ICA
-
 KAIST builds a high-resolution 3D holographic sensor using a single mask
Holographic cameras can provide more realistic images than ordinary cameras thanks to their ability to acquire 3D information about objects. However, existing holographic cameras use interferometers that measure the wavelength and refraction of light through the interference of light waves, which makes them complex and sensitive to their surrounding environment.
On August 23, a KAIST research team led by Professor YongKeun Park from the Department of Physics announced a new leap forward in 3D holographic imaging sensor technology.
The team proposed an innovative holographic camera technology that does not use complex interferometry. Instead, it uses a mask to precisely measure the phase information of light and reconstruct the 3D information of an object with higher accuracy.
< Figure 1. Structure and principle of the proposed holographic camera. The amplitude and phase information of light scattered from a holographic camera can be measured. >
The team used a mask that fulfills certain mathematical conditions and incorporated it into an ordinary camera, and the light scattered from a laser is measured through the mask and analyzed using a computer. This does not require a complex interferometer and allows the phase information of light to be collected through a simplified optical system. With this technique, the mask that is placed between the two lenses and behind an object plays an important role. The mask selectively filters specific parts of light,, and the intensity of the light passing through the lens can be measured using an ordinary commercial camera. This technique combines the image data received from the camera with the unique pattern received from the mask and reconstructs an object’s precise 3D information using an algorithm.
This method allows a high-resolution 3D image of an object to be captured in any position. In practical situations, one can construct a laser-based holographic 3D image sensor by adding a mask with a simple design to a general image sensor. This makes the design and construction of the optical system much easier. In particular, this novel technology can capture high-resolution holographic images of objects moving at high speeds, which widens its potential field of application.
< Figure 2. A moving doll captured by a conventional camera and the proposed holographic camera. When taking a picture without focusing on the object, only a blurred image of the doll can be obtained from a general camera, but the proposed holographic camera can restore the blurred image of the doll into a clear image. >
The results of this study, conducted by Dr. Jeonghun Oh from the KAIST Department of Physics as the first author, were published in Nature Communications on August 12 under the title, "Non-interferometric stand-alone single-shot holographic camera using reciprocal diffractive imaging".
Dr. Oh said, “The holographic camera module we are suggesting can be built by adding a filter to an ordinary camera, which would allow even non-experts to handle it easily in everyday life if it were to be commercialized.” He added, “In particular, it is a promising candidate with the potential to replace existing remote sensing technologies.”
This research was supported by the National Research Foundation’s Leader Research Project, the Korean Ministry of Science and ICT’s Core Hologram Technology Support Project, and the Nano and Material Technology Development Project.
2023.09.05 View 8892
KAIST builds a high-resolution 3D holographic sensor using a single mask
Holographic cameras can provide more realistic images than ordinary cameras thanks to their ability to acquire 3D information about objects. However, existing holographic cameras use interferometers that measure the wavelength and refraction of light through the interference of light waves, which makes them complex and sensitive to their surrounding environment.
On August 23, a KAIST research team led by Professor YongKeun Park from the Department of Physics announced a new leap forward in 3D holographic imaging sensor technology.
The team proposed an innovative holographic camera technology that does not use complex interferometry. Instead, it uses a mask to precisely measure the phase information of light and reconstruct the 3D information of an object with higher accuracy.
< Figure 1. Structure and principle of the proposed holographic camera. The amplitude and phase information of light scattered from a holographic camera can be measured. >
The team used a mask that fulfills certain mathematical conditions and incorporated it into an ordinary camera, and the light scattered from a laser is measured through the mask and analyzed using a computer. This does not require a complex interferometer and allows the phase information of light to be collected through a simplified optical system. With this technique, the mask that is placed between the two lenses and behind an object plays an important role. The mask selectively filters specific parts of light,, and the intensity of the light passing through the lens can be measured using an ordinary commercial camera. This technique combines the image data received from the camera with the unique pattern received from the mask and reconstructs an object’s precise 3D information using an algorithm.
This method allows a high-resolution 3D image of an object to be captured in any position. In practical situations, one can construct a laser-based holographic 3D image sensor by adding a mask with a simple design to a general image sensor. This makes the design and construction of the optical system much easier. In particular, this novel technology can capture high-resolution holographic images of objects moving at high speeds, which widens its potential field of application.
< Figure 2. A moving doll captured by a conventional camera and the proposed holographic camera. When taking a picture without focusing on the object, only a blurred image of the doll can be obtained from a general camera, but the proposed holographic camera can restore the blurred image of the doll into a clear image. >
The results of this study, conducted by Dr. Jeonghun Oh from the KAIST Department of Physics as the first author, were published in Nature Communications on August 12 under the title, "Non-interferometric stand-alone single-shot holographic camera using reciprocal diffractive imaging".
Dr. Oh said, “The holographic camera module we are suggesting can be built by adding a filter to an ordinary camera, which would allow even non-experts to handle it easily in everyday life if it were to be commercialized.” He added, “In particular, it is a promising candidate with the potential to replace existing remote sensing technologies.”
This research was supported by the National Research Foundation’s Leader Research Project, the Korean Ministry of Science and ICT’s Core Hologram Technology Support Project, and the Nano and Material Technology Development Project.
2023.09.05 View 8892 -
 KAIST Research Team Develops World’s First Humanoid Pilot, PIBOT
In the Spring of last year, the legendary, fictional pilot “Maverick” flew his plane in the film “Top Gun: Maverick” that drew crowds to theatres around the world. This year, the appearance of a humanoid pilot, PIBOT, has stolen the spotlight at KAIST.
< Photo 1. Humanoid pilot robot, PIBOT >
A KAIST research team has developed a humanoid robot that can understand manuals written in natural language and fly a plane on its own. The team also announced their plans to commercialize the humanoid pilot.
< Photo 2. PIBOT on flight simulator (view from above) >
The project was led by KAIST Professor David Hyunchul Shim, and was conducted as a joint research project with Professors Jaegul Choo, Kuk-Jin Yoon, and Min Jun Kim. The study was supported by Future Challenge Funding under the project title, “Development of Human-like Pilot Robot based on Natural Language Processing”. The team utilized AI and robotics technologies, and demonstrated that the humanoid could sit itself in a real cockpit and operate the various pieces of equipment without modifying any part of the aircraft. This is a fundamental difference that distinguishes this technology from existing autopilot functions or unmanned aircrafts.
< Photo 3. PIBOT operating a flight simulator (side) >
The KAIST team’s humanoid pilot is still under development but it can already remember Jeppeson charts from all around the world, which is impossible for human pilots to do, and fly without error. In particular, it can make use of recent ChatGPT technology to remember the full Quick Reference Handbook (QRF) and respond immediately to various situations, as well as calculate safe routes in real time based on the flight status of the aircraft, with emergency response times quicker than human pilots.
Furthermore, while existing robots usually carry out repeated motions in a fixed position, PIBOT can analyze the state of the cockpit as well as the situation outside the aircraft using an embedded camera. PIBOT can accurately control the various switches in the cockpit and, using high-precision control technology, it can accurately control its robotic arms and hands even during harsh turbulence.
< Photo 4. PIBOT on-board KLA-100, Korea’s first light aircraft >
The humanoid pilot is currently capable of carrying out all operations from starting the aircraft to taxiing, takeoff and landing, cruising, and cycling using a flight control simulator. The research team plans to use the humanoid pilot to fly a real-life light aircraft to verify its abilities. Prof. Shim explained, “Humanoid pilot robots do not require the modification of existing aircrafts and can be applied immediately to automated flights. They are therefore highly applicable and practical. We expect them to be applied into various other vehicles like cars and military trucks since they can control a wide range of equipment. They will particularly be particularly helpful in situations where military resources are severely depleted.”
This research was supported by Future Challenge Funding (total: 5.7 bn KRW) from the Agency for Defense Development. The project started in 2022 as a joint research project by Prof. David Hyunchul Shim (chief of research) from the KAIST School of Electrical Engineering (EE), Prof. Jaegul Choo from the Kim Jaechul Graduate School of AI at KAIST, Prof. Kuk-Jin Yoon from the KAIST Department of Mechanical Engineering, and Prof. Min Jun Kim from the KAIST School of EE. The project is to be completed by 2026 and the involved researchers are also considering commercialization strategies for both military and civil use.
2023.08.03 View 15578
KAIST Research Team Develops World’s First Humanoid Pilot, PIBOT
In the Spring of last year, the legendary, fictional pilot “Maverick” flew his plane in the film “Top Gun: Maverick” that drew crowds to theatres around the world. This year, the appearance of a humanoid pilot, PIBOT, has stolen the spotlight at KAIST.
< Photo 1. Humanoid pilot robot, PIBOT >
A KAIST research team has developed a humanoid robot that can understand manuals written in natural language and fly a plane on its own. The team also announced their plans to commercialize the humanoid pilot.
< Photo 2. PIBOT on flight simulator (view from above) >
The project was led by KAIST Professor David Hyunchul Shim, and was conducted as a joint research project with Professors Jaegul Choo, Kuk-Jin Yoon, and Min Jun Kim. The study was supported by Future Challenge Funding under the project title, “Development of Human-like Pilot Robot based on Natural Language Processing”. The team utilized AI and robotics technologies, and demonstrated that the humanoid could sit itself in a real cockpit and operate the various pieces of equipment without modifying any part of the aircraft. This is a fundamental difference that distinguishes this technology from existing autopilot functions or unmanned aircrafts.
< Photo 3. PIBOT operating a flight simulator (side) >
The KAIST team’s humanoid pilot is still under development but it can already remember Jeppeson charts from all around the world, which is impossible for human pilots to do, and fly without error. In particular, it can make use of recent ChatGPT technology to remember the full Quick Reference Handbook (QRF) and respond immediately to various situations, as well as calculate safe routes in real time based on the flight status of the aircraft, with emergency response times quicker than human pilots.
Furthermore, while existing robots usually carry out repeated motions in a fixed position, PIBOT can analyze the state of the cockpit as well as the situation outside the aircraft using an embedded camera. PIBOT can accurately control the various switches in the cockpit and, using high-precision control technology, it can accurately control its robotic arms and hands even during harsh turbulence.
