes. In this research, a KAIST team provided a systematic way to yield OLEDs with an external quantum efficiency (EQE) greater than 50% with an external scattering medium.
Having properties suitable for thin and flexible devices, OLEDs are popular light sources for displays, such as mobile devices and high quality TVs. In recent years, numerous efforts have been made to apply OLEDs in lighting as well as light sources for vehicles.
For such applications, high efficiency is of the upmost importance for the successful deployment of light sources. Thanks to continuous research and the development of OLEDs, their efficiency is steadily on the rise, and a level equivalent to inorganic LEDs has been demonstrated in some reports.
However, these highly efficient OLEDs were often achieved with a macroscopic lens or complex internal nanostructures, which undermines the key advantages of OLEDs as an affordable planar light sources and tends to hinder their stable operation, thus putting a limitation to their commercialization.
Among various methods proven effective for OLED light extraction, a team led by Professor Seunghyup Yoo at the School of Electrical Engineering focused on the external scattering-based approach, as it can maintain planar geometry and compatibility with flexibility. It is also able to be fabricated on a large scale at a low cost and causes no interference with electrical properties of OLEDs.
Conventionally, research on enhancing OLED light extraction using light scattering has been conducted empirically in many cases. This time, the team developed comprehensive and analytical methodology to theoretically predict structures that maximize efficiency.
Considering OLEDs with the external scattering layers as a whole rather than two separate entities, the researchers combined the mathematical description of the scattering phenomena with the optical model for light emission within an OLED to rapidly predict the characteristics of many devices with various structures. Based on this approach, the team theoretically predicted the optimal combination of scattering layers and OLED architectures that can lead to the maximum efficiency.
Following this theoretical prediction, the team experimentally produced the optimal light scattering film and incorporated it to OLEDs with orange emitters having a high degree of horizontal dipole orientation. As a result, the team successfully realized OLEDs exhibiting EQE of 56% and power efficiency of 221 lm/W. This is one of the highest efficiencies ever realized for an OLED unit device without the help of a macroscopic lens or internal light extraction structures.
Professor Yoo said, “There are various technologies developed for improving OLED light extraction efficiency; nevertheless, most of them have not reached a level of practical use. This research mainly provides a systematic way to attain an EQE of 50% or higher in OLEDs while keeping in mind the constraints for commercialization. The approach shown here can readily be applied to lighting devices or sensors of wearable devices.”.
This research, co-led by Professor Jang-Joo Kim from Seoul National University and Professor Yun-Hi Kim from Gyeongsang National University, was published in Nature Communications on August 10, 2018. (J. Song et al. Nature Communications, 9, 3207. DOI: 10.1038/s41467-018-05671-x)
Figure 1.Photographs of OLEDs with SiO₂ -embedded scattering layers according to scatterance
2018.10.26 View 9087 -
 Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 6680
Skin Hardness to Estimate Better Human Thermal Status
(Professor Young-Ho Cho and Researcher Sunghyun Yoon)
Under the same temperature and humidity, human thermal status may vary due to individual body constitution and climatic environment. A KAIST research team previously developed a wearable sweat rate sensor for human thermal comfort monitoring. Furthering the development, this time they proposed skin hardness as an additional, independent physiological sign to estimate human thermal status more accurately. This novel approach can be applied to developing systems incorporating human-machine interaction, which requires accurate information about human thermal status.
Professor Young-Ho Cho and his team from the Department of Bio and Brain Engineering had previously studied skin temperature and sweat rate to determine human thermal comfort, and developed a watch-type sweat rate sensor that accurately and steadily detects thermal comfort last February (title: Wearable Sweat Rate Sensors for Human Thermal Comfort Monitoring ).
However, skin temperature and sweat rate are still not enough to estimate exact human thermal comfort. Hence, an additional indicator is required for enhancing the accuracy and reliability of the estimation and the team selected skin hardness. When people feel hot or cold, arrector pili muscles connected to hair follicles contract and expand, and skin hardness comes from this contraction and relaxation of the muscles. Based on the phenomenon of changing skin hardness, the team proposed skin hardness as a new indicator for measuring human thermal sensation.
