Cell
-
 Lead-free, Efficient Perovskite for Photovoltaic Cells
(Clockwise from left: Post-doc Researcher Lamjed Debbichi, Master’s Candidate Songju Lee, Professor Min Seok Jang and Professor Hyungjun Kim)
A KAIST research team has proposed a perovskite material, Cs2Au2I6 that serves as a potential active material for highly efficient lead-free thin-film photovoltaic devices. This material is expected to lay the foundation to overcome previously known limitations of perovskite including its stability and toxicity issues.
As strong candidates for next-generation high-efficiency photovoltaic cells, perovskite photovoltaic cells have a maximum photoconversion efficiency of 22%, comparable to high-performance crystalline silicon photovoltaic cells. In addition, perovskite-based cells can be fabricated at low temperatures, thereby bringing about dramatic cost reductions.
However, it has been noted that conventional organic-inorganic hybrid perovskite materials exhibit low stability, eventually degrading their performance and making them unfit for continued use. Moreover, their inclusion of lead has undermined their environmental friendliness.
In light of this, a joint team led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Min Seok Jang from the School of Electrical Engineering has analyzed a previously discovered perovskite material, Cs2Au2I6, consisting of only inorganic substances and investigated its suitability for application in thin-film photovoltaic devices. Theoretical investigations suggests that this new perovskite material is not only as efficient but also more stable and environment friendly compared to the conventional perovskite materials. For this analysis, the team developed multiscale multiphysics simulation frameworks. Atomic-scale first-principle quantum calculations were carried out to study the optical properties of the proposed material, and device-scale electromagnetic simulations were conducted to suggest that the material could indeed serve as a promising photovoltaic substance at the device level.
From this point onward, the research team plans to extend the study in two directions: an empirical study to apply the perovskite material in real-world photovoltaic cells and a theoretical analysis to find the optimal and highly stable material for photovoltaic cells. The team said, “Perovskite materials are highly efficient, but in order to completely replace the conventional solar cells, their stability and toxicity issues must first be resolved.” They added that this research is expected to accelerate related studies in pursuit of high-efficiency, environment-friendly perovskite materials.
This research, led by post-doc researcher Lamjed Debbichi and master’s candidate Songju Lee, was selected as the front cover article of Advanced Materials on March 22.
Figure 1. Cover of Advanced Materials
Figure 2. Schematic of full solar cell device structure
2018.06.08 View 10420
Lead-free, Efficient Perovskite for Photovoltaic Cells
(Clockwise from left: Post-doc Researcher Lamjed Debbichi, Master’s Candidate Songju Lee, Professor Min Seok Jang and Professor Hyungjun Kim)
A KAIST research team has proposed a perovskite material, Cs2Au2I6 that serves as a potential active material for highly efficient lead-free thin-film photovoltaic devices. This material is expected to lay the foundation to overcome previously known limitations of perovskite including its stability and toxicity issues.
As strong candidates for next-generation high-efficiency photovoltaic cells, perovskite photovoltaic cells have a maximum photoconversion efficiency of 22%, comparable to high-performance crystalline silicon photovoltaic cells. In addition, perovskite-based cells can be fabricated at low temperatures, thereby bringing about dramatic cost reductions.
However, it has been noted that conventional organic-inorganic hybrid perovskite materials exhibit low stability, eventually degrading their performance and making them unfit for continued use. Moreover, their inclusion of lead has undermined their environmental friendliness.
In light of this, a joint team led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Min Seok Jang from the School of Electrical Engineering has analyzed a previously discovered perovskite material, Cs2Au2I6, consisting of only inorganic substances and investigated its suitability for application in thin-film photovoltaic devices. Theoretical investigations suggests that this new perovskite material is not only as efficient but also more stable and environment friendly compared to the conventional perovskite materials. For this analysis, the team developed multiscale multiphysics simulation frameworks. Atomic-scale first-principle quantum calculations were carried out to study the optical properties of the proposed material, and device-scale electromagnetic simulations were conducted to suggest that the material could indeed serve as a promising photovoltaic substance at the device level.
From this point onward, the research team plans to extend the study in two directions: an empirical study to apply the perovskite material in real-world photovoltaic cells and a theoretical analysis to find the optimal and highly stable material for photovoltaic cells. The team said, “Perovskite materials are highly efficient, but in order to completely replace the conventional solar cells, their stability and toxicity issues must first be resolved.” They added that this research is expected to accelerate related studies in pursuit of high-efficiency, environment-friendly perovskite materials.
This research, led by post-doc researcher Lamjed Debbichi and master’s candidate Songju Lee, was selected as the front cover article of Advanced Materials on March 22.
Figure 1. Cover of Advanced Materials
Figure 2. Schematic of full solar cell device structure
2018.06.08 View 10420 -
 Activation of Bystander Immune Cells during Acute Hepatitis A.
A KAIST research team has identified a process of tissue damage caused by bystander immune cells in acute viral infections. This research will pave the way for research to understand the principles of tissue damage in viral infections and immune diseases, and can point toward a possible therapeutic target for the treatment.
Upon viral infection, viral replication itself destroys human cells, but in some cases, viral replication is not the direct cause of the tissue damage. In particular, the destruction of infected cells is the primary cause of tissue damage during non-cytopathic viral infections such as hepatitis A virus, hepatitis B virus and hepatitis C virus. However, the underlying pathological mechanisms involved in the tissue damage during viral infections have not been fully elucidated.
Specificity is one of the most important characteristics of the immune system. In general, infection from a certain virus specifically activates immune cells targeting the virus, while other immune cells specific to different viruses remain inactive.
An immune cell not specific to an infected virus is called a bystander immune cell. A phenomenon that activates irrelevant immune cells not originally targeting the infecting virus, called the activation of bystander immune cells, is already known to the world; however, its clinical significance has not been investigated thoroughly.
Professor Eui-Cheol Shin and Professor Su-Hyung Park from the Graduate School of Medical Science and Engineering analyzed patients with acute hepatitis A, in collaboration with Chung-Ang University Hospital.
The team found not only immune cells specifically targeting the hepatitis A virus were activated, but also bystander immune cells were activated and involved in the damaging of liver tissues during acute hepatitis A.
According to the research, when a person is infected with hepatitis A virus, hepatitis A virus-infected cells produce IL-15, which induces the activation of bystander immune cells. Activated bystander immune cells exert innate-like cytotoxicity, triggered by activating receptors NKG2D and NKp30 and this can lead to liver injury.