< Photo 4. PIBOT on-board KLA-100, Korea’s first light aircraft >
The humanoid pilot is currently capable of carrying out all operations from starting the aircraft to taxiing, takeoff and landing, cruising, and cycling using a flight control simulator. The research team plans to use the humanoid pilot to fly a real-life light aircraft to verify its abilities. Prof. Shim explained, “Humanoid pilot robots do not require the modification of existing aircrafts and can be applied immediately to automated flights. They are therefore highly applicable and practical. We expect them to be applied into various other vehicles like cars and military trucks since they can control a wide range of equipment. They will particularly be particularly helpful in situations where military resources are severely depleted.”
This research was supported by Future Challenge Funding (total: 5.7 bn KRW) from the Agency for Defense Development. The project started in 2022 as a joint research project by Prof. David Hyunchul Shim (chief of research) from the KAIST School of Electrical Engineering (EE), Prof. Jaegul Choo from the Kim Jaechul Graduate School of AI at KAIST, Prof. Kuk-Jin Yoon from the KAIST Department of Mechanical Engineering, and Prof. Min Jun Kim from the KAIST School of EE. The project is to be completed by 2026 and the involved researchers are also considering commercialization strategies for both military and civil use.
2023.08.03 View 15578 -
 KAIST research team develops a forgery prevention technique using salmon DNA
The authenticity scandal that plagued the artwork “Beautiful Woman” by Kyung-ja Chun for 30 years shows how concerns about replicas can become a burden to artists, as most of them are not experts in the field of anti-counterfeiting. To solve this problem, artist-friendly physical unclonable functions (PUFs) based on optical techniques instead of electronic ones, which can be applied immediately onto artwork through brushstrokes are needed.
On May 23, a KAIST research team led by Professor Dong Ki Yoon in the Department of Chemistry revealed the development of a proprietary technology for security and certification using random patterns that occur during the self-assembly of soft materials.
With the development of the Internet of Things in recent years, various electronic devices and services can now be connected to the internet and carry out new innovative functions. However, counterfeiting technologies that infringe on individuals’ privacy have also entered the marketplace.
The technique developed by the research team involves random and spontaneous patterns that naturally occur during the self-assembly of two different types of soft materials, which can be used in the same way as human fingerprints for non-replicable security. This is very significant in that even non-experts in the field of security can construct anti-counterfeiting systems through simple actions like drawing a picture.
The team developed two unique methods. The first method uses liquid crystals. When liquid crystals become trapped in patterned substrates, they induce the symmetrical destruction of the structure and create a maze-like topology (Figure 1). The research team defined the pathways open to the right as 0 (blue), and those open to the left as 1 (red), and confirmed that the structure could be converted into a digital code composed of 0’s and 1’s that can serve as a type of fingerprint through object recognition using machine learning. This groundbreaking technique can be utilized by non-experts, as it does not require complex semiconductor patterns that are required by existing technology, and can be observed through the level of resolution of a smartphone camera. In particular, this technique can reconstruct information more easily than conventional methods that use semiconductor chips.
< Figure 1. Security technology using the maze made up of magnetically-assembled structures formed on a substrate patterned with liquid crystal materials. >
The second method uses DNA extracted from salmon. The DNA can be dissolved in water and applied with a brush to induce bulking instability, which forms random patterns similar to a zebra’s stripes. Here, the patterns create ridge endings and bifurcation, which are characteristics in fingerprints, and these can also be digitalized into 0’s and 1’s through machine learning. The research team applied conventional fingerprint recognition technology to this patterning technique and demonstrated its use as an artificial fingerprint. This method can be easily carried out using a brush, and the solution can be mixed into various colors and used as a new security ink.
< Figure 2. Technology to produce security ink using DNA polymers extracted from salmon >
This new security technology developed by the research team uses only simple organic materials and requires basic manufacturing processes, making it possible to enhance security at a low cost. In addition, users can produce patterns in the shapes and sizes they want, and even if the patterns are made in the same way, their randomness makes each individual pattern different. This provides high levels of security and gives the technique enhanced marketability.
Professor Dong Ki Yoon said, “These studies have taken the randomness that naturally occurs during self-assembly to create non-replicable patterns that can act like human fingerprints.” He added, “These ideas will be the cornerstone of technology that applies the many randomities that exist in nature to security systems.”
The two studies were published in the journal Advanced Materials under the titles “1Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media” and “2Paintable Physical Unclonable Function Using DNA” on May 6 and 5, respectively.
Author Information: 1Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon*, and Dong Ki Yoon*/ 2Soon Mo Park†, Geonhyeong Park†, Dong Ki Yoon*: †co-first authors, *corresponding author
This research was funded by the Center for Multiscale Chiral Architectures and supported by the Ministry of Science and ICT-Korea Research Foundation, BRIDGE Convergent Research and Development Program, the Running Together Project, and the Samsung Future Technology Development Program.
< Figure 1-1. A scene from the schematic animation of the process of Blues (0) and Reds (1) forming the PUF by exploring the maze. From "Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media" by Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon, Dong Ki Yoon. https://doi.org/10.1002/adma.202303077 >
< Figure 2-1. A schematic diagram of the formation of digital fingerprints formed using the DNA ink. From "Paintable Physical Unclonable Function Using DNA" by Soon Mo Park, Geonhyeong Park, Dong Ki Yoon. https://doi.org/10.1002/adma.202302135 >
2023.06.08 View 8516
KAIST research team develops a forgery prevention technique using salmon DNA
The authenticity scandal that plagued the artwork “Beautiful Woman” by Kyung-ja Chun for 30 years shows how concerns about replicas can become a burden to artists, as most of them are not experts in the field of anti-counterfeiting. To solve this problem, artist-friendly physical unclonable functions (PUFs) based on optical techniques instead of electronic ones, which can be applied immediately onto artwork through brushstrokes are needed.
On May 23, a KAIST research team led by Professor Dong Ki Yoon in the Department of Chemistry revealed the development of a proprietary technology for security and certification using random patterns that occur during the self-assembly of soft materials.
With the development of the Internet of Things in recent years, various electronic devices and services can now be connected to the internet and carry out new innovative functions. However, counterfeiting technologies that infringe on individuals’ privacy have also entered the marketplace.
The technique developed by the research team involves random and spontaneous patterns that naturally occur during the self-assembly of two different types of soft materials, which can be used in the same way as human fingerprints for non-replicable security. This is very significant in that even non-experts in the field of security can construct anti-counterfeiting systems through simple actions like drawing a picture.
The team developed two unique methods. The first method uses liquid crystals. When liquid crystals become trapped in patterned substrates, they induce the symmetrical destruction of the structure and create a maze-like topology (Figure 1). The research team defined the pathways open to the right as 0 (blue), and those open to the left as 1 (red), and confirmed that the structure could be converted into a digital code composed of 0’s and 1’s that can serve as a type of fingerprint through object recognition using machine learning. This groundbreaking technique can be utilized by non-experts, as it does not require complex semiconductor patterns that are required by existing technology, and can be observed through the level of resolution of a smartphone camera. In particular, this technique can reconstruct information more easily than conventional methods that use semiconductor chips.
< Figure 1. Security technology using the maze made up of magnetically-assembled structures formed on a substrate patterned with liquid crystal materials. >
The second method uses DNA extracted from salmon. The DNA can be dissolved in water and applied with a brush to induce bulking instability, which forms random patterns similar to a zebra’s stripes. Here, the patterns create ridge endings and bifurcation, which are characteristics in fingerprints, and these can also be digitalized into 0’s and 1’s through machine learning. The research team applied conventional fingerprint recognition technology to this patterning technique and demonstrated its use as an artificial fingerprint. This method can be easily carried out using a brush, and the solution can be mixed into various colors and used as a new security ink.
< Figure 2. Technology to produce security ink using DNA polymers extracted from salmon >
This new security technology developed by the research team uses only simple organic materials and requires basic manufacturing processes, making it possible to enhance security at a low cost. In addition, users can produce patterns in the shapes and sizes they want, and even if the patterns are made in the same way, their randomness makes each individual pattern different. This provides high levels of security and gives the technique enhanced marketability.
Professor Dong Ki Yoon said, “These studies have taken the randomness that naturally occurs during self-assembly to create non-replicable patterns that can act like human fingerprints.” He added, “These ideas will be the cornerstone of technology that applies the many randomities that exist in nature to security systems.”
The two studies were published in the journal Advanced Materials under the titles “1Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media” and “2Paintable Physical Unclonable Function Using DNA” on May 6 and 5, respectively.
Author Information: 1Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon*, and Dong Ki Yoon*/ 2Soon Mo Park†, Geonhyeong Park†, Dong Ki Yoon*: †co-first authors, *corresponding author
This research was funded by the Center for Multiscale Chiral Architectures and supported by the Ministry of Science and ICT-Korea Research Foundation, BRIDGE Convergent Research and Development Program, the Running Together Project, and the Samsung Future Technology Development Program.
< Figure 1-1. A scene from the schematic animation of the process of Blues (0) and Reds (1) forming the PUF by exploring the maze. From "Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media" by Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon, Dong Ki Yoon. https://doi.org/10.1002/adma.202303077 >
< Figure 2-1. A schematic diagram of the formation of digital fingerprints formed using the DNA ink. From "Paintable Physical Unclonable Function Using DNA" by Soon Mo Park, Geonhyeong Park, Dong Ki Yoon. https://doi.org/10.1002/adma.202302135 >
2023.06.08 View 8516 -
 KAIST Team Develops Highly-Sensitive Wearable Piezoelectric Blood Pressure Sensor for Continuous Health Monitoring
- A collaborative research team led by KAIST Professor Keon Jae Lee verifies the accuracy of the highly-sensitive sensor through clinical trials
- Commercialization of the watch and patch-type sensor is in progress
A KAIST research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering and the College of Medicine of the Catholic University of Korea has developed a highly sensitive, wearable piezoelectric blood pressure sensor.
Blood pressure is a critical indicator for assessing general health and predicting stroke or heart failure. In particular, cardiovascular disease is the leading cause of global death, therefore, periodic measurement of blood pressure is crucial for personal healthcare.
Recently, there has been a growing interest in healthcare devices for continuous blood pressure monitoring. Although smart watches using LED-based photoplethysmography (PPG) technology have been on market, these devices have been limited by the accuracy constraints of optical sensors, making it hard to meet the international standards of automatic sphygmomanometers.