With this new estimation model using three physiological signs for estimating human thermal status, the team conducted human experiments and verified that skin hardness is effective and independent from the two conventional physiological signs. Adding skin hardness to the conventional model can reduce errors by 23.5%, which makes its estimation more reliable.
The team will develop a sensor that detects skin hardness and applies it to cognitive air-conditioning and heating systems that better interact with humans than existing systems.
Professor Cho said, “Introducing this new indicator, skin hardness, elevates the reliability of measuring human thermal comfort regardless of individual body constitution and climatic environment. Based on this method, we can develop a personalized air conditioning and heating system that will allow affective interaction between humans and machines by sharing both physical and mental health conditions and emotions.”
This research, led by researchers Sunghyun Yoon and Jai Kyoung Sim, was published in Scientific Reports, Vol.8, Article No.12027 on August 13, 2018. (pp.1-6)
Figure 1. Measuring human thermal status through skin hardness
Figure 2. The instrument used for measuring human thermal status through skin hardness
2018.10.17 View 6680 -
 Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 6676
Trigger of the Hyperactivation of Fibrosis Identified
(Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering)
Scientists have been investigating the negative effects that the hyperactivation of fibrosis has on fibrotic diseases and cancer. A KAIST research team unveiled a positive feedback loop that bi-stably activates fibroblasts in collaboration with Samsung Medical Center. This finding will contribute to developing therapeutic targets for both fibrosis and cancer.
Human fibroblasts are dormant in normal tissue, but show radical activation during wound healing. However, the principle that induces their explosive activation has not yet been identified.
Here, Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering, in collaboration with Professor Seok-Hyung Kim from Samsung Medical Center, discovered the principle of a circuit that continuously activates fibroblasts.
They constructed a positive feedback loops (PFLs) where Twist1, Prrx1, and Tenascin-C (TNC) molecules consecutively activate fibroblasts. They confirmed that these are the main inducers of fibroblast activation by conducting various experiments, including molecular biological tests, mathematical modeling, animal testing, and computer simulations to conclude that they are the main inducers of fibroblast activation.
According to their research, Twist 1 is a key regulator of cancer-associated fibroblasts, which directly upregulates Prrx1 and then triggers TNC, which also increases Twist1 expression. This circuit consequently forms a Twist-Prrx1-TNC positive feedback loop.
Activated fibroblasts need to be deactivated after wounds are healed. However, if the PFLs continue, the fibroblasts become the major cause of worsening fibrotic diseases and cancers.
Therefore, the team expects that Twist1-Prrx1-TNC positive PFLs will be applied for novel and effective therapeutic targeting of fibrotic diseases and cancers.
This research was published in Nature Communications on August 1, 2018.
Figure 1. Twist1 increases tenascin-c expression in cancer-associated fibroblasts. Twist1 is a potent but indirect inducer of tenascin-c (TNC), which is essential for maintaining Twist1 expression in cancer-associated fibroblasts (CAFs).
Figure 2. Summary of the study. The Twist1-Prrx1-TNC positive feedback regulation provides clues for understanding the activation of fibroblasts during wound healing under normal conditions, as well as abnormally activated fibroblasts in pathological conditions such as cancerous and fibrotic diseases. Under normal conditions, the PFL acts as a reversible bistable switch by which the activation of fibroblasts is “ON" above a sufficient level of stimulation and “OFF" for the withdrawal of the stimulus. However, this switch can be permanently turned on under pathologic conditions by continued activation of the PFL, resulting in sustained proliferation of fibroblasts.
2018.10.11 View 6676 -
 Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5617
Effective Drug Delivery to Heart with Tannic Acid
(Professor Haeshin Lee from the Department of Chemistry) Typical methods of drug delivery to the heart require surgical procedures involving incisions in the chest wall and bones. To efficiently treat cardiovascular and related vascular diseases without surgery, a KAIST research team developed a heart-targeting drug delivery technology using tannin acid via intravenous systemic injection. This method can be applied to the development of a variety of new protein-based drugs.
Cardiovascular-circulatory disease is currently the second leading cause of death in Korea. A typical example of this disease is myocardial infarction caused by poor oxygen and nutrient supply due to narrowed coronary arteries and poor blood flow to the heart.