Through describing the cause of excessive tissue damage during acute viral hepatitis, the research outcome is expected to provide critical contributions for the development of potential therapeutic intervention that can minimize tissue damage caused by viral hepatitis and immune disorders.
Professor Shin said, “This is a novel research case that discovered the clinical significance of bystander immune cell activation, which was previously unknown. We will continue to work on establishing treatments which could prevent tissue damage in viral and immune diseases in the future.”
This research was published in Immunity on January 2.
Figure 1. Graphical abstract
2018.03.06 View 7524
Activation of Bystander Immune Cells during Acute Hepatitis A.
A KAIST research team has identified a process of tissue damage caused by bystander immune cells in acute viral infections. This research will pave the way for research to understand the principles of tissue damage in viral infections and immune diseases, and can point toward a possible therapeutic target for the treatment.
Upon viral infection, viral replication itself destroys human cells, but in some cases, viral replication is not the direct cause of the tissue damage. In particular, the destruction of infected cells is the primary cause of tissue damage during non-cytopathic viral infections such as hepatitis A virus, hepatitis B virus and hepatitis C virus. However, the underlying pathological mechanisms involved in the tissue damage during viral infections have not been fully elucidated.
Specificity is one of the most important characteristics of the immune system. In general, infection from a certain virus specifically activates immune cells targeting the virus, while other immune cells specific to different viruses remain inactive.
An immune cell not specific to an infected virus is called a bystander immune cell. A phenomenon that activates irrelevant immune cells not originally targeting the infecting virus, called the activation of bystander immune cells, is already known to the world; however, its clinical significance has not been investigated thoroughly.
Professor Eui-Cheol Shin and Professor Su-Hyung Park from the Graduate School of Medical Science and Engineering analyzed patients with acute hepatitis A, in collaboration with Chung-Ang University Hospital.
The team found not only immune cells specifically targeting the hepatitis A virus were activated, but also bystander immune cells were activated and involved in the damaging of liver tissues during acute hepatitis A.
According to the research, when a person is infected with hepatitis A virus, hepatitis A virus-infected cells produce IL-15, which induces the activation of bystander immune cells. Activated bystander immune cells exert innate-like cytotoxicity, triggered by activating receptors NKG2D and NKp30 and this can lead to liver injury.
Through describing the cause of excessive tissue damage during acute viral hepatitis, the research outcome is expected to provide critical contributions for the development of potential therapeutic intervention that can minimize tissue damage caused by viral hepatitis and immune disorders.
Professor Shin said, “This is a novel research case that discovered the clinical significance of bystander immune cell activation, which was previously unknown. We will continue to work on establishing treatments which could prevent tissue damage in viral and immune diseases in the future.”
This research was published in Immunity on January 2.
Figure 1. Graphical abstract
2018.03.06 View 7524 -
 Cellular Mechanism for Severe Viral Hepatitis Identified
(Professor Shin(left) and Professor Jung)
KAIST medical scientists identified a cellular mechanism causing inflammatory changes in regulatory T cells that can lead to severe viral hepatitis. Research on this mechanism will help further understand the nature of various inflammatory diseases and lead to the development of relevant clinical treatments.
It is known that activated immune cells of patients with viral hepatitis destroy hepatocyte, but its regulatory mechanism has not yet been described.
Regulatory T cells inhibit activation of other immune cells and thus are important for homeostasis of the immune system. However, recent studies contradictorily show that immune inhibitory functions of regulatory T cells weaken in inflammatory conditions and the cells secrete inflammatory cytokines in response. Meanwhile, such a phenomenon was not observed in viral hepatitis including types A, B and C.
The team focused on changes in regulatory T cells in patients with viral hepatitis and discovered that regulatory T cells undergo inflammatory changes to secrete inflammatory cytokines (protein secreted by immune cells) called TNF. They also proved regulatory T cells that secrete TNF contribute to the progression of viral hepatitis.
The team confirmed that regulatory T cells of acute hepatitis A patients have reduced immune-inhibitory functions. Instead, their regulatory T cells secrete TNF. Through this research, the team identified a molecular mechanism for changes in regulatory T cells and identified the transcription factor regulating the process. Furthermore, the team found similar changes to be also present in hepatitis B and C patients.
A KAIST immunology research team led by Professors Eui-Cheol Shin and Min Kyung Jung at the Graduate School of Medical Science & Engineering conducted this translational research with teams from Chungnam National University and Yonsei University to identify the mechanism in humans, instead of using animal models. The research was described in Gastroenterology last December.
Professor Shin said, “This is the first research on regulatory T cells that contributes to hepatocyte damage in viral hepatitis.” He continued, “It is significant for identifying the cells and the molecules that can be used as effective treatment targets for viral hepatitis in the future. This research was funded by the Samsung Science and Technology Foundation.
(Figure1: Treg cells from acute hepatitis A (AHA) patients produce tumor necrosis factor (TNF) andhave reduced suppressive activity. These changes are due to a decrease in FoxP3 transcription factor and an increase in RORγt transcription factor. TNF-producing Treg cells are associated with severe liver injury in AHA patients.)
(Figure 2: A higher proportion of Treg cells from patients with acute hepatitis A, compared with healthy controls, produced TNF upon stimulation with anti-CD3 and anti-CD2. This study reports the presence and the significance of TNF-producing Treg cells for the first time in human patients.)
2018.01.18 View 9485
Cellular Mechanism for Severe Viral Hepatitis Identified
(Professor Shin(left) and Professor Jung)
KAIST medical scientists identified a cellular mechanism causing inflammatory changes in regulatory T cells that can lead to severe viral hepatitis. Research on this mechanism will help further understand the nature of various inflammatory diseases and lead to the development of relevant clinical treatments.
It is known that activated immune cells of patients with viral hepatitis destroy hepatocyte, but its regulatory mechanism has not yet been described.
Regulatory T cells inhibit activation of other immune cells and thus are important for homeostasis of the immune system. However, recent studies contradictorily show that immune inhibitory functions of regulatory T cells weaken in inflammatory conditions and the cells secrete inflammatory cytokines in response. Meanwhile, such a phenomenon was not observed in viral hepatitis including types A, B and C.
The team focused on changes in regulatory T cells in patients with viral hepatitis and discovered that regulatory T cells undergo inflammatory changes to secrete inflammatory cytokines (protein secreted by immune cells) called TNF. They also proved regulatory T cells that secrete TNF contribute to the progression of viral hepatitis.