Professor Lee’s team has developed the wearable piezoelectric blood pressure sensor by transferring a highly sensitive, inorganic piezoelectric membrane from bulk sapphire substrates to flexible substrates. Ultrathin piezoelectric sensors with a thickness of several micrometers (one hundredth of the human hair) exhibit conformal contact with the skin to successfully collect accurate blood pressure from the subtle pulsation of the blood vessels.
Clinical trial at the St. Mary’s Hospital of the Catholic University validated the accuracy of blood pressure sensor at par with international standard with errors within ±5 mmHg and a standard deviation under 8 mmHg for both systolic and diastolic blood pressure. In addition, the research team successfully embedded the sensor on a watch-type product to enable continuous monitoring of blood pressure.
Prof. Keon Jae Lee said, “Major target of our healthcare devices is hypertensive patients for their daily medical check-up. We plan to develop a comfortable patch-type sensor to monitor blood pressure during sleep and have a start-up company commercialize these watch and patch-type products soon.”
This result titled “Clinical validation of wearable piezoelectric blood pressure sensor for health monitoring” was published in the online issue of Advanced Materials on March 24th, 2023. (DOI: 10.1002/adma.202301627)
Figure 1. Schematic illustration of the overall concept for a wearable piezoelectric blood pressure sensor (WPBPS).
Figure 2. Wearable piezoelectric blood pressure sensor (WPBPS) mounted on a watch (a) Schematic design of the WPBPS-embedded wristwatch. (b) Block diagram of the wireless communication circuit, which filters, amplifies, and transmits wireless data to portable devices. (c) Pulse waveforms transmitted from the wristwatch to the portable device by the wireless communication circuit. The inset shows a photograph of monitoring a user’s beat-to-beat pulses and their corresponding BP values in real time using the developed WPBPS-mounted wristwatch.
2023.04.17 View 9536
KAIST Team Develops Highly-Sensitive Wearable Piezoelectric Blood Pressure Sensor for Continuous Health Monitoring
- A collaborative research team led by KAIST Professor Keon Jae Lee verifies the accuracy of the highly-sensitive sensor through clinical trials
- Commercialization of the watch and patch-type sensor is in progress
A KAIST research team led by Professor Keon Jae Lee from the Department of Materials Science and Engineering and the College of Medicine of the Catholic University of Korea has developed a highly sensitive, wearable piezoelectric blood pressure sensor.
Blood pressure is a critical indicator for assessing general health and predicting stroke or heart failure. In particular, cardiovascular disease is the leading cause of global death, therefore, periodic measurement of blood pressure is crucial for personal healthcare.
Recently, there has been a growing interest in healthcare devices for continuous blood pressure monitoring. Although smart watches using LED-based photoplethysmography (PPG) technology have been on market, these devices have been limited by the accuracy constraints of optical sensors, making it hard to meet the international standards of automatic sphygmomanometers.
Professor Lee’s team has developed the wearable piezoelectric blood pressure sensor by transferring a highly sensitive, inorganic piezoelectric membrane from bulk sapphire substrates to flexible substrates. Ultrathin piezoelectric sensors with a thickness of several micrometers (one hundredth of the human hair) exhibit conformal contact with the skin to successfully collect accurate blood pressure from the subtle pulsation of the blood vessels.
Clinical trial at the St. Mary’s Hospital of the Catholic University validated the accuracy of blood pressure sensor at par with international standard with errors within ±5 mmHg and a standard deviation under 8 mmHg for both systolic and diastolic blood pressure. In addition, the research team successfully embedded the sensor on a watch-type product to enable continuous monitoring of blood pressure.
Prof. Keon Jae Lee said, “Major target of our healthcare devices is hypertensive patients for their daily medical check-up. We plan to develop a comfortable patch-type sensor to monitor blood pressure during sleep and have a start-up company commercialize these watch and patch-type products soon.”
This result titled “Clinical validation of wearable piezoelectric blood pressure sensor for health monitoring” was published in the online issue of Advanced Materials on March 24th, 2023. (DOI: 10.1002/adma.202301627)
Figure 1. Schematic illustration of the overall concept for a wearable piezoelectric blood pressure sensor (WPBPS).
Figure 2. Wearable piezoelectric blood pressure sensor (WPBPS) mounted on a watch (a) Schematic design of the WPBPS-embedded wristwatch. (b) Block diagram of the wireless communication circuit, which filters, amplifies, and transmits wireless data to portable devices. (c) Pulse waveforms transmitted from the wristwatch to the portable device by the wireless communication circuit. The inset shows a photograph of monitoring a user’s beat-to-beat pulses and their corresponding BP values in real time using the developed WPBPS-mounted wristwatch.
2023.04.17 View 9536 -
 KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7647
KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7647 -
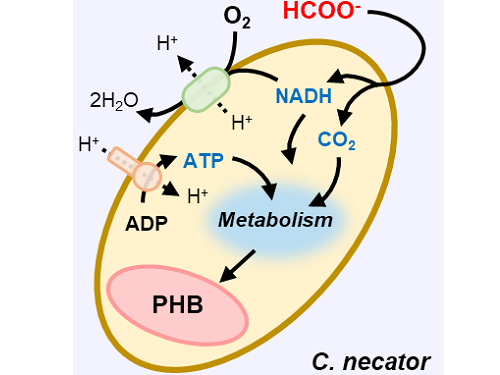 A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11855
A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11855 -
 Using light to throw and catch atoms to open up a new chapter for quantum computing
The technology to move and arrange atoms, the most basic component of a quantum computer, is very important to Rydberg quantum computing research. However, to place the atoms at the desired location, the atoms must be captured and transported one by one using a highly focused laser beam, commonly referred to as an optical tweezer. and, the quantum information of the atoms is likely to change midway.
KAIST (President Kwang Hyung Lee) announced on the 27th that a research team led by Professor Jaewook Ahn of the Department of Physics developed a technology to throw and receive rubidium atoms one by one using a laser beam.
The research team developed a method to throw and receive atoms which would minimize the time the optical tweezers are in contact with the atoms in which the quantum information the atoms carry may change. The research team used the characteristic that the rubidium atoms, which are kept at a very low temperature of 40μK below absolute zero, move very sensitively to the electromagnetic force applied by light along the focal point of the light tweezers.
The research team accelerated the laser of an optical tweezer to give an optical kick to an atom to send it to a target, then caught the flying atom with another optical tweezer to stop it. The atom flew at a speed of 65 cm/s, and traveled up to 4.2 μm. Compared to the existing technique of guiding the atoms with the optical tweezers, the technique of throwing and receiving atoms eliminates the need to calculate the transporting path for the tweezers, and makes it easier to fix the defects in the atomic arrangement. As a result, it is effective in generating and maintaining a large number of atomic arrangements, and when the technology is used to throw and receive flying atom qubits, it will be used in studying new and more powerful quantum computing methods that presupposes the structural changes in quantum arrangements.
"This technology will be used to develop larger and more powerful Rydberg quantum computers," says Professor Jaewook Ahn. “In a Rydberg quantum computer,” he continues, “atoms are arranged to store quantum information and interact with neighboring atoms through electromagnetic forces to perform quantum computing. The method of throwing an atom away for quick reconstruction the quantum array can be an effective way to fix an error in a quantum computer that requires a removal or replacement of an atom.”
The research, which was conducted by doctoral students Hansub Hwang and Andrew Byun of the Department of Physics at KAIST and Sylvain de Léséleuc, a researcher at the National Institute of Natural Sciences in Japan, was published in the international journal, Optica, 0n March 9th. (Paper title: Optical tweezers throw and catch single atoms).
This research was carried out with the support of the Samsung Science & Technology Foundation.
<Figure 1> A schematic diagram of the atom catching and throwing technique. The optical tweezer on the left kicks the atom to throw it into a trajectory to have the tweezer on the right catch it to stop it.
2023.03.28 View 7818
Using light to throw and catch atoms to open up a new chapter for quantum computing
The technology to move and arrange atoms, the most basic component of a quantum computer, is very important to Rydberg quantum computing research. However, to place the atoms at the desired location, the atoms must be captured and transported one by one using a highly focused laser beam, commonly referred to as an optical tweezer. and, the quantum information of the atoms is likely to change midway.
KAIST (President Kwang Hyung Lee) announced on the 27th that a research team led by Professor Jaewook Ahn of the Department of Physics developed a technology to throw and receive rubidium atoms one by one using a laser beam.
The research team developed a method to throw and receive atoms which would minimize the time the optical tweezers are in contact with the atoms in which the quantum information the atoms carry may change. The research team used the characteristic that the rubidium atoms, which are kept at a very low temperature of 40μK below absolute zero, move very sensitively to the electromagnetic force applied by light along the focal point of the light tweezers.
The research team accelerated the laser of an optical tweezer to give an optical kick to an atom to send it to a target, then caught the flying atom with another optical tweezer to stop it. The atom flew at a speed of 65 cm/s, and traveled up to 4.2 μm. Compared to the existing technique of guiding the atoms with the optical tweezers, the technique of throwing and receiving atoms eliminates the need to calculate the transporting path for the tweezers, and makes it easier to fix the defects in the atomic arrangement. As a result, it is effective in generating and maintaining a large number of atomic arrangements, and when the technology is used to throw and receive flying atom qubits, it will be used in studying new and more powerful quantum computing methods that presupposes the structural changes in quantum arrangements.
"This technology will be used to develop larger and more powerful Rydberg quantum computers," says Professor Jaewook Ahn. “In a Rydberg quantum computer,” he continues, “atoms are arranged to store quantum information and interact with neighboring atoms through electromagnetic forces to perform quantum computing. The method of throwing an atom away for quick reconstruction the quantum array can be an effective way to fix an error in a quantum computer that requires a removal or replacement of an atom.”
The research, which was conducted by doctoral students Hansub Hwang and Andrew Byun of the Department of Physics at KAIST and Sylvain de Léséleuc, a researcher at the National Institute of Natural Sciences in Japan, was published in the international journal, Optica, 0n March 9th. (Paper title: Optical tweezers throw and catch single atoms).
This research was carried out with the support of the Samsung Science & Technology Foundation.
<Figure 1> A schematic diagram of the atom catching and throwing technique. The optical tweezer on the left kicks the atom to throw it into a trajectory to have the tweezer on the right catch it to stop it.