Although there have been numerous research projects to develop chemotherapeutic drugs and therapeutic proteins, clinics still rely on surgical procedures. Drug delivery can be an alternative, but it is quite challenging because ceaseless dynamic cycles of the heart and massive exchanges of blood mean administered therapeutics do not stay inside the heart very long.
Professor Haeshin Lee from the Department of Chemistry and his team employed tannic acid (TA), which is known for giving bitter taste to wines. It is one of the most abundant polyphenols and can be easily found in plants, such as fruits, vegetables, cacao, and others. TA has also been used as a multifunctional coating molecule.
Using these properties of TA, the team complexed protein and peptide therapeutics with tannic acid and succeeded in targeting protein and peptide therapeutics to the heart. TA, coated on the surface of a granulated protein complex, helps maintain cardiac function because it adheres to extracellular matrices, elastin, and collagens in heart tissues allowing the protein to stay attached to the heart tissue for a longer period.
The team confirmed that these Tannic-acid-modified proteins stay in blood vessels five days longer than with protein-only injections. Additionally they found that TA-protein complexes do not show any cardiac toxicity and do not cause noticeable pathology.
The team has been continuously developing biomaterials for medical applications by testing various polyphenolic materials that feature adhesive and coating properties, including tannic acid. They have injected a mixture of TA and fibroblast growth factors (FGF) into animal models with myocardial infarctions. After four weeks, they confirmed that the infarction was reduced and the left ventricular pressure and cardiac output were almost normalized.
Professor Lee said, “Although there have been numerous drugs related to heart disease, so far there has not been efficient drug delivery to the heart so this technology will be able to reformulate existing drugs into new and more efficient drugs.”
This research, jointly led by Dr. Ki-Suk Kim from the Predictive Model Research Center, was published in Nature Biomedical Engineering on April 30 ( http://www.nature.com/articles/s41551-018-0227-9 ).
Figure 1. Schematic for the heart-targeting mechanism of TANNylated protein nanocomplexes: (1) size-dependent permeation, (2) phenolic (that is, TA), and (3) internalization by internalization by myoblasts
Figure 2. Effect of TA based protein complexes on cardiac cell transport efficiency and viral gene expression efficiency and therapeutic function in animal models with myocardial infarction
2018.09.18 View 5617 -
 Electron Heating in Weakly Ionized Collisional Plasmas
(from left: Professor Wonho Choe and Research Professor Sanghoo Park)
A KAIST research team successfully identified the underlying principles behind electron heating, which is one of the most important phenomena in plasmas. As the electric heating determines wide range of physical and chemical properties of plasmas, this outcome will allow relevant industries to extend and effectively customize a range of plasma characteristics for their specific needs.
Plasma, frequently called the fourth state of matter, can be mostly formed by artificially energizing gases in standard temperature (25°C) and pressure (1 atm) range. Among the many types of plasma, atmospheric-pressure plasmas have been gaining a great deal of attention due to their unique features and applicability in various scientific and industrial fields.
Because plasma characteristics strongly depends on gas pressure in the sub-atmospheric to atmospheric pressure range, characterizing the plasma at different pressures is a prerequisite for understanding the fundamental principles of plasmas and for their industrial applications.
In that sense, information on the spatio-temporal evolution in the electron density and temperature is very important because various physical and chemical reactions within a plasma arise from electrons. Hence, electron heating has been an interesting topic in the field of plasma.
Because collisions between free electrons and neutral gases are frequent under atmospheric-pressure conditions, there are physical limits to measuring the electron density and temperature in plasmas using conventional diagnostic tools, thus the principles behind free electron heating could not be experimentally revealed.
Moreover, lacking information on a key parameter of electron heating and its controlling methods is troublesome and limit improving the reactivity and applicability of such plasmas.
To address these issues, Professor Wonho Choe and his team from the Department of Nuclear and Quantum Engineering employed neutral bremsstrahlung-based electron diagnostics in order to accurately examine the electron density and temperature in target plasmas. In addition, a novel imaging diagnostics for two dimensional distribution of electron information was developed.
Using the diagnostic technique they developed, the team measured the nanosecond-resolved electron temperature in weakly ionized collisional plasmas, and they succeeded in revealing the spatiotemporal distribution and the fundamental principle involved in the electron heating process.
The team successfully revealed the fundamental principle of the electron heating process under atmospheric to sub-atmospheric pressure (0.25-1atm) conditions through conducting the experiment on the spatiotemporal evolution of electron temperature.