The team confirmed that regulatory T cells of acute hepatitis A patients have reduced immune-inhibitory functions. Instead, their regulatory T cells secrete TNF. Through this research, the team identified a molecular mechanism for changes in regulatory T cells and identified the transcription factor regulating the process. Furthermore, the team found similar changes to be also present in hepatitis B and C patients.
A KAIST immunology research team led by Professors Eui-Cheol Shin and Min Kyung Jung at the Graduate School of Medical Science & Engineering conducted this translational research with teams from Chungnam National University and Yonsei University to identify the mechanism in humans, instead of using animal models. The research was described in Gastroenterology last December.
Professor Shin said, “This is the first research on regulatory T cells that contributes to hepatocyte damage in viral hepatitis.” He continued, “It is significant for identifying the cells and the molecules that can be used as effective treatment targets for viral hepatitis in the future. This research was funded by the Samsung Science and Technology Foundation.
(Figure1: Treg cells from acute hepatitis A (AHA) patients produce tumor necrosis factor (TNF) andhave reduced suppressive activity. These changes are due to a decrease in FoxP3 transcription factor and an increase in RORγt transcription factor. TNF-producing Treg cells are associated with severe liver injury in AHA patients.)
(Figure 2: A higher proportion of Treg cells from patients with acute hepatitis A, compared with healthy controls, produced TNF upon stimulation with anti-CD3 and anti-CD2. This study reports the presence and the significance of TNF-producing Treg cells for the first time in human patients.)
2018.01.18 View 9485 -
 President Shin Reaffirms Innovation Initiatives in New Year Speech
(President Shin and representatives of faculty, students, staff celebrate the New Year in a reception held on January 2 at the auditorium.)
The KAIST community gathered to celebrate a fresh start for the year 2018. At the ceremony, held in the auditorium on January 2, members of KAIST community reaffirmed their commitment to be the trailblazers of Korea and beyond through unwavering innovations.
President Sung-Chul Shin presented his new vision and plan in his New Year speech, which focused on innovation for enhancing institutional competitiveness and global visibility. He said that as you are the future of KAIST, KAIST is the future of Korea. KAIST’s vision for a better future will have a significant impact on national progress and beyond. He stressed that innovation in the five pillars of education, research, technology commercialization, globalization, and future strategy will further advance the excellence of KAIST.
At the ceremony, President Shin also presented the award for ‘the KAISTian of the Year’ to Professor YongKeun Park of the Department of Physics. The annual award recognizes a distinguished professor whose academic accomplishments made the most significant impact.
In his New Year speech, President Shin said that the year 2018 will provide an opportunity to take a leap forward for becoming a ‘Global Value Creative, World-Leading University. The Vision 2031 Committee endorsed the five innovation initiatives to fulfill KAIST’s long-term vision and will open its recommendations to the public on March 20.
Educational innovation tops the initiatives. President Shin explained that the future of Korea is in the hands of talented individuals in science and technology, emphasizing the need to nurture creative, transdisciplinary talents with the capacity to enhance the social value of science and technology.
To this end, KAIST will establish a new undergraduate non-departmental program for transdisciplinary education. This plan will eventually provide students with more options in choosing their major, as well as help students build a strong foundation in basic science and engineering and encourage multidisciplinary approaches.
For creating an innovative institutional research infrastructure, KAIST plans to build a Network of Excellence for the Fourth Industrial Revolution (NExFire) for convergence research. The plan of ‘Cross-Generational Collaborative Labs,’ will bring out a new collaboration platform by pairing up senior and junior faculty. President Shin said it will be a stepping stone to extend the spectrum of knowledge without any cessation.
For technology commercialization, KAIST will maximize its intellectual property and economic value by stimulating technology-invested companies and startups. Close cooperation with venture capitalists at home and abroad will further accelerate the commercialization drive at KAIST.
Saying that the globalization is no long an option but a necessity, he stressed KAIST will strengthen its efforts to established a bilingual campus. “KAIST will make every effort to create a more welcoming and comfortable atmosphere for the international community and their families. We will expand benefits to our international community, such as access to the KAIST Child Care Center and collaboration with the Taejon Christian International School (TCIS),” he said. President Shin added he will further expand global networks and partnerships this year, participating in a diverse range of international events at home and abroad for increasing global visibility.
He also said that well-designed future strategies will complete innovation initiatives. The Future Strategy Research Center will serve as a think tank for identifying future agendas, establishing strategies and advocating for them.
In addition to the five innovation initiatives, President Shin emphasized a new organizational culture that embraces inclusiveness and mutual respect among all of the members of KAIST.
“So far, the ideal qualifications expected of KAISTians have included creativity and a challenging spirit. From now on, we will nurture talents with a focus on the 3Cs: Creativity, Challenge, and Caring. I would like to make a campus in which all members care for each other to help attain mutual growth with warmth and respect," he said.
For the full text, Click
2018.01.02 View 11337
President Shin Reaffirms Innovation Initiatives in New Year Speech
(President Shin and representatives of faculty, students, staff celebrate the New Year in a reception held on January 2 at the auditorium.)
The KAIST community gathered to celebrate a fresh start for the year 2018. At the ceremony, held in the auditorium on January 2, members of KAIST community reaffirmed their commitment to be the trailblazers of Korea and beyond through unwavering innovations.
President Sung-Chul Shin presented his new vision and plan in his New Year speech, which focused on innovation for enhancing institutional competitiveness and global visibility. He said that as you are the future of KAIST, KAIST is the future of Korea. KAIST’s vision for a better future will have a significant impact on national progress and beyond. He stressed that innovation in the five pillars of education, research, technology commercialization, globalization, and future strategy will further advance the excellence of KAIST.
At the ceremony, President Shin also presented the award for ‘the KAISTian of the Year’ to Professor YongKeun Park of the Department of Physics. The annual award recognizes a distinguished professor whose academic accomplishments made the most significant impact.
In his New Year speech, President Shin said that the year 2018 will provide an opportunity to take a leap forward for becoming a ‘Global Value Creative, World-Leading University. The Vision 2031 Committee endorsed the five innovation initiatives to fulfill KAIST’s long-term vision and will open its recommendations to the public on March 20.
Educational innovation tops the initiatives. President Shin explained that the future of Korea is in the hands of talented individuals in science and technology, emphasizing the need to nurture creative, transdisciplinary talents with the capacity to enhance the social value of science and technology.
To this end, KAIST will establish a new undergraduate non-departmental program for transdisciplinary education. This plan will eventually provide students with more options in choosing their major, as well as help students build a strong foundation in basic science and engineering and encourage multidisciplinary approaches.