2023.03.28 View 7818 -
 KAIST Holds 2023 Commencement Ceremony
< Photo 1. On the 17th, KAIST held the 2023 Commencement Ceremony for a total of 2,870 students, including 691 doctors. >
KAIST held its 2023 commencement ceremony at the Sports Complex of its main campus in Daejeon at 2 p.m. on February 27. It was the first commencement ceremony to invite all its graduates since the start of COVID-19 quarantine measures.
KAIST awarded a total of 2,870 degrees including 691 PhD degrees, 1,464 master’s degrees, and 715 bachelor’s degrees, which adds to the total of 74,999 degrees KAIST has conferred since its foundation in 1971, which includes 15,772 PhD, 38,360 master’s and 20,867 bachelor’s degrees.
This year’s Cum Laude, Gabin Ryu, from the Department of Mechanical Engineering received the Minister of Science and ICT Award. Seung-ju Lee from the School of Computing received the Chairman of the KAIST Board of Trustees Award, while Jantakan Nedsaengtip, an international student from Thailand received the KAIST Presidential Award, and Jaeyong Hwang from the Department of Physics and Junmo Lee from the Department of Industrial and Systems Engineering each received the President of the Alumni Association Award and the Chairman of the KAIST Development Foundation Award, respectively.
Minister Jong-ho Lee of the Ministry of Science and ICT awarded the recipients of the academic awards and delivered a congratulatory speech.
Yujin Cha from the Department of Bio and Brain Engineering, who received a PhD degree after 19 years since his entrance to KAIST as an undergraduate student in 2004 gave a speech on behalf of the graduates to move and inspire the graduates and the guests.
After Cha received a bachelor’s degree from the Department of Nuclear and Quantum Engineering, he entered a medical graduate school and became a radiation oncology specialist. But after experiencing the death of a young patient who suffered from osteosarcoma, he returned to his alma mater to become a scientist. As he believes that science and technology is the ultimate solution to the limitations of modern medicine, he started as a PhD student at the Department of Bio and Brain Engineering in 2018, hoping to find such solutions.
During his course, he identified the characteristics of the decision-making process of doctors during diagnosis, and developed a brain-inspired AI algorithm. It is an original and challenging study that attempted to develop a fundamental machine learning theory from the data he collected from 200 doctors of different specialties.
Cha said, “Humans and AI can cooperate by humans utilizing the unique learning abilities of AI to develop our expertise, while AIs can mimic us humans’ learning abilities to improve.” He added, “My ultimate goal is to develop technology to a level at which humans and machines influence each other and ‘coevolve’, and applying it not only to medicine, but in all areas.”
Cha, who is currently an assistant professor at the KAIST Biomedical Research Center, has also written Artificial Intelligence for Doctors in 2017 to help medical personnel use AI in clinical fields, and the book was selected as one of the 2018 Sejong Books in the academic category.
During his speech at this year’s commencement ceremony, he shared that “there are so many things in the world that are difficult to solve and many things to solve them with, but I believe the things that can really broaden the horizons of the world and find fundamental solutions to the problems at hand are science and technology.”
Meanwhile, singer-songwriter Sae Byul Park who studied at the KAIST Graduate School of Culture Technology will also receive her PhD degree.
Natural language processing (NLP) is a field in AI that teaches a computer to understand and analyze human language that is actively being studied. An example of NLP is ChatGTP, which recently received a lot of attention. For her research, Park analyzed music rather than language using NLP technology.
To analyze music, which is in the form of sound, using the methods for NLP, it is necessary to rebuild notes and beats into a form of words or sentences as in a language. For this, Park designed an algorithm called Mel2Word and applied it to her research.
She also suggested that by converting melodies into texts for analysis, one would be able to quantitatively express music as sentences or words with meaning and context rather than as simple sounds representing a certain note.
Park said, “music has always been considered as a product of subjective emotion, but this research provides a framework that can calculate and analyze music.”
Park’s study can later be developed into a tool to measure the similarities between musical work, as well as a piece’s originality, artistry and popularity, and it can be used as a clue to explore the fundamental principles of how humans respond to music from a cognitive science perspective.
Park began her Ph.D. program in 2014, while carrying on with her musical activities as well as public and university lectures alongside, and dealing with personally major events including marriage and childbirth during the course of years. She already met the requirements to receive her degree in 2019, but delayed her graduation in order to improve the level of completion of her research, and finally graduated with her current achievements after nine years.
Professor Juhan Nam, who supervised Park’s research, said, “Park, who has a bachelor’s degree in psychology, later learned to code for graduate school, and has complete high-quality research in the field of artificial intelligence.” He added, “Though it took a long time, her attitude of not giving up until the end as a researcher is also excellent.”
Sae Byul Park is currently lecturing courses entitled Culture Technology and Music Information Retrieval at the Underwood International College of Yonsei University.
Park said, “the 10 or so years I’ve spent at KAIST as a graduate student was a time I could learn and prosper not only academically but from all angles of life.” She added, “having received a doctorate degree is not the end, but a ‘commencement’. Therefore, I will start to root deeper from the seeds I sowed and work harder as a both a scholar and an artist.”
< Photo 2. From left) Yujin Cha (Valedictorian, Medical-Scientist Program Ph.D. graduate), Saebyeol Park (a singer-songwriter, Ph.D. graduate from the Graduate School of Culture and Technology), Junseok Moon and Inah Seo (the two highlighted CEO graduates from the Department of Management Engineering's master’s program) >
Young entrepreneurs who dream of solving social problems will also be wearing their graduation caps. Two such graduates are Jun-seok Moon and Inah Seo, receiving their master’s degrees in social entrepreneurship MBA from the KAIST College of Business.
Before entrance, Moon ran a café helping African refugees stand on their own feet. Then, he entered KAIST to later expand his business and learn social entrepreneurship in order to sustainably help refugees in the blind spots of human rights and welfare.
During his master’s course, Moon realized that he could achieve active carbon reduction by changing the coffee alone, and switched his business field and founded Equal Table. The amount of carbon an individual can reduce by refraining from using a single paper cup is 10g, while changing the coffee itself can reduce it by 300g.
1kg of coffee emits 15kg of carbon over the course of its production, distribution, processing, and consumption, but Moon produces nearly carbon-neutral coffee beans by having innovated the entire process. In particular, the company-to-company ESG business solution is Moon’s new start-up area. It provides companies with carbon-reduced coffee made by roasting raw beans from carbon-neutral certified farms with 100% renewable energy, and shows how much carbon has been reduced in its making. Equal Table will launch the service this month in collaboration with SK Telecom, its first partner.
Inah Seo, who also graduated with Moon, founded Conscious Wear to start a fashion business reducing environmental pollution. In order to realize her mission, she felt the need to gain the appropriate expertise in management, and enrolled for the social entrepreneurship MBA.
Out of the various fashion industries, Seo focused on the leather market, which is worth 80 trillion won. Due to thickness or contamination issues, only about 60% of animal skin fabric is used, and the rest is discarded. Heavy metals are used during such processes, which also directly affects the environment.
During the social entrepreneurship MBA course, Seo collaborated with SK Chemicals, which had links through the program, and launched eco-friendly leather bags. The bags used discarded leather that was recycled by grinding and reprocessing into a biomaterial called PO3G. It was the first case in which PO3G that is over 90% biodegradable was applied to regenerated leather. In other words, it can reduce environmental pollution in the processing and disposal stages, while also reducing carbon emissions and water usage by one-tenth compared to existing cowhide products.
The social entrepreneurship MBA course, from which Moon and Seo graduated, will run in integration with the Graduate School of Green Growth as an Impact MBA program starting this year. KAIST plans to steadily foster entrepreneurs who will lead meaningful changes in the environment and society as well as economic values through innovative technologies and ideas.
< Photo 3. NYU President Emeritus John Sexton (left), who received this year's honorary doctorate of science, poses with President Kwang Hyung Lee >
Meanwhile, during this day’s commencement ceremony, KAIST also presented President Emeritus John Sexton of New York University with an honorary doctorate in science. He was recognized for laying the foundation for the cooperation between KAIST and New York University, such as promoting joint campuses.
< Photo 4. At the commencement ceremony of KAIST held on the 17th, President Kwang Hyung Lee is encouraging the graduates with his commencement address. >
President Kwang Hyung Lee emphasized in his commencement speech that, “if you can draw up the future and work hard toward your goal, the future can become a work of art that you create with your own hands,” and added, “Never stop on the journey toward your dreams, and do not give up even when you are met with failure. Failure happens to everyone, all the time. The important thing is to know 'why you failed', and to use those elements of failure as the driving force for the next try.”
2023.02.20 View 22024
KAIST Holds 2023 Commencement Ceremony
< Photo 1. On the 17th, KAIST held the 2023 Commencement Ceremony for a total of 2,870 students, including 691 doctors. >
KAIST held its 2023 commencement ceremony at the Sports Complex of its main campus in Daejeon at 2 p.m. on February 27. It was the first commencement ceremony to invite all its graduates since the start of COVID-19 quarantine measures.
KAIST awarded a total of 2,870 degrees including 691 PhD degrees, 1,464 master’s degrees, and 715 bachelor’s degrees, which adds to the total of 74,999 degrees KAIST has conferred since its foundation in 1971, which includes 15,772 PhD, 38,360 master’s and 20,867 bachelor’s degrees.
This year’s Cum Laude, Gabin Ryu, from the Department of Mechanical Engineering received the Minister of Science and ICT Award. Seung-ju Lee from the School of Computing received the Chairman of the KAIST Board of Trustees Award, while Jantakan Nedsaengtip, an international student from Thailand received the KAIST Presidential Award, and Jaeyong Hwang from the Department of Physics and Junmo Lee from the Department of Industrial and Systems Engineering each received the President of the Alumni Association Award and the Chairman of the KAIST Development Foundation Award, respectively.
Minister Jong-ho Lee of the Ministry of Science and ICT awarded the recipients of the academic awards and delivered a congratulatory speech.
Yujin Cha from the Department of Bio and Brain Engineering, who received a PhD degree after 19 years since his entrance to KAIST as an undergraduate student in 2004 gave a speech on behalf of the graduates to move and inspire the graduates and the guests.