Their findings of the underlying research data on free electrons in weakly ionized collisional plasmas will contribute to enhancing the field of plasma science and their commercial applications.
Professor Choe said, “The results of this study provide a clear picture of electron heating in weakly ionized plasmas under conditions where collisions between free electrons and neutral particles are frequent. We hope this study will be informative and helpful in utilizing and commercializing atmospheric-pressure plasma sources in the near future.”
Articles related to this research, led by Research Professor Sanghoo Park, were published in Scientific Reports on May 14 and July 5.
Figure 1. Nanosecond-resolved visualization of the electron heating structure. Spatiotemporal evolution of 514.5-nm continuum radiation,Te, Ar I emission
Figure 2. Nanosecond-resolved visualization of electron heating. Spatiotemporal evolution of neutral bremsstrahlung at 514.5 nm
2018.09.10 View 7185
Electron Heating in Weakly Ionized Collisional Plasmas
(from left: Professor Wonho Choe and Research Professor Sanghoo Park)
A KAIST research team successfully identified the underlying principles behind electron heating, which is one of the most important phenomena in plasmas. As the electric heating determines wide range of physical and chemical properties of plasmas, this outcome will allow relevant industries to extend and effectively customize a range of plasma characteristics for their specific needs.
Plasma, frequently called the fourth state of matter, can be mostly formed by artificially energizing gases in standard temperature (25°C) and pressure (1 atm) range. Among the many types of plasma, atmospheric-pressure plasmas have been gaining a great deal of attention due to their unique features and applicability in various scientific and industrial fields.
Because plasma characteristics strongly depends on gas pressure in the sub-atmospheric to atmospheric pressure range, characterizing the plasma at different pressures is a prerequisite for understanding the fundamental principles of plasmas and for their industrial applications.
In that sense, information on the spatio-temporal evolution in the electron density and temperature is very important because various physical and chemical reactions within a plasma arise from electrons. Hence, electron heating has been an interesting topic in the field of plasma.
Because collisions between free electrons and neutral gases are frequent under atmospheric-pressure conditions, there are physical limits to measuring the electron density and temperature in plasmas using conventional diagnostic tools, thus the principles behind free electron heating could not be experimentally revealed.
Moreover, lacking information on a key parameter of electron heating and its controlling methods is troublesome and limit improving the reactivity and applicability of such plasmas.
To address these issues, Professor Wonho Choe and his team from the Department of Nuclear and Quantum Engineering employed neutral bremsstrahlung-based electron diagnostics in order to accurately examine the electron density and temperature in target plasmas. In addition, a novel imaging diagnostics for two dimensional distribution of electron information was developed.
Using the diagnostic technique they developed, the team measured the nanosecond-resolved electron temperature in weakly ionized collisional plasmas, and they succeeded in revealing the spatiotemporal distribution and the fundamental principle involved in the electron heating process.
The team successfully revealed the fundamental principle of the electron heating process under atmospheric to sub-atmospheric pressure (0.25-1atm) conditions through conducting the experiment on the spatiotemporal evolution of electron temperature.
Their findings of the underlying research data on free electrons in weakly ionized collisional plasmas will contribute to enhancing the field of plasma science and their commercial applications.
Professor Choe said, “The results of this study provide a clear picture of electron heating in weakly ionized plasmas under conditions where collisions between free electrons and neutral particles are frequent. We hope this study will be informative and helpful in utilizing and commercializing atmospheric-pressure plasma sources in the near future.”
Articles related to this research, led by Research Professor Sanghoo Park, were published in Scientific Reports on May 14 and July 5.