For creating an innovative institutional research infrastructure, KAIST plans to build a Network of Excellence for the Fourth Industrial Revolution (NExFire) for convergence research. The plan of ‘Cross-Generational Collaborative Labs,’ will bring out a new collaboration platform by pairing up senior and junior faculty. President Shin said it will be a stepping stone to extend the spectrum of knowledge without any cessation.
For technology commercialization, KAIST will maximize its intellectual property and economic value by stimulating technology-invested companies and startups. Close cooperation with venture capitalists at home and abroad will further accelerate the commercialization drive at KAIST.
Saying that the globalization is no long an option but a necessity, he stressed KAIST will strengthen its efforts to established a bilingual campus. “KAIST will make every effort to create a more welcoming and comfortable atmosphere for the international community and their families. We will expand benefits to our international community, such as access to the KAIST Child Care Center and collaboration with the Taejon Christian International School (TCIS),” he said. President Shin added he will further expand global networks and partnerships this year, participating in a diverse range of international events at home and abroad for increasing global visibility.
He also said that well-designed future strategies will complete innovation initiatives. The Future Strategy Research Center will serve as a think tank for identifying future agendas, establishing strategies and advocating for them.
In addition to the five innovation initiatives, President Shin emphasized a new organizational culture that embraces inclusiveness and mutual respect among all of the members of KAIST.
“So far, the ideal qualifications expected of KAISTians have included creativity and a challenging spirit. From now on, we will nurture talents with a focus on the 3Cs: Creativity, Challenge, and Caring. I would like to make a campus in which all members care for each other to help attain mutual growth with warmth and respect," he said.
For the full text, Click
2018.01.02 View 11337 -
 Unlocking the Keys to Parkinson's Disease
A KAIST research team has identified a new mechanism that causes the hallmark symptoms of Parkinson’s disease, namely tremors, rigidity, and loss of voluntary movement.
The discovery, made in collaboration with Nanyang Technological University in Singapore, presents a new perspective to three decades of conventional wisdom in Parkinson’s disease research. It also opens up new avenues that can help alleviate the motor problems suffered by patients of the disease, which reportedly number more than 10 million worldwide. The research was published in Neuron on August 30.
The research team was led by Professor Daesoo Kim from the Department of Biological Sciences at KAIST and Professor George Augustine from the Lee Kong Chian School of Medicine at NTU. Dr. Jeongjin Kim, a former postdoctoral fellow at KAIST who now works at the Korea Institute of Science and Technology (KIST), is the lead author.
It is known that Parkinson’s disease is caused by a lack of dopamine, a chemical in the brain that transmits neural signals. However, it remains unknown how the disease causes the motor
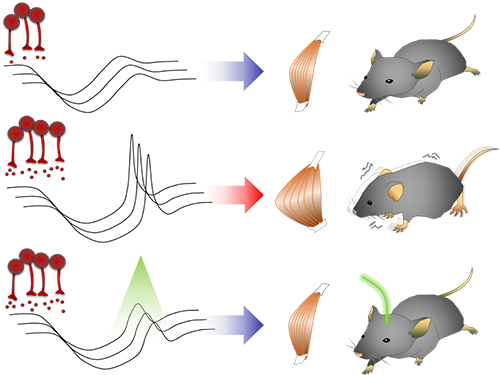
Smooth, voluntary movements, such as reaching for a cup of coffee, are controlled by the basal ganglia, which issue instructions via neurons (nerve cells that process and transmit information in the brain) in the thalamus to the cortex. These instructions come in two types: one that triggers a response (excitatory signals) and the other that suppresses a response (inhibitory signals). Proper balance between the two controls movement.
A low level of dopamine causes the basal ganglia to severely inhibit target neurons in the thalamus, called an inhibition. Scientists have long assumed that this stronger inhibition causes the motor problems of Parkinson’s disease patients.
To test this assumption, the research team used optogenetic technology in an animal model to study the effects of this increased inhibition of the thalamus and ultimately movement. Optogenetics is the use of light to control the activity of specific types of neurons within the brain.
They found that when signals from the basal ganglia are more strongly activated by light, the target neurons in the thalamus paradoxically became hyperactive. Called rebound excitation, this hyperactivity produced abnormal muscular stiffness and tremor. Such motor problems are very similar to the symptoms of Parkinson’s disease patients. When this hyperactivity of thalamic neurons is suppressed by light, mice show normal movments without Parkinson’s disease symptoms. Reducing the levels of activity back to normal caused the motor symptoms to stop, proving that the hyperactivity caused the motor problems experienced by Parkinson’s disease patients.
Professor Kim at KAIST said, “This study overturns three decades of consensus on the provenance of Parkinsonian symptoms.” The lead author, Dr Jeongjin Kim said, “The therapeutic implications of this study for the treatment of Parkinsonian symptoms are profound. It may soon become possible to remedy movement disorders without using L-DOPA, a pre-cursor to dopamine.”
Professor Augustine at NTU added, “Our findings are a breakthrough, both for understanding how the brain normally controls the movement of our body and how this control goes awry during Parkinson’s disease and related dopamine-deficiency disorders.”
The study took five years to complete, and includes researchers from the Department of Bio & Brain Engineering at KAIST.
The research team will move forward by investigating how hyperactivity in neurons in the thalamus leads to abnormal movement, as well as developing therapeutic strategies for the disease by targeting this neural mechanism.
Figure abstract: Inhibitory inputs from the basal ganglia inhibit thalamic neurons (upper). In low-dopamine states, like PD, rebound firing follows inhibition and causes movement disorders (middle). The inhibition of rebound firing alleviates PD-like symptoms in a mouse model of PD.
2017.09.22 View 11675
Unlocking the Keys to Parkinson's Disease
A KAIST research team has identified a new mechanism that causes the hallmark symptoms of Parkinson’s disease, namely tremors, rigidity, and loss of voluntary movement.
The discovery, made in collaboration with Nanyang Technological University in Singapore, presents a new perspective to three decades of conventional wisdom in Parkinson’s disease research. It also opens up new avenues that can help alleviate the motor problems suffered by patients of the disease, which reportedly number more than 10 million worldwide. The research was published in Neuron on August 30.
The research team was led by Professor Daesoo Kim from the Department of Biological Sciences at KAIST and Professor George Augustine from the Lee Kong Chian School of Medicine at NTU. Dr. Jeongjin Kim, a former postdoctoral fellow at KAIST who now works at the Korea Institute of Science and Technology (KIST), is the lead author.