After Cha received a bachelor’s degree from the Department of Nuclear and Quantum Engineering, he entered a medical graduate school and became a radiation oncology specialist. But after experiencing the death of a young patient who suffered from osteosarcoma, he returned to his alma mater to become a scientist. As he believes that science and technology is the ultimate solution to the limitations of modern medicine, he started as a PhD student at the Department of Bio and Brain Engineering in 2018, hoping to find such solutions.
During his course, he identified the characteristics of the decision-making process of doctors during diagnosis, and developed a brain-inspired AI algorithm. It is an original and challenging study that attempted to develop a fundamental machine learning theory from the data he collected from 200 doctors of different specialties.
Cha said, “Humans and AI can cooperate by humans utilizing the unique learning abilities of AI to develop our expertise, while AIs can mimic us humans’ learning abilities to improve.” He added, “My ultimate goal is to develop technology to a level at which humans and machines influence each other and ‘coevolve’, and applying it not only to medicine, but in all areas.”
Cha, who is currently an assistant professor at the KAIST Biomedical Research Center, has also written Artificial Intelligence for Doctors in 2017 to help medical personnel use AI in clinical fields, and the book was selected as one of the 2018 Sejong Books in the academic category.
During his speech at this year’s commencement ceremony, he shared that “there are so many things in the world that are difficult to solve and many things to solve them with, but I believe the things that can really broaden the horizons of the world and find fundamental solutions to the problems at hand are science and technology.”
Meanwhile, singer-songwriter Sae Byul Park who studied at the KAIST Graduate School of Culture Technology will also receive her PhD degree.
Natural language processing (NLP) is a field in AI that teaches a computer to understand and analyze human language that is actively being studied. An example of NLP is ChatGTP, which recently received a lot of attention. For her research, Park analyzed music rather than language using NLP technology.
To analyze music, which is in the form of sound, using the methods for NLP, it is necessary to rebuild notes and beats into a form of words or sentences as in a language. For this, Park designed an algorithm called Mel2Word and applied it to her research.
She also suggested that by converting melodies into texts for analysis, one would be able to quantitatively express music as sentences or words with meaning and context rather than as simple sounds representing a certain note.
Park said, “music has always been considered as a product of subjective emotion, but this research provides a framework that can calculate and analyze music.”
Park’s study can later be developed into a tool to measure the similarities between musical work, as well as a piece’s originality, artistry and popularity, and it can be used as a clue to explore the fundamental principles of how humans respond to music from a cognitive science perspective.
Park began her Ph.D. program in 2014, while carrying on with her musical activities as well as public and university lectures alongside, and dealing with personally major events including marriage and childbirth during the course of years. She already met the requirements to receive her degree in 2019, but delayed her graduation in order to improve the level of completion of her research, and finally graduated with her current achievements after nine years.
Professor Juhan Nam, who supervised Park’s research, said, “Park, who has a bachelor’s degree in psychology, later learned to code for graduate school, and has complete high-quality research in the field of artificial intelligence.” He added, “Though it took a long time, her attitude of not giving up until the end as a researcher is also excellent.”
Sae Byul Park is currently lecturing courses entitled Culture Technology and Music Information Retrieval at the Underwood International College of Yonsei University.
Park said, “the 10 or so years I’ve spent at KAIST as a graduate student was a time I could learn and prosper not only academically but from all angles of life.” She added, “having received a doctorate degree is not the end, but a ‘commencement’. Therefore, I will start to root deeper from the seeds I sowed and work harder as a both a scholar and an artist.”
< Photo 2. From left) Yujin Cha (Valedictorian, Medical-Scientist Program Ph.D. graduate), Saebyeol Park (a singer-songwriter, Ph.D. graduate from the Graduate School of Culture and Technology), Junseok Moon and Inah Seo (the two highlighted CEO graduates from the Department of Management Engineering's master’s program) >
Young entrepreneurs who dream of solving social problems will also be wearing their graduation caps. Two such graduates are Jun-seok Moon and Inah Seo, receiving their master’s degrees in social entrepreneurship MBA from the KAIST College of Business.
Before entrance, Moon ran a café helping African refugees stand on their own feet. Then, he entered KAIST to later expand his business and learn social entrepreneurship in order to sustainably help refugees in the blind spots of human rights and welfare.
During his master’s course, Moon realized that he could achieve active carbon reduction by changing the coffee alone, and switched his business field and founded Equal Table. The amount of carbon an individual can reduce by refraining from using a single paper cup is 10g, while changing the coffee itself can reduce it by 300g.
1kg of coffee emits 15kg of carbon over the course of its production, distribution, processing, and consumption, but Moon produces nearly carbon-neutral coffee beans by having innovated the entire process. In particular, the company-to-company ESG business solution is Moon’s new start-up area. It provides companies with carbon-reduced coffee made by roasting raw beans from carbon-neutral certified farms with 100% renewable energy, and shows how much carbon has been reduced in its making. Equal Table will launch the service this month in collaboration with SK Telecom, its first partner.
Inah Seo, who also graduated with Moon, founded Conscious Wear to start a fashion business reducing environmental pollution. In order to realize her mission, she felt the need to gain the appropriate expertise in management, and enrolled for the social entrepreneurship MBA.
Out of the various fashion industries, Seo focused on the leather market, which is worth 80 trillion won. Due to thickness or contamination issues, only about 60% of animal skin fabric is used, and the rest is discarded. Heavy metals are used during such processes, which also directly affects the environment.
During the social entrepreneurship MBA course, Seo collaborated with SK Chemicals, which had links through the program, and launched eco-friendly leather bags. The bags used discarded leather that was recycled by grinding and reprocessing into a biomaterial called PO3G. It was the first case in which PO3G that is over 90% biodegradable was applied to regenerated leather. In other words, it can reduce environmental pollution in the processing and disposal stages, while also reducing carbon emissions and water usage by one-tenth compared to existing cowhide products.
The social entrepreneurship MBA course, from which Moon and Seo graduated, will run in integration with the Graduate School of Green Growth as an Impact MBA program starting this year. KAIST plans to steadily foster entrepreneurs who will lead meaningful changes in the environment and society as well as economic values through innovative technologies and ideas.
< Photo 3. NYU President Emeritus John Sexton (left), who received this year's honorary doctorate of science, poses with President Kwang Hyung Lee >
Meanwhile, during this day’s commencement ceremony, KAIST also presented President Emeritus John Sexton of New York University with an honorary doctorate in science. He was recognized for laying the foundation for the cooperation between KAIST and New York University, such as promoting joint campuses.
< Photo 4. At the commencement ceremony of KAIST held on the 17th, President Kwang Hyung Lee is encouraging the graduates with his commencement address. >
President Kwang Hyung Lee emphasized in his commencement speech that, “if you can draw up the future and work hard toward your goal, the future can become a work of art that you create with your own hands,” and added, “Never stop on the journey toward your dreams, and do not give up even when you are met with failure. Failure happens to everyone, all the time. The important thing is to know 'why you failed', and to use those elements of failure as the driving force for the next try.”
2023.02.20 View 22024 -
 Scientists re-writes FDA-recommended equation to improve estimation of drug-drug interaction
Drugs absorbed into the body are metabolized and thus removed by enzymes from several organs like the liver. How fast a drug is cleared out of the system can be affected by other drugs that are taken together because added substance can increase the amount of enzyme secretion in the body. This dramatically decreases the concentration of a drug, reducing its efficacy, often leading to the failure of having any effect at all. Therefore, accurately predicting the clearance rate in the presence of drug-drug interaction* is critical in the process of drug prescription and development of a new drug in order to ensure its efficacy and/or to avoid unwanted side-effects.
*Drug-drug interaction: In terms of metabolism, drug-drug interaction is a phenomenon in which one drug changes the metabolism of another drug to promote or inhibit its excretion from the body when two or more drugs are taken together. As a result, it increases the toxicity of medicines or causes loss of efficacy.
Since it is practically impossible to evaluate all interactions between new drug candidates and all marketed drugs during the development process, the FDA recommends indirect evaluation of drug interactions using a formula suggested in their guidance, first published in 1997, revised in January of 2020, in order to evaluate drug interactions and minimize side effects of having to use more than one type of drugs at once.
The formula relies on the 110-year-old Michaelis-Menten (MM) model, which has a fundamental limit of making a very broad and groundless assumption on the part of the presence of the enzymes that metabolizes the drug. While MM equation has been one of the most widely known equations in biochemistry used in more than 220,000 published papers, the MM equation is accurate only when the concentration of the enzyme that metabolizes the drug is almost non-existent, causing the accuracy of the equation highly unsatisfactory – only 38 percent of the predictions had less than two-fold errors.
“To make up for the gap, researcher resorted to plugging in scientifically unjustified constants into the equation,” Professor Jung-woo Chae of Chungnam National University College of Pharmacy said. “This is comparable to having to have the epicyclic orbits introduced to explain the motion of the planets back in the days in order to explain the now-defunct Ptolemaic theory, because it was 'THE' theory back then.”
< (From left) Ph.D. student Yun Min Song (KAIST, co-first authors), Professor Sang Kyum Kim (Chungnam National University, co-corresponding author), Jae Kyoung Kim, CI (KAIST, co-corresponding author), Professor Jung-woo Chae (Chungnam National University, co-corresponding author), Ph.D. students Quyen Thi Tran and Ngoc-Anh Thi Vu (Chungnam National University, co-first authors) >
A joint research team composed of mathematicians from the Biomedical Mathematics Group within the Institute for Basic Science (IBS) and the Korea Advanced Institute of Science and Technology (KAIST) and pharmacological scientists from the Chungnam National University reported that they identified the major causes of the FDA-recommended equation’s inaccuracies and presented a solution.
When estimating the gut bioavailability (Fg), which is the key parameter of the equation, the fraction absorbed from the gut lumen (Fa) is usually assumed to be 1. However, many experiments have shown that Fa is less than 1, obviously since it can’t be expected that all of the orally taken drugs to be completely absorbed by the intestines. To solve this problem, the research team used an “estimated Fa” value based on factors such as the drug’s transit time, intestine radius, and permeability values and used it to re-calculate Fg.