Figure 1. Nanosecond-resolved visualization of the electron heating structure. Spatiotemporal evolution of 514.5-nm continuum radiation,Te, Ar I emission
Figure 2. Nanosecond-resolved visualization of electron heating. Spatiotemporal evolution of neutral bremsstrahlung at 514.5 nm
2018.09.10 View 7185 -
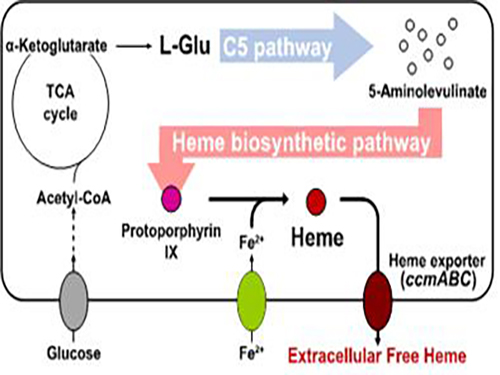 Metabolic Engineering of E. coli for the Secretory Production of Free Haem
Researchers of KAIST have defined a novel strategy for the secretory production of free haem using engineered Escherichia coli (E. coli) strains. They utilized the C5 pathway, the optimized downstream pathways, and the haem exporter to construct a recombinant micro-organism producing extracellular haem using fed-batch fermentation. This is the first report to extracellularly produce haem using engineered E. coli.
This strategy will expedite the efficient production of free haem to serve as a bioavailable iron-supplying agent and an important prosthetic group of multiple hemoproteins for medical uses. This study, led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, was published in Nature Catalysis on Aug. 28.
Haem, an organometallic compound complexed with a ferrous ion, is an essential molecule delivering oxygen in the blood of many animals. It is also a key component of electron transport chains responsible for the respiration of aerobic organisms including diverse bacteria. It is now being widely applied as a bioavailable iron-supplying agent in the healthcare and dietary supplement industries. The demand for haem and the need for the efficient production of this compound continue to grow.
Many previous researchers have attempted to produce free haem using engineered E. coli. However, none of the studies was successful in producing free haem extracellularly, requiring an additional step to extract the accumulated haem from cells for subsequent uses. The secretion of haem in the form of haem peptides or proteins also requires an extraction step to isolate the free haem from the secreted products. Thus, the secretory production of free haem is an important task for the economical production of haem that is suitable for human consumption.
Although some researchers could produce intracellular haem using recombinant E. coli strains, its final titer was extremely low, resulting from the use of sub-optimal metabolic pathways. Furthermore, the addition of the precursors L-glycine and succinate was deemed undesirable for massive industrial production. Thus, it is necessary to construct an optimized haem biosynthetic pathway to enable the efficient production of haem and examine the consequent secretion of free haem.
To address this issue, the KAIST team used multiple strategies to produce extracellular free haem by enhancing its biosynthesis in E. coli. First, the capacities of the C4 and C5 pathways to produce aminolevulinate (ALA) without feeding precursors were examined. After confirming the superior performance of the C5 pathway over the C4 pathway, the metabolic genes of the C5 pathway and downstream pathways for haem biosynthesis were overexpressed. Then, the metabolic pathways were optimized by adjusting the expression levels of the relevant genes and disrupting the putative haem degradation enzyme encoded by the yfeX gene.
Consequently, the resulting engineered strain secreted a significant amount of haem to the medium. Subsequent optimization of the cultivation conditions and the supplementation of nitrogen sources further increased both the titer of the total free haem and the amount of free haem secreted to the medium. Finally, the overexpression of the ccmABC genes encoding the haem exporter further enhanced the production and secretion of haem, producing the highest titer of haem both intracellularly and extracellularly from glucose.
Professor Lee said, “The eco-friendly and sustainable chemical industry is a key global agenda every nation faces. We are conducting research to bio-synthesize high concentrations, high yields, and high productivity in natural products. This novel technology will serve as an opportunity to advance the biochemical industry moving forward.”
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea. Further Contact: Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea ( leesy@kaist.ac.kr+82-42-350-3930).
2018.08.28 View 6112
Metabolic Engineering of E. coli for the Secretory Production of Free Haem
Researchers of KAIST have defined a novel strategy for the secretory production of free haem using engineered Escherichia coli (E. coli) strains. They utilized the C5 pathway, the optimized downstream pathways, and the haem exporter to construct a recombinant micro-organism producing extracellular haem using fed-batch fermentation. This is the first report to extracellularly produce haem using engineered E. coli.
This strategy will expedite the efficient production of free haem to serve as a bioavailable iron-supplying agent and an important prosthetic group of multiple hemoproteins for medical uses. This study, led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, was published in Nature Catalysis on Aug. 28.