It is known that Parkinson’s disease is caused by a lack of dopamine, a chemical in the brain that transmits neural signals. However, it remains unknown how the disease causes the motor
Smooth, voluntary movements, such as reaching for a cup of coffee, are controlled by the basal ganglia, which issue instructions via neurons (nerve cells that process and transmit information in the brain) in the thalamus to the cortex. These instructions come in two types: one that triggers a response (excitatory signals) and the other that suppresses a response (inhibitory signals). Proper balance between the two controls movement.
A low level of dopamine causes the basal ganglia to severely inhibit target neurons in the thalamus, called an inhibition. Scientists have long assumed that this stronger inhibition causes the motor problems of Parkinson’s disease patients.
To test this assumption, the research team used optogenetic technology in an animal model to study the effects of this increased inhibition of the thalamus and ultimately movement. Optogenetics is the use of light to control the activity of specific types of neurons within the brain.
They found that when signals from the basal ganglia are more strongly activated by light, the target neurons in the thalamus paradoxically became hyperactive. Called rebound excitation, this hyperactivity produced abnormal muscular stiffness and tremor. Such motor problems are very similar to the symptoms of Parkinson’s disease patients. When this hyperactivity of thalamic neurons is suppressed by light, mice show normal movments without Parkinson’s disease symptoms. Reducing the levels of activity back to normal caused the motor symptoms to stop, proving that the hyperactivity caused the motor problems experienced by Parkinson’s disease patients.
Professor Kim at KAIST said, “This study overturns three decades of consensus on the provenance of Parkinsonian symptoms.” The lead author, Dr Jeongjin Kim said, “The therapeutic implications of this study for the treatment of Parkinsonian symptoms are profound. It may soon become possible to remedy movement disorders without using L-DOPA, a pre-cursor to dopamine.”
Professor Augustine at NTU added, “Our findings are a breakthrough, both for understanding how the brain normally controls the movement of our body and how this control goes awry during Parkinson’s disease and related dopamine-deficiency disorders.”
The study took five years to complete, and includes researchers from the Department of Bio & Brain Engineering at KAIST.
The research team will move forward by investigating how hyperactivity in neurons in the thalamus leads to abnormal movement, as well as developing therapeutic strategies for the disease by targeting this neural mechanism.
Figure abstract: Inhibitory inputs from the basal ganglia inhibit thalamic neurons (upper). In low-dopamine states, like PD, rebound firing follows inhibition and causes movement disorders (middle). The inhibition of rebound firing alleviates PD-like symptoms in a mouse model of PD.
2017.09.22 View 11675 -
 Humicotta Wins the Silver Prize at the 2017 IDEA
The 3D-printed ceramic humidifier made by the research team led by Professor Sang-Min Bae won the silver prize at the 2017 International Design Excellence Awards (IDEA). Professor Bae’s ID+IM team was also listed as winners of three more appropriate technology designs at the IDEA. The awards, sponsored by the Industrial Designers Society of America, are one of the three prestigious design awards including the Red Dot Design Award and the iF Design Award in Germany.
The silver prize winner in the category of home and bath, Humicotta is an energy-efficient, bacteria free, and easy to clean humidifier. It includes a base module and filter. The base is a cylindrical pedestal with a built-in fan on which the filter is placed. The filter is a 3D-printed honeycomb structure made of diatomite. When water is added, the honeycomb structure and porous terracotta maximize natural humidification. It also offers an open platform service that customizes the filters or provides files that users can use their own 3D printer.
Professor Bae’s team has worked on philanthropy design using appropriate technology as their main topic for years. Their designs have been recognized at prestigious global design awards events, winning more than 50 prizes with innovative designs made for addressing various global and social problems.
The Light Funnel is a novel type of lighting device designed for off-grid areas of Africa. It helps to maximize the natural light effect in the daytime without any drastic home renovations. It consists of a transparent acrylic sphere and a reflective pathway. After filling the acrylic sphere with water and placing it on a rooftop, sunlight passes into the house through the water inside the sphere. It provides a lighted environment nine times brighter than without it. Also, once installed, it can be used almost permanently.
The Maasai Smart Cane is made using wood sticks purchased through fair trade with the Maasai tribe. GPS is installed into the grip of the birch-tree cane, so that cane users can send a signal when in an emergency situation. All of the proceeds of this product go to the tribe.
S.Cone is a first aid kit made in collaboration with Samsung Fire and Marine Insurance. The traffic cone-shaped kit is designed to help users handle an emergency situation intact and safe. The S.Cone has unique versions for fires, car accidents, and marine accidents. For example, the S.Cone for fires is equipped with a small fire extinguisher, smoke mask, and fire blanket. The cap of the S.Cone also functions as an IoT station connecting the fire and gas detector with smart phones.
Professor Bae said of his team’s winning design products, “By making the data public, any person can design their own humidifier if they have access to a 3D-printer. We want it to be a very accessible product for the public. The Light Funnel and Maasai Smart Cane are designed for economically-marginalized populations and the elderly. We will continue to make the best designed products serving the marginalized 90% of the population around the world.”
2017.09.14 View 29834
Humicotta Wins the Silver Prize at the 2017 IDEA
The 3D-printed ceramic humidifier made by the research team led by Professor Sang-Min Bae won the silver prize at the 2017 International Design Excellence Awards (IDEA). Professor Bae’s ID+IM team was also listed as winners of three more appropriate technology designs at the IDEA. The awards, sponsored by the Industrial Designers Society of America, are one of the three prestigious design awards including the Red Dot Design Award and the iF Design Award in Germany.
The silver prize winner in the category of home and bath, Humicotta is an energy-efficient, bacteria free, and easy to clean humidifier. It includes a base module and filter. The base is a cylindrical pedestal with a built-in fan on which the filter is placed. The filter is a 3D-printed honeycomb structure made of diatomite. When water is added, the honeycomb structure and porous terracotta maximize natural humidification. It also offers an open platform service that customizes the filters or provides files that users can use their own 3D printer.
Professor Bae’s team has worked on philanthropy design using appropriate technology as their main topic for years. Their designs have been recognized at prestigious global design awards events, winning more than 50 prizes with innovative designs made for addressing various global and social problems.
The Light Funnel is a novel type of lighting device designed for off-grid areas of Africa. It helps to maximize the natural light effect in the daytime without any drastic home renovations. It consists of a transparent acrylic sphere and a reflective pathway. After filling the acrylic sphere with water and placing it on a rooftop, sunlight passes into the house through the water inside the sphere. It provides a lighted environment nine times brighter than without it. Also, once installed, it can be used almost permanently.