Also, taking a different approach from the MM equation, the team used an alternative model they derived in a previous study back in 2020, which can more accurately predict the drug metabolism rate regardless of the enzyme concentration. Combining these changes, the modified equation with re-calculated Fg had a dramatically increased accuracy of the resulting estimate. The existing FDA formula predicted drug interactions within a 2-fold margin of error at the rate of 38%, whereas the accuracy rate of the revised formula reached 80%.
“Such drastic improvement in drug-drug interaction prediction accuracy is expected to make great contribution to increasing the success rate of new drug development and drug efficacy in clinical trials. As the results of this study were published in one of the top clinical pharmacology journal, it is expected that the FDA guidance will be revised according to the results of this study.” said Professor Sang Kyum Kim from Chungnam National University College of Pharmacy.
Furthermore, this study highlights the importance of collaborative research between research groups in vastly different disciplines, in a field that is as dynamic as drug interactions.
“Thanks to the collaborative research between mathematics and pharmacy, we were able to recify the formula that we have accepted to be the right answer for so long to finally grasp on the leads toward healthier life for mankind.,” said Professor Jae Kyung Kim. He continued, “I hope seeing a ‘K-formula’ entered into the US FDA guidance one day.”
The results of this study were published in the online edition of Clinical Pharmacology and Therapeutics (IF 7.051), an authoritative journal in the field of clinical pharmacology, on December 15, 2022 (Korean time).
Thesis Title: Beyond the Michaelis-Menten: Accurate Prediction of Drug Interactions through Cytochrome P450 3A4 Induction (doi: 10.1002/cpt.2824)
< Figure 1. The formula proposed by the FDA guidance for predicting drug-drug interactions (top) and the formula newly derived by the researchers (bottom). AUCR (the ratio of substrate area under the plasma concentration-time curve) represents the rate of change in drug concentration due to drug interactions. The research team more than doubled the accuracy of drug interaction prediction compared to the existing formula. >
< Figure 2. Existing FDA formulas tend to underestimate the extent of drug-drug interactions (gray dots) than the actual measured values. On the other hand, the newly derived equation (red dot) has a prediction rate that is within the error range of 2 times (0.5 to 2 times) of the measured value, and is more than twice as high as the existing equation. The solid line in the figure represents the predicted value that matches the measured value. The dotted line represents the predicted value with an error of 0.5 to 2 times. >
For further information or to request media assistance, please contact Jae Kyoung Kim at Biomedical Mathematics Group, Institute for Basic Science (IBS) (jaekkim@ibs.re.kr) or William I. Suh at the IBS Communications Team (willisuh@ibs.re.kr).
- About the Institute for Basic Science (IBS)
IBS was founded in 2011 by the government of the Republic of Korea with the sole purpose of driving forward the development of basic science in South Korea. IBS has 4 research institutes and 33 research centers as of January 2023. There are eleven physics, three mathematics, five chemistry, nine life science, two earth science, and three interdisciplinary research centers.
2023.01.18 View 14479
Scientists re-writes FDA-recommended equation to improve estimation of drug-drug interaction
Drugs absorbed into the body are metabolized and thus removed by enzymes from several organs like the liver. How fast a drug is cleared out of the system can be affected by other drugs that are taken together because added substance can increase the amount of enzyme secretion in the body. This dramatically decreases the concentration of a drug, reducing its efficacy, often leading to the failure of having any effect at all. Therefore, accurately predicting the clearance rate in the presence of drug-drug interaction* is critical in the process of drug prescription and development of a new drug in order to ensure its efficacy and/or to avoid unwanted side-effects.
*Drug-drug interaction: In terms of metabolism, drug-drug interaction is a phenomenon in which one drug changes the metabolism of another drug to promote or inhibit its excretion from the body when two or more drugs are taken together. As a result, it increases the toxicity of medicines or causes loss of efficacy.
Since it is practically impossible to evaluate all interactions between new drug candidates and all marketed drugs during the development process, the FDA recommends indirect evaluation of drug interactions using a formula suggested in their guidance, first published in 1997, revised in January of 2020, in order to evaluate drug interactions and minimize side effects of having to use more than one type of drugs at once.
The formula relies on the 110-year-old Michaelis-Menten (MM) model, which has a fundamental limit of making a very broad and groundless assumption on the part of the presence of the enzymes that metabolizes the drug. While MM equation has been one of the most widely known equations in biochemistry used in more than 220,000 published papers, the MM equation is accurate only when the concentration of the enzyme that metabolizes the drug is almost non-existent, causing the accuracy of the equation highly unsatisfactory – only 38 percent of the predictions had less than two-fold errors.
“To make up for the gap, researcher resorted to plugging in scientifically unjustified constants into the equation,” Professor Jung-woo Chae of Chungnam National University College of Pharmacy said. “This is comparable to having to have the epicyclic orbits introduced to explain the motion of the planets back in the days in order to explain the now-defunct Ptolemaic theory, because it was 'THE' theory back then.”
< (From left) Ph.D. student Yun Min Song (KAIST, co-first authors), Professor Sang Kyum Kim (Chungnam National University, co-corresponding author), Jae Kyoung Kim, CI (KAIST, co-corresponding author), Professor Jung-woo Chae (Chungnam National University, co-corresponding author), Ph.D. students Quyen Thi Tran and Ngoc-Anh Thi Vu (Chungnam National University, co-first authors) >
A joint research team composed of mathematicians from the Biomedical Mathematics Group within the Institute for Basic Science (IBS) and the Korea Advanced Institute of Science and Technology (KAIST) and pharmacological scientists from the Chungnam National University reported that they identified the major causes of the FDA-recommended equation’s inaccuracies and presented a solution.
When estimating the gut bioavailability (Fg), which is the key parameter of the equation, the fraction absorbed from the gut lumen (Fa) is usually assumed to be 1. However, many experiments have shown that Fa is less than 1, obviously since it can’t be expected that all of the orally taken drugs to be completely absorbed by the intestines. To solve this problem, the research team used an “estimated Fa” value based on factors such as the drug’s transit time, intestine radius, and permeability values and used it to re-calculate Fg.
Also, taking a different approach from the MM equation, the team used an alternative model they derived in a previous study back in 2020, which can more accurately predict the drug metabolism rate regardless of the enzyme concentration. Combining these changes, the modified equation with re-calculated Fg had a dramatically increased accuracy of the resulting estimate. The existing FDA formula predicted drug interactions within a 2-fold margin of error at the rate of 38%, whereas the accuracy rate of the revised formula reached 80%.
“Such drastic improvement in drug-drug interaction prediction accuracy is expected to make great contribution to increasing the success rate of new drug development and drug efficacy in clinical trials. As the results of this study were published in one of the top clinical pharmacology journal, it is expected that the FDA guidance will be revised according to the results of this study.” said Professor Sang Kyum Kim from Chungnam National University College of Pharmacy.
Furthermore, this study highlights the importance of collaborative research between research groups in vastly different disciplines, in a field that is as dynamic as drug interactions.
“Thanks to the collaborative research between mathematics and pharmacy, we were able to recify the formula that we have accepted to be the right answer for so long to finally grasp on the leads toward healthier life for mankind.,” said Professor Jae Kyung Kim. He continued, “I hope seeing a ‘K-formula’ entered into the US FDA guidance one day.”
The results of this study were published in the online edition of Clinical Pharmacology and Therapeutics (IF 7.051), an authoritative journal in the field of clinical pharmacology, on December 15, 2022 (Korean time).
Thesis Title: Beyond the Michaelis-Menten: Accurate Prediction of Drug Interactions through Cytochrome P450 3A4 Induction (doi: 10.1002/cpt.2824)
< Figure 1. The formula proposed by the FDA guidance for predicting drug-drug interactions (top) and the formula newly derived by the researchers (bottom). AUCR (the ratio of substrate area under the plasma concentration-time curve) represents the rate of change in drug concentration due to drug interactions. The research team more than doubled the accuracy of drug interaction prediction compared to the existing formula. >
< Figure 2. Existing FDA formulas tend to underestimate the extent of drug-drug interactions (gray dots) than the actual measured values. On the other hand, the newly derived equation (red dot) has a prediction rate that is within the error range of 2 times (0.5 to 2 times) of the measured value, and is more than twice as high as the existing equation. The solid line in the figure represents the predicted value that matches the measured value. The dotted line represents the predicted value with an error of 0.5 to 2 times. >
For further information or to request media assistance, please contact Jae Kyoung Kim at Biomedical Mathematics Group, Institute for Basic Science (IBS) (jaekkim@ibs.re.kr) or William I. Suh at the IBS Communications Team (willisuh@ibs.re.kr).
- About the Institute for Basic Science (IBS)
IBS was founded in 2011 by the government of the Republic of Korea with the sole purpose of driving forward the development of basic science in South Korea. IBS has 4 research institutes and 33 research centers as of January 2023. There are eleven physics, three mathematics, five chemistry, nine life science, two earth science, and three interdisciplinary research centers.
2023.01.18 View 14479 -
 A Quick but Clingy Creepy-Crawler that will MARVEL You
Engineered by KAIST Mechanics, a quadrupedal robot climbs steel walls and crawls across metal ceilings at the fastest speed that the world has ever seen.
< Photo 1. (From left) KAIST ME Prof. Hae-Won Park, Ph.D. Student Yong Um, Ph.D. Student Seungwoo Hong >
- Professor Hae-Won Park's team at the Department of Mechanical Engineering developed a quadrupedal robot that can move at a high speed on ferrous walls and ceilings.
- It is expected to make a wide variety of contributions as it is to be used to conduct inspections and repairs of large steel structures such as ships, bridges, and transmission towers, offering an alternative to dangerous or risky activities required in hazardous environments while maintaining productivity and efficiency through automation and unmanning of such operations.
- The study was published as the cover paper of the December issue of Science Robotics.
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor Hae-Won Park of the Department of Mechanical Engineering developed a quadrupedal walking robot that can move at high speed on steel walls and ceilings named M.A.R.V.E.L. - rightly so as it is a Magnetically Adhesive Robot for Versatile and Expeditious Locomotion as described in their paper, “Agile and Versatile Climbing on Ferromagnetic Surfaces with a Quadrupedal Robot.” (DOI: 10.1126/scirobotics.add1017)
To make this happen, Professor Park's research team developed a foot pad that can quickly turn the magnetic adhesive force on and off while retaining high adhesive force even on an uneven surface through the use of the Electro-Permanent Magnet (EPM), a device that can magnetize and demagnetize an electromagnet with little power, and the Magneto-Rheological Elastomer (MRE), an elastic material made by mixing a magnetic response factor, such as iron powder, with an elastic material, such as rubber, which they mounted on a small quadrupedal robot they made in-house, at their own laboratory. These walking robots are expected to be put into a wide variety of usage, including being programmed to perform inspections, repairs, and maintenance tasks on large structures made of steel, such as ships, bridges, transmission towers, large storage areas, and construction sites.