Haem, an organometallic compound complexed with a ferrous ion, is an essential molecule delivering oxygen in the blood of many animals. It is also a key component of electron transport chains responsible for the respiration of aerobic organisms including diverse bacteria. It is now being widely applied as a bioavailable iron-supplying agent in the healthcare and dietary supplement industries. The demand for haem and the need for the efficient production of this compound continue to grow.
Many previous researchers have attempted to produce free haem using engineered E. coli. However, none of the studies was successful in producing free haem extracellularly, requiring an additional step to extract the accumulated haem from cells for subsequent uses. The secretion of haem in the form of haem peptides or proteins also requires an extraction step to isolate the free haem from the secreted products. Thus, the secretory production of free haem is an important task for the economical production of haem that is suitable for human consumption.
Although some researchers could produce intracellular haem using recombinant E. coli strains, its final titer was extremely low, resulting from the use of sub-optimal metabolic pathways. Furthermore, the addition of the precursors L-glycine and succinate was deemed undesirable for massive industrial production. Thus, it is necessary to construct an optimized haem biosynthetic pathway to enable the efficient production of haem and examine the consequent secretion of free haem.
To address this issue, the KAIST team used multiple strategies to produce extracellular free haem by enhancing its biosynthesis in E. coli. First, the capacities of the C4 and C5 pathways to produce aminolevulinate (ALA) without feeding precursors were examined. After confirming the superior performance of the C5 pathway over the C4 pathway, the metabolic genes of the C5 pathway and downstream pathways for haem biosynthesis were overexpressed. Then, the metabolic pathways were optimized by adjusting the expression levels of the relevant genes and disrupting the putative haem degradation enzyme encoded by the yfeX gene.
Consequently, the resulting engineered strain secreted a significant amount of haem to the medium. Subsequent optimization of the cultivation conditions and the supplementation of nitrogen sources further increased both the titer of the total free haem and the amount of free haem secreted to the medium. Finally, the overexpression of the ccmABC genes encoding the haem exporter further enhanced the production and secretion of haem, producing the highest titer of haem both intracellularly and extracellularly from glucose.
Professor Lee said, “The eco-friendly and sustainable chemical industry is a key global agenda every nation faces. We are conducting research to bio-synthesize high concentrations, high yields, and high productivity in natural products. This novel technology will serve as an opportunity to advance the biochemical industry moving forward.”
This work was supported by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation (NRF) of Korea. Further Contact: Dr. Sang Yup Lee, Distinguished Professor, KAIST, Daejeon, Korea ( leesy@kaist.ac.kr+82-42-350-3930).
2018.08.28 View 6112 -
 A Breakthrough for Understanding Glioblastoma: Origin Cells for Deadly Brain Tumors Identified
Figure 1. The pattern of GBM genesis is similar to that of firework. The bottom canon represents the first occurrence of the SVZ mutated cell.
A new study by KAIST researchers identified where the mutation causing glioblastoma starts. According to the study, neural stem cells away from the tumor mass are the cells of origin that contain mutation drivers for glioblastoma, one of the most aggressive brain tumor. This breakthrough research, reported in Nature on August 1, gives insights for understanding why glioblastomas almost always grow back, even after surgery, and suggests novel ways to treat glioblastoma, which was previously thought to be incurable.
Like most cancers, glioblastoma is treated with surgery to remove as much of the tumor as possible, then radiation and chemotherapy. However, it almost always returns in less than a year and its median survival time is only 15 months. Precision therapeutic approaches targeting tumors themselves didn’t lead to any breakthroughs.
Professor Jeong Ho Lee’s team at the Graduate School of Medical Science and Engineering described direct genetic evidence through the deep sequencing of all triple-matched samples: normal SVZ tissue away from the tumor mass, tumor tissue, and normal cortical tissue. The research team studied 28 patients with glioblastomas and other types of brain tumors who underwent supra-total resection or other surgical resections of tumors, providing access to normal subventricular zone (SVZ) tissue (where neural stem cells are located) away from the tumor mass. The researchers used various deep and single cell sequencing technologies to conduct comparative DNA analysis on the samples from the patient’s SVZ tissue and tumors.