The Maasai Smart Cane is made using wood sticks purchased through fair trade with the Maasai tribe. GPS is installed into the grip of the birch-tree cane, so that cane users can send a signal when in an emergency situation. All of the proceeds of this product go to the tribe.
S.Cone is a first aid kit made in collaboration with Samsung Fire and Marine Insurance. The traffic cone-shaped kit is designed to help users handle an emergency situation intact and safe. The S.Cone has unique versions for fires, car accidents, and marine accidents. For example, the S.Cone for fires is equipped with a small fire extinguisher, smoke mask, and fire blanket. The cap of the S.Cone also functions as an IoT station connecting the fire and gas detector with smart phones.
Professor Bae said of his team’s winning design products, “By making the data public, any person can design their own humidifier if they have access to a 3D-printer. We want it to be a very accessible product for the public. The Light Funnel and Maasai Smart Cane are designed for economically-marginalized populations and the elderly. We will continue to make the best designed products serving the marginalized 90% of the population around the world.”
2017.09.14 View 29834 -
 Professor Jin Woo Kim Wins the 14th Macrogen Scientist Award
Professor Jin Woo Kim of the Department of Biological Sciences at KAIST received the 14th Macrogen Scientist Award at the 2017 KSMCB International Conference held in COEX on September 12, 2017.
The award is given by the Korean Society for Molecular and Cellular Biology (KSMCB) and sponsored by Macrogen, a service provider of genome research. The award was established in 2004 to recognize biological scientists who have accomplished excellent performance in the field of basic life sciences.
Professor Kim has achieved outstanding research performances on nerve development, such as identifying the cause of senile retinal degenerative disease and finding retinal nerve cells that distinguish light and darkness in dark conditions.
Recently, he discovered intercellular communication, which controls the development of retinal neurons. His findings have contributed to addressing the principles of maintenance and regeneration of retinal neurons.
Since joining KAIST, he has presented approximately 20 papers and published in numerous international journals including Cell Reports, Genes and Development, and EMBO Journal. Moreover, he delivered special lectures at international conferences, universities, and institutes around the world.
2017.09.14 View 11166
Professor Jin Woo Kim Wins the 14th Macrogen Scientist Award
Professor Jin Woo Kim of the Department of Biological Sciences at KAIST received the 14th Macrogen Scientist Award at the 2017 KSMCB International Conference held in COEX on September 12, 2017.
The award is given by the Korean Society for Molecular and Cellular Biology (KSMCB) and sponsored by Macrogen, a service provider of genome research. The award was established in 2004 to recognize biological scientists who have accomplished excellent performance in the field of basic life sciences.
Professor Kim has achieved outstanding research performances on nerve development, such as identifying the cause of senile retinal degenerative disease and finding retinal nerve cells that distinguish light and darkness in dark conditions.
Recently, he discovered intercellular communication, which controls the development of retinal neurons. His findings have contributed to addressing the principles of maintenance and regeneration of retinal neurons.
Since joining KAIST, he has presented approximately 20 papers and published in numerous international journals including Cell Reports, Genes and Development, and EMBO Journal. Moreover, he delivered special lectures at international conferences, universities, and institutes around the world.
2017.09.14 View 11166 -
 Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 12611
Cooperative Tumor Cell Membrane-Targeted Phototherapy
A KAIST research team led by Professor Ji-Ho Park in the Bio and Brain Engineering Department at KAIST developed a technology for the effective treatment of cancer by delivering synthetic receptors throughout tumor tissue. The study, led by Ph.D. candidate Heegon Kim, was published online in Nature Communications on June 19.
Cancer targeted therapy generally refers to therapy targeting specific molecules that are involved in the growth and generation of cancer. The targeted delivery of therapeutics using targeting agents such as antibodies or nanomaterials has improved the precision and safety of cancer therapy.
However, the paucity and heterogeneity of identified molecular targets within tumors have resulted in poor and uneven distribution of targeted agents, thus compromising treatment outcomes.
To solve this problem, the team constructed a cooperative targeting system in which synthetic and biological nanocomponents participate together in the tumor cell membrane-selective localization of synthetic receptors to amplify the subsequent targeting of therapeutics. Here, synthetic and biological nanocomponents refer to liposomes and extracellular vesicles, respectively.
The synthetic receptors are first delivered selectively to tumor cell membranes in the perivascular region using liposomes. By hitchhiking with extracellular vesicles secreted by the cells, the synthetic receptors are transferred to neighboring cells and further spread throughout the tumor tissues where the molecular targets are limited.
Hitchhiking extracellular vesicles for delivery of synthetic receptors was possible since extracellular vesicles, such as exosomes, mediate intercellular communications by transferring various biological components such as lipids, cytosolic proteins, and RNA through a membrane fusion process. They also play a supportive role in promoting tumor progression in that tumor-derived extracellular vesicles deliver oncogenic signals to normal host cells.
The team showed that this tumor cell membrane-targeted delivery of synthetic receptors led to a uniform distribution of synthetic receptors throughout a tumor and subsequently led to enhanced phototherapeutic efficacy of the targeted photosensitizer.
Professor Park said, “The cooperative tumor targeting system is expected to be applied in treating various diseases that are hard to target.”
The research was funded by the Basic Science Research Program through the National Research Foundation funded by the Ministry of Science, ICT & Future Planning, and the National R&D Program for Cancer Control funded by the Ministry for Health and Welfare.
(Ph.D. candidates Hee Gon Kim (left) and Chanhee Oh)
Figure 1. A schematic of a cooperative tumor targeting system via delivery of synthetic receptors.
Figure 2. A confocal microscopic image of a tumor section after cooperative targeting by synthetic receptor delivery. Green and magenta represent vessels and therapeutic agents inside a tumor respectively.
2017.07.07 View 12611 -
 Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
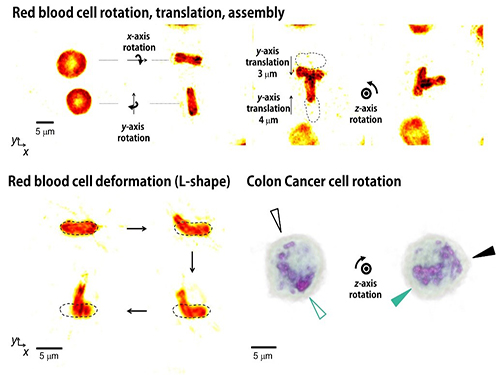
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9806
Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9806 -
 The Antibody That Normalizes Tumor Vessels
Researchers also discover that their antisepsis antibody reduces glioma, lung and breast cancer progression in mice.