This study, in which Seungwoo Hong and Yong Um of the Department of Mechanical Engineering participated as co-first authors, was published as the cover paper in the December issue of Science Robotics.
< Image on the Cover of 2022 December issue of Science Robotics >
Existing wall-climbing robots use wheels or endless tracks, so their mobility is limited on surfaces with steps or irregularities. On the other hand, walking robots for climbing can expect improved mobility in obstacle terrain, but have disadvantages in that they have significantly slower moving speeds or cannot perform various movements.
In order to enable fast movement of the walking robot, the sole of the foot must have strong adhesion force and be able to control the adhesion to quickly switch from sticking to the surface or to be off of it. In addition, it is necessary to maintain the adhesion force even on a rough or uneven surface.
To solve this problem, the research team used the EPM and MRE for the first time in designing the soles of walking robots. An EPM is a magnet that can turn on and off the electromagnetic force with a short current pulse. Unlike general electromagnets, it has the advantage that it does not require energy to maintain the magnetic force. The research team proposed a new EPM with a rectangular structure arrangement, enabling faster switching while significantly lowering the voltage required for switching compared to existing electromagnets.
In addition, the research team was able to increase the frictional force without significantly reducing the magnetic force of the sole by covering the sole with an MRE. The proposed sole weighs only 169 g, but provides a vertical gripping force of about *535 Newtons (N) and a frictional force of 445 N, which is sufficient gripping force for a quadrupedal robot weighing 8 kg.
* 535 N converted to kg is 54.5 kg, and 445 N is 45.4 kg. In other words, even if an external force of up to 54.5 kg in the vertical direction and up to 45.4 kg in the horizontal direction is applied (or even if a corresponding weight is hung), the sole of the foot does not come off the steel plate.
MARVEL climbed up a vertical wall at high speed at a speed of 70 cm per second, and was able to walk while hanging upside down from the ceiling at a maximum speed of 50 cm per second. This is the world's fastest speed for a walking climbing robot. In addition, the research team demonstrated that the robot can climb at a speed of up to 35 cm even on a surface that is painted, dirty with dust and the rust-tainted surfaces of water tanks, proving the robot's performance in a real environment. It was experimentally demonstrated that the robot not only exhibited high speed, but also can switch from floor to wall and from wall to ceiling, and overcome 5-cm high obstacles protruding from walls without difficulty.
The new climbing quadrupedal robot is expected to be widely used for inspection, repair, and maintenance of large steel structures such as ships, bridges, transmission towers, oil pipelines, large storage areas, and construction sites. As the works required in these places involves risks such as falls, suffocation and other accidents that may result in serious injuries or casualties, the need for automation is of utmost urgency.
One of the first co-authors of the paper, a Ph.D. student, Yong Um of KAIST’s Department of Mechanical Engineering, said, "By the use of the magnetic soles made up of the EPM and MRE and the non-linear model predictive controller suitable for climbing, the robot can speedily move through a variety of ferromagnetic surfaces including walls and ceilings, not just level grounds. We believe this would become a cornerstone that will expand the mobility and the places of pedal-mobile robots can venture into." He added, “These robots can be put into good use in executing dangerous and difficult tasks on steel structures in places like the shipbuilding yards.”
This research was carried out with support from the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers and Korea Shipbuilding & Offshore Engineering Co., Ltd..
< Figure 1. The quadrupedal robot (MARVEL) walking over various ferrous surfaces. (A) vertical wall (B) ceiling. (C) over obstacles on a vertical wall (D) making floor-to-wall and wall-to-ceiling transitions (E) moving over a storage tank (F) walking on a wall with a 2-kg weight and over a ceiling with a 3-kg load. >
< Figure 2. Description of the magnetic foot (A) Components of the magnet sole: ankle, Square Eletro-Permanent Magnet(S-EPM), MRE footpad. (B) Components of the S-EPM and MRE footpad. (C) Working principle of the S-EPM. When the magnetization direction is aligned as shown in the left figure, magnetic flux comes out of the keeper and circulates through the steel plate, generating holding force (ON state). Conversely, if the magnetization direction is aligned as shown in the figure on the right, the magnetic flux circulates inside the S-EPM and the holding force disappears (OFF state). >
Video Introduction: Agile and versatile climbing on ferromagnetic surfaces with a quadrupedal robot - YouTube
2022.12.30 View 18651
A Quick but Clingy Creepy-Crawler that will MARVEL You
Engineered by KAIST Mechanics, a quadrupedal robot climbs steel walls and crawls across metal ceilings at the fastest speed that the world has ever seen.
< Photo 1. (From left) KAIST ME Prof. Hae-Won Park, Ph.D. Student Yong Um, Ph.D. Student Seungwoo Hong >
- Professor Hae-Won Park's team at the Department of Mechanical Engineering developed a quadrupedal robot that can move at a high speed on ferrous walls and ceilings.
- It is expected to make a wide variety of contributions as it is to be used to conduct inspections and repairs of large steel structures such as ships, bridges, and transmission towers, offering an alternative to dangerous or risky activities required in hazardous environments while maintaining productivity and efficiency through automation and unmanning of such operations.
- The study was published as the cover paper of the December issue of Science Robotics.
KAIST (President Kwang Hyung Lee) announced on the 26th that a research team led by Professor Hae-Won Park of the Department of Mechanical Engineering developed a quadrupedal walking robot that can move at high speed on steel walls and ceilings named M.A.R.V.E.L. - rightly so as it is a Magnetically Adhesive Robot for Versatile and Expeditious Locomotion as described in their paper, “Agile and Versatile Climbing on Ferromagnetic Surfaces with a Quadrupedal Robot.” (DOI: 10.1126/scirobotics.add1017)
To make this happen, Professor Park's research team developed a foot pad that can quickly turn the magnetic adhesive force on and off while retaining high adhesive force even on an uneven surface through the use of the Electro-Permanent Magnet (EPM), a device that can magnetize and demagnetize an electromagnet with little power, and the Magneto-Rheological Elastomer (MRE), an elastic material made by mixing a magnetic response factor, such as iron powder, with an elastic material, such as rubber, which they mounted on a small quadrupedal robot they made in-house, at their own laboratory. These walking robots are expected to be put into a wide variety of usage, including being programmed to perform inspections, repairs, and maintenance tasks on large structures made of steel, such as ships, bridges, transmission towers, large storage areas, and construction sites.
This study, in which Seungwoo Hong and Yong Um of the Department of Mechanical Engineering participated as co-first authors, was published as the cover paper in the December issue of Science Robotics.
< Image on the Cover of 2022 December issue of Science Robotics >
Existing wall-climbing robots use wheels or endless tracks, so their mobility is limited on surfaces with steps or irregularities. On the other hand, walking robots for climbing can expect improved mobility in obstacle terrain, but have disadvantages in that they have significantly slower moving speeds or cannot perform various movements.
In order to enable fast movement of the walking robot, the sole of the foot must have strong adhesion force and be able to control the adhesion to quickly switch from sticking to the surface or to be off of it. In addition, it is necessary to maintain the adhesion force even on a rough or uneven surface.
To solve this problem, the research team used the EPM and MRE for the first time in designing the soles of walking robots. An EPM is a magnet that can turn on and off the electromagnetic force with a short current pulse. Unlike general electromagnets, it has the advantage that it does not require energy to maintain the magnetic force. The research team proposed a new EPM with a rectangular structure arrangement, enabling faster switching while significantly lowering the voltage required for switching compared to existing electromagnets.
In addition, the research team was able to increase the frictional force without significantly reducing the magnetic force of the sole by covering the sole with an MRE. The proposed sole weighs only 169 g, but provides a vertical gripping force of about *535 Newtons (N) and a frictional force of 445 N, which is sufficient gripping force for a quadrupedal robot weighing 8 kg.
* 535 N converted to kg is 54.5 kg, and 445 N is 45.4 kg. In other words, even if an external force of up to 54.5 kg in the vertical direction and up to 45.4 kg in the horizontal direction is applied (or even if a corresponding weight is hung), the sole of the foot does not come off the steel plate.
MARVEL climbed up a vertical wall at high speed at a speed of 70 cm per second, and was able to walk while hanging upside down from the ceiling at a maximum speed of 50 cm per second. This is the world's fastest speed for a walking climbing robot. In addition, the research team demonstrated that the robot can climb at a speed of up to 35 cm even on a surface that is painted, dirty with dust and the rust-tainted surfaces of water tanks, proving the robot's performance in a real environment. It was experimentally demonstrated that the robot not only exhibited high speed, but also can switch from floor to wall and from wall to ceiling, and overcome 5-cm high obstacles protruding from walls without difficulty.
The new climbing quadrupedal robot is expected to be widely used for inspection, repair, and maintenance of large steel structures such as ships, bridges, transmission towers, oil pipelines, large storage areas, and construction sites. As the works required in these places involves risks such as falls, suffocation and other accidents that may result in serious injuries or casualties, the need for automation is of utmost urgency.
One of the first co-authors of the paper, a Ph.D. student, Yong Um of KAIST’s Department of Mechanical Engineering, said, "By the use of the magnetic soles made up of the EPM and MRE and the non-linear model predictive controller suitable for climbing, the robot can speedily move through a variety of ferromagnetic surfaces including walls and ceilings, not just level grounds. We believe this would become a cornerstone that will expand the mobility and the places of pedal-mobile robots can venture into." He added, “These robots can be put into good use in executing dangerous and difficult tasks on steel structures in places like the shipbuilding yards.”
This research was carried out with support from the National Research Foundation of Korea's Basic Research in Science & Engineering Program for Mid-Career Researchers and Korea Shipbuilding & Offshore Engineering Co., Ltd..