They reported that normal SVZ tissue away from the tumor in 56.3% of patients with glioblastoma already contained low-level glioblastoma driver mutations that were observed at high levels in their matching tumors. Furthermore, the research team generated a genome edited mouse carrying glioblastoma mutations in the SVZ and showed that neural stem cells with mutations migrate from the SVZ lead to the development of glioblastomas in distant brain regions. (See the image below)
Professor Lee conducted this study in collaboration with Professor Seok-Gu Kang of the Brain Tumor Center at Severance Hospital of Yonsei University. He said, “It’s easier to understand when we compare it to fireworks. Every flare flying around sky can be likened to cancer cells even though the fireworks are triggered on the ground. We found the trigger.” The identification of this mutation pathway of glioblastomas will lead to a new paradigm for therapeutic strategies. He added, “Now, we can focus on interrupting the recurrence and evolution of glioblastomas.”
Professor Lee has investigated mutations arising in the brain for a decade. He is developing innovative diagnostics and therapeutics for untreatable brain disorders including intractable epilepsy and glioblastoma at a tech-startup, SoVarGen. “All technologies we used during the research were transferred to the company. This research gave us very good momentum to reach the next phase of our startup,” he remarked.
Figure 2. Genetic analysis of tumor-free SVZ tissue and matching tumor tissue from GBM patients.
Figure 3. Glioma progression in genome edited mice carrying GBM mutations in the SVZ
2018.08.02 View 12618
A Breakthrough for Understanding Glioblastoma: Origin Cells for Deadly Brain Tumors Identified
Figure 1. The pattern of GBM genesis is similar to that of firework. The bottom canon represents the first occurrence of the SVZ mutated cell.
A new study by KAIST researchers identified where the mutation causing glioblastoma starts. According to the study, neural stem cells away from the tumor mass are the cells of origin that contain mutation drivers for glioblastoma, one of the most aggressive brain tumor. This breakthrough research, reported in Nature on August 1, gives insights for understanding why glioblastomas almost always grow back, even after surgery, and suggests novel ways to treat glioblastoma, which was previously thought to be incurable.
Like most cancers, glioblastoma is treated with surgery to remove as much of the tumor as possible, then radiation and chemotherapy. However, it almost always returns in less than a year and its median survival time is only 15 months. Precision therapeutic approaches targeting tumors themselves didn’t lead to any breakthroughs.
Professor Jeong Ho Lee’s team at the Graduate School of Medical Science and Engineering described direct genetic evidence through the deep sequencing of all triple-matched samples: normal SVZ tissue away from the tumor mass, tumor tissue, and normal cortical tissue. The research team studied 28 patients with glioblastomas and other types of brain tumors who underwent supra-total resection or other surgical resections of tumors, providing access to normal subventricular zone (SVZ) tissue (where neural stem cells are located) away from the tumor mass. The researchers used various deep and single cell sequencing technologies to conduct comparative DNA analysis on the samples from the patient’s SVZ tissue and tumors.
They reported that normal SVZ tissue away from the tumor in 56.3% of patients with glioblastoma already contained low-level glioblastoma driver mutations that were observed at high levels in their matching tumors. Furthermore, the research team generated a genome edited mouse carrying glioblastoma mutations in the SVZ and showed that neural stem cells with mutations migrate from the SVZ lead to the development of glioblastomas in distant brain regions. (See the image below)
Professor Lee conducted this study in collaboration with Professor Seok-Gu Kang of the Brain Tumor Center at Severance Hospital of Yonsei University. He said, “It’s easier to understand when we compare it to fireworks. Every flare flying around sky can be likened to cancer cells even though the fireworks are triggered on the ground. We found the trigger.” The identification of this mutation pathway of glioblastomas will lead to a new paradigm for therapeutic strategies. He added, “Now, we can focus on interrupting the recurrence and evolution of glioblastomas.”
Professor Lee has investigated mutations arising in the brain for a decade. He is developing innovative diagnostics and therapeutics for untreatable brain disorders including intractable epilepsy and glioblastoma at a tech-startup, SoVarGen. “All technologies we used during the research were transferred to the company. This research gave us very good momentum to reach the next phase of our startup,” he remarked.
Figure 2. Genetic analysis of tumor-free SVZ tissue and matching tumor tissue from GBM patients.
Figure 3. Glioma progression in genome edited mice carrying GBM mutations in the SVZ
2018.08.02 View 12618 -
 Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10354
Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10354