A research team at the Center for Vascular Research within the Institute for Basic Science (IBS) discovered that the antisepsis antibody ABTAA (Ang2-Binding and Tie2-Activating Antibody) reduces tumor volume and improves the delivery of anti-cancer drugs. Published in Cancer Cell, this study demonstrates that ABTAA restores the structural and functional integrity of tumor blood vessels in three different tumor models: breast, lungs, and brain.
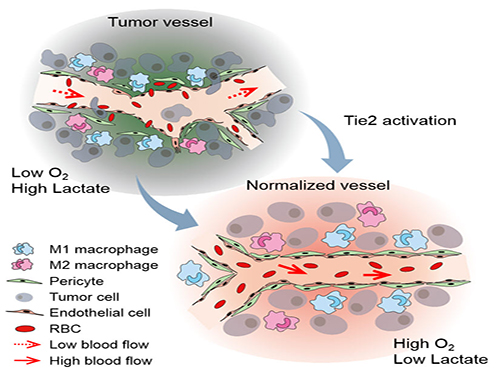
Blood vessels inside and around an established tumor can be described as a chaotic and dysfunctional labyrinth. While the inner walls of healthy blood vessels are surrounded and supported by endothelial cells and other cells called pericytes, in the established tumor, the endothelial junctions are broken apart and pericytes are also detached. Blood flow into and from the tumor is severely retarded and tumor vessels lacking an intact vessel wall become leaky. This microenvironment causes limited drug delivery to the tumor and leads to inadequate oxygen supply (hypoxia) and even metastasis.
The research team led by Professor Gou-Young Koh at KAIST’s Graduate School of Medical Science and Engineering found that the antibody ABTAA normalizes the tumor vessels and hence, change the whole tumor microenvironment. “We call it normalization of tumor vessels, because it resembles closely the wall architecture of healthy, normal vessels,” explains PARK Jin-Sung, first author of the study. And continues: “Tumor can adapt to hypoxia and get more aggressive, so we tried to prevent this transition by normalizing tumor vessels. ABTAA changes the whole tumor environment, oxygenation status and level of lactate, so that the immune cells and drugs can reach the core regions of the tumor more easily. In this way, we create a favorable ground for tumor treatment.”
In an attempt to generate antibodies targeting the protein Ang2, which is specifically expressed by endothelial cells in stressful conditions like in tumor, the team unexpectedly discovered that ABTAA has a peculiar way of working and a dual function. ABTAA indeed not only blocks Ang2, but also activates Tie2 at the same time. Tie2 is a receptor present on the cell membrane of endothelial cells. ABTAA causes Ang2 to cluster together and to strongly activate Tie2 receptors. “If we activate Tie2, we can efficiently normalize tumor vessels, enhance drug delivery and change the whole microenvironment,” explains KOH Gou Young, Director of the Center for Vascular Research.
Several pharmaceutical companies are developing Ang2-blocking antibodies to cure cancer. However, even if these antibodies significantly inhibit tumor progression, they do not stop tumor hypoxia. Moreover, most of the anti-cancer drugs target the tumor at its early stage, when tumors are still hard to diagnose. ABTAA, instead, works with tumors that are already rooted: “When the tumor is established, hypoxia is the main driver of tumor progression. So, if we eliminate hypoxia, we make the tumor milder, by reducing its progression and metastasis,” comments Koh.
Figure: Schematic drawing of a blood vessel around tumors before and after treatment with ABTAA. The picture above shows a typical tumor vasculature characterized by damaged walls, red blood cells leakage and detached pericytes. Activating Tie2 on endothelial cells with the antibody ABTAA restores the normal vessel architecture: endothelial and pericytes on the vessel walls are stabilized, the delivery of blood is improved, and the anticancer drugs are more likely to reach the tumor core.
The researchers tested ABTAA in mice with three different types of tumors that show high levels of Ang2: glioma (a type of a brain tumor), lung carcinoma, and breast cancer. They also compared the effect of ABTAA with ABA, another antibody that blocks Ang2 but misses the Tie2 activating properties. In all three cases, ABTAA was superior to ABA in inducing tumor vessel normalization, which led to a better delivery of the anti-cancer drugs into the tumor core region.
Glioma is one of the so-called intractable diseases, because of its poor prognosis and treatment. Professor Koh’s team found that the glioma volume was reduced 39% by ABTAA and 17% by ABA. ABTAA profoundly reduced vascular leakage and edema formation in glioma through promoting vascular tightening. Moreover, when ABTAA was administered together with the chemotherapeutic drug temozolomide (TMZ), the tumor volume reduces further (76% by ABTAA+TMZ, 51% by ABA+TMZ, and 36% by TMZ).
In the Lewis Lung Carcinoma (LLC) tumor model, the team administered ABTAA together with a chemotherapeutic drug called cisplatin (Cpt) and observed a greater suppression of tumor growth (52%) compared with the controls and increased overall survival. Moreover, ABTAA+Cpt led to a marked increase in necrotic area within tumors.
Finally, in a spontaneous breast cancer model, ABTAA delayed tumor growth and enhanced the anti-tumor effect of Cpt.
Courtesy of the Institute for Basic Sciences (IBS)
Figure: The antibody ABTAA alone and in combination with other anti-cancer drugs have a beneficial effect in reducing tumor volume. ABTAA was tested in mice with brain tumor (glioma), lung or breast cancer. The image shows the improvements: reduction in glioma tumor size, reduction in metastatic colonies in lung tumor and decrease in necrotic regions in breast tumor.
In the future, the team would like to further understand the underlying relationship between faulty blood vessels and diseases. “We would like to apply this antibody to an organ that is rich in blood vessels, that is the eye, and see if this antibody can be useful to treat eye diseases such as age-related macular degeneration and diabetic retinopathy,” concludes Koh.
Professor Gou-Young Koh (left) and Jin-Sung Park (right)
2016.12.16 View 9717
The Antibody That Normalizes Tumor Vessels
Researchers also discover that their antisepsis antibody reduces glioma, lung and breast cancer progression in mice.
A research team at the Center for Vascular Research within the Institute for Basic Science (IBS) discovered that the antisepsis antibody ABTAA (Ang2-Binding and Tie2-Activating Antibody) reduces tumor volume and improves the delivery of anti-cancer drugs. Published in Cancer Cell, this study demonstrates that ABTAA restores the structural and functional integrity of tumor blood vessels in three different tumor models: breast, lungs, and brain.
Blood vessels inside and around an established tumor can be described as a chaotic and dysfunctional labyrinth. While the inner walls of healthy blood vessels are surrounded and supported by endothelial cells and other cells called pericytes, in the established tumor, the endothelial junctions are broken apart and pericytes are also detached. Blood flow into and from the tumor is severely retarded and tumor vessels lacking an intact vessel wall become leaky. This microenvironment causes limited drug delivery to the tumor and leads to inadequate oxygen supply (hypoxia) and even metastasis.
The research team led by Professor Gou-Young Koh at KAIST’s Graduate School of Medical Science and Engineering found that the antibody ABTAA normalizes the tumor vessels and hence, change the whole tumor microenvironment. “We call it normalization of tumor vessels, because it resembles closely the wall architecture of healthy, normal vessels,” explains PARK Jin-Sung, first author of the study. And continues: “Tumor can adapt to hypoxia and get more aggressive, so we tried to prevent this transition by normalizing tumor vessels. ABTAA changes the whole tumor environment, oxygenation status and level of lactate, so that the immune cells and drugs can reach the core regions of the tumor more easily. In this way, we create a favorable ground for tumor treatment.”
In an attempt to generate antibodies targeting the protein Ang2, which is specifically expressed by endothelial cells in stressful conditions like in tumor, the team unexpectedly discovered that ABTAA has a peculiar way of working and a dual function. ABTAA indeed not only blocks Ang2, but also activates Tie2 at the same time. Tie2 is a receptor present on the cell membrane of endothelial cells. ABTAA causes Ang2 to cluster together and to strongly activate Tie2 receptors. “If we activate Tie2, we can efficiently normalize tumor vessels, enhance drug delivery and change the whole microenvironment,” explains KOH Gou Young, Director of the Center for Vascular Research.
Several pharmaceutical companies are developing Ang2-blocking antibodies to cure cancer. However, even if these antibodies significantly inhibit tumor progression, they do not stop tumor hypoxia. Moreover, most of the anti-cancer drugs target the tumor at its early stage, when tumors are still hard to diagnose. ABTAA, instead, works with tumors that are already rooted: “When the tumor is established, hypoxia is the main driver of tumor progression. So, if we eliminate hypoxia, we make the tumor milder, by reducing its progression and metastasis,” comments Koh.
Figure: Schematic drawing of a blood vessel around tumors before and after treatment with ABTAA. The picture above shows a typical tumor vasculature characterized by damaged walls, red blood cells leakage and detached pericytes. Activating Tie2 on endothelial cells with the antibody ABTAA restores the normal vessel architecture: endothelial and pericytes on the vessel walls are stabilized, the delivery of blood is improved, and the anticancer drugs are more likely to reach the tumor core.
The researchers tested ABTAA in mice with three different types of tumors that show high levels of Ang2: glioma (a type of a brain tumor), lung carcinoma, and breast cancer. They also compared the effect of ABTAA with ABA, another antibody that blocks Ang2 but misses the Tie2 activating properties. In all three cases, ABTAA was superior to ABA in inducing tumor vessel normalization, which led to a better delivery of the anti-cancer drugs into the tumor core region.
Glioma is one of the so-called intractable diseases, because of its poor prognosis and treatment. Professor Koh’s team found that the glioma volume was reduced 39% by ABTAA and 17% by ABA. ABTAA profoundly reduced vascular leakage and edema formation in glioma through promoting vascular tightening. Moreover, when ABTAA was administered together with the chemotherapeutic drug temozolomide (TMZ), the tumor volume reduces further (76% by ABTAA+TMZ, 51% by ABA+TMZ, and 36% by TMZ).
In the Lewis Lung Carcinoma (LLC) tumor model, the team administered ABTAA together with a chemotherapeutic drug called cisplatin (Cpt) and observed a greater suppression of tumor growth (52%) compared with the controls and increased overall survival. Moreover, ABTAA+Cpt led to a marked increase in necrotic area within tumors.
Finally, in a spontaneous breast cancer model, ABTAA delayed tumor growth and enhanced the anti-tumor effect of Cpt.
Courtesy of the Institute for Basic Sciences (IBS)
Figure: The antibody ABTAA alone and in combination with other anti-cancer drugs have a beneficial effect in reducing tumor volume. ABTAA was tested in mice with brain tumor (glioma), lung or breast cancer. The image shows the improvements: reduction in glioma tumor size, reduction in metastatic colonies in lung tumor and decrease in necrotic regions in breast tumor.
In the future, the team would like to further understand the underlying relationship between faulty blood vessels and diseases. “We would like to apply this antibody to an organ that is rich in blood vessels, that is the eye, and see if this antibody can be useful to treat eye diseases such as age-related macular degeneration and diabetic retinopathy,” concludes Koh.
Professor Gou-Young Koh (left) and Jin-Sung Park (right)
2016.12.16 View 9717 -
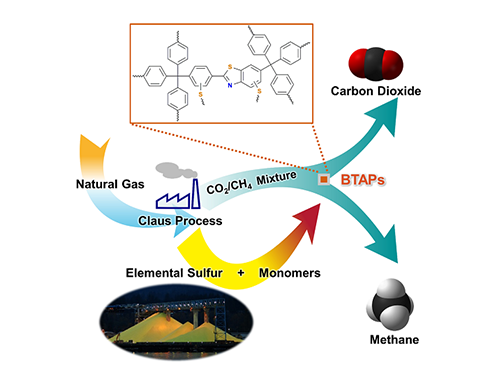 Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9912
Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 9912 -
 KAIST Team Develops Semi-Transparent Solar Cells with Thermal Mirror Capability
A research team led by KAIST and Sungkyunkwan University professors has created semi-transparent perovskite solar cells that demonstrate high-power conversion efficiency and transmit visible light while blocking infrared light, making them great candidates for solar windows.
Modern architects prefer to build exteriors designed with glass mainly from artistic or cost perspectives. Scientists, however, go one step further and see opportunities from its potential ability to harness solar energy. Researchers have thus explored ways to make solar cells transparent or semi-transparent as a substitute material for glass, but this has proven to be a challenging task because solar cells need to absorb sunlight to generate electricity, and when they are transparent, it reduces their energy efficiency.
Typical solar cells today are made of crystalline silicon, but it is difficult to make them translucent. Semi-transparent solar cells under development use, for example, organic or dye-sensitized materials, but compared to crystalline silicon-based cells, their power-conversion efficiencies are relatively low