< Figure 1. The quadrupedal robot (MARVEL) walking over various ferrous surfaces. (A) vertical wall (B) ceiling. (C) over obstacles on a vertical wall (D) making floor-to-wall and wall-to-ceiling transitions (E) moving over a storage tank (F) walking on a wall with a 2-kg weight and over a ceiling with a 3-kg load. >
< Figure 2. Description of the magnetic foot (A) Components of the magnet sole: ankle, Square Eletro-Permanent Magnet(S-EPM), MRE footpad. (B) Components of the S-EPM and MRE footpad. (C) Working principle of the S-EPM. When the magnetization direction is aligned as shown in the left figure, magnetic flux comes out of the keeper and circulates through the steel plate, generating holding force (ON state). Conversely, if the magnetization direction is aligned as shown in the figure on the right, the magnetic flux circulates inside the S-EPM and the holding force disappears (OFF state). >
Video Introduction: Agile and versatile climbing on ferromagnetic surfaces with a quadrupedal robot - YouTube
2022.12.30 View 18651 -
 Professor Shinhyun Choi’s team, selected for Nature Communications Editors’ highlight
[ From left, Ph.D. candidates See-On Park and Hakcheon Jeong, along with Master's student Jong-Yong Park and Professor Shinhyun Choi ]
See-On Park, Hakcheon Jeong, Jong-Yong Park - a team of researchers under the leadership of Professor Shinhyun Choi of the School of Electrical Engineering, developed a highly reliable variable resistor (memristor) array that simulates the behavior of neurons using a metal oxide layer with an oxygen concentration gradient, and published their work in Nature Communications. The study was selected as the Nature Communications' Editor's highlight, and as the featured article posted on the main page of the journal's website.
Link : https://www.nature.com/ncomms/
[ Figure 1. The featured image on the main page of the Nature Communications' website introducing the research by Professor Choi's team on the memristor for artificial neurons ]
Thesis title: Experimental demonstration of highly reliable dynamic memristor for artificial neuron and neuromorphic computing.
( https://doi.org/10.1038/s41467-022-30539-6 )
At KAIST, their research was introduced on the 2022 Fall issue of Breakthroughs, the biannual newsletter published by KAIST College of Engineering.
This research was conducted with the support from the Samsung Research Funding & Incubation Center of Samsung Electronics.
2022.11.01 View 10100
Professor Shinhyun Choi’s team, selected for Nature Communications Editors’ highlight
[ From left, Ph.D. candidates See-On Park and Hakcheon Jeong, along with Master's student Jong-Yong Park and Professor Shinhyun Choi ]
See-On Park, Hakcheon Jeong, Jong-Yong Park - a team of researchers under the leadership of Professor Shinhyun Choi of the School of Electrical Engineering, developed a highly reliable variable resistor (memristor) array that simulates the behavior of neurons using a metal oxide layer with an oxygen concentration gradient, and published their work in Nature Communications. The study was selected as the Nature Communications' Editor's highlight, and as the featured article posted on the main page of the journal's website.
Link : https://www.nature.com/ncomms/
[ Figure 1. The featured image on the main page of the Nature Communications' website introducing the research by Professor Choi's team on the memristor for artificial neurons ]
Thesis title: Experimental demonstration of highly reliable dynamic memristor for artificial neuron and neuromorphic computing.
( https://doi.org/10.1038/s41467-022-30539-6 )
At KAIST, their research was introduced on the 2022 Fall issue of Breakthroughs, the biannual newsletter published by KAIST College of Engineering.
This research was conducted with the support from the Samsung Research Funding & Incubation Center of Samsung Electronics.
2022.11.01 View 10100 -
 Phage resistant Escherichia coli strains developed to reduce fermentation failure
A genome engineering-based systematic strategy for developing phage resistant Escherichia coli strains has been successfully developed through the collaborative efforts of a team led by Professor Sang Yup Lee, Professor Shi Chen, and Professor Lianrong Wang. This study by Xuan Zou et al. was published in Nature Communications in August 2022 and featured in Nature Communications Editors’ Highlights. The collaboration by the School of Pharmaceutical Sciences at Wuhan University, the First Affiliated Hospital of Shenzhen University, and the KAIST Department of Chemical and Biomolecular Engineering has made an important advance in the metabolic engineering and fermentation industry as it solves a big problem of phage infection causing fermentation failure.
Systems metabolic engineering is a highly interdisciplinary field that has made the development of microbial cell factories to produce various bioproducts including chemicals, fuels, and materials possible in a sustainable and environmentally friendly way, mitigating the impact of worldwide resource depletion and climate change. Escherichia coli is one of the most important chassis microbial strains, given its wide applications in the bio-based production of a diverse range of chemicals and materials. With the development of tools and strategies for systems metabolic engineering using E. coli, a highly optimized and well-characterized cell factory will play a crucial role in converting cheap and readily available raw materials into products of great economic and industrial value.
However, the consistent problem of phage contamination in fermentation imposes a devastating impact on host cells and threatens the productivity of bacterial bioprocesses in biotechnology facilities, which can lead to widespread fermentation failure and immeasurable economic loss. Host-controlled defense systems can be developed into effective genetic engineering solutions to address bacteriophage contamination in industrial-scale fermentation; however, most of the resistance mechanisms only narrowly restrict phages and their effect on phage contamination will be limited.
There have been attempts to develop diverse abilities/systems for environmental adaptation or antiviral defense. The team’s collaborative efforts developed a new type II single-stranded DNA phosphorothioation (Ssp) defense system derived from E. coli 3234/A, which can be used in multiple industrial E. coli strains (e.g., E. coli K-12, B and W) to provide broad protection against various types of dsDNA coliphages. Furthermore, they developed a systematic genome engineering strategy involving the simultaneous genomic integration of the Ssp defense module and mutations in components that are essential to the phage life cycle. This strategy can be used to transform E. coli hosts that are highly susceptible to phage attack into strains with powerful restriction effects on the tested bacteriophages. This endows hosts with strong resistance against a wide spectrum of phage infections without affecting bacterial growth and normal physiological function. More importantly, the resulting engineered phage-resistant strains maintained the capabilities of producing the desired chemicals and recombinant proteins even under high levels of phage cocktail challenge, which provides crucial protection against phage attacks.
This is a major step forward, as it provides a systematic solution for engineering phage-resistant bacterial strains, especially industrial bioproduction strains, to protect cells from a wide range of bacteriophages. Considering the functionality of this engineering strategy with diverse E. coli strains, the strategy reported in this study can be widely extended to other bacterial species and industrial applications, which will be of great interest to researchers in academia and industry alike.
Fig. A schematic model of the systematic strategy for engineering phage-sensitive industrial E. coli strains into strains with broad antiphage activities. Through the simultaneous genomic integration of a DNA phosphorothioation-based Ssp defense module and mutations of components essential for the phage life cycle, the engineered E. coli strains show strong resistance against diverse phages tested and maintain the capabilities of producing example recombinant proteins, even under high levels of phage cocktail challenge.
2022.08.23 View 15138
Phage resistant Escherichia coli strains developed to reduce fermentation failure
A genome engineering-based systematic strategy for developing phage resistant Escherichia coli strains has been successfully developed through the collaborative efforts of a team led by Professor Sang Yup Lee, Professor Shi Chen, and Professor Lianrong Wang. This study by Xuan Zou et al. was published in Nature Communications in August 2022 and featured in Nature Communications Editors’ Highlights. The collaboration by the School of Pharmaceutical Sciences at Wuhan University, the First Affiliated Hospital of Shenzhen University, and the KAIST Department of Chemical and Biomolecular Engineering has made an important advance in the metabolic engineering and fermentation industry as it solves a big problem of phage infection causing fermentation failure.
Systems metabolic engineering is a highly interdisciplinary field that has made the development of microbial cell factories to produce various bioproducts including chemicals, fuels, and materials possible in a sustainable and environmentally friendly way, mitigating the impact of worldwide resource depletion and climate change. Escherichia coli is one of the most important chassis microbial strains, given its wide applications in the bio-based production of a diverse range of chemicals and materials. With the development of tools and strategies for systems metabolic engineering using E. coli, a highly optimized and well-characterized cell factory will play a crucial role in converting cheap and readily available raw materials into products of great economic and industrial value.
However, the consistent problem of phage contamination in fermentation imposes a devastating impact on host cells and threatens the productivity of bacterial bioprocesses in biotechnology facilities, which can lead to widespread fermentation failure and immeasurable economic loss. Host-controlled defense systems can be developed into effective genetic engineering solutions to address bacteriophage contamination in industrial-scale fermentation; however, most of the resistance mechanisms only narrowly restrict phages and their effect on phage contamination will be limited.
There have been attempts to develop diverse abilities/systems for environmental adaptation or antiviral defense. The team’s collaborative efforts developed a new type II single-stranded DNA phosphorothioation (Ssp) defense system derived from E. coli 3234/A, which can be used in multiple industrial E. coli strains (e.g., E. coli K-12, B and W) to provide broad protection against various types of dsDNA coliphages. Furthermore, they developed a systematic genome engineering strategy involving the simultaneous genomic integration of the Ssp defense module and mutations in components that are essential to the phage life cycle. This strategy can be used to transform E. coli hosts that are highly susceptible to phage attack into strains with powerful restriction effects on the tested bacteriophages. This endows hosts with strong resistance against a wide spectrum of phage infections without affecting bacterial growth and normal physiological function. More importantly, the resulting engineered phage-resistant strains maintained the capabilities of producing the desired chemicals and recombinant proteins even under high levels of phage cocktail challenge, which provides crucial protection against phage attacks.
This is a major step forward, as it provides a systematic solution for engineering phage-resistant bacterial strains, especially industrial bioproduction strains, to protect cells from a wide range of bacteriophages. Considering the functionality of this engineering strategy with diverse E. coli strains, the strategy reported in this study can be widely extended to other bacterial species and industrial applications, which will be of great interest to researchers in academia and industry alike.
Fig. A schematic model of the systematic strategy for engineering phage-sensitive industrial E. coli strains into strains with broad antiphage activities. Through the simultaneous genomic integration of a DNA phosphorothioation-based Ssp defense module and mutations of components essential for the phage life cycle, the engineered E. coli strains show strong resistance against diverse phages tested and maintain the capabilities of producing example recombinant proteins, even under high levels of phage cocktail challenge.
2022.08.23 View 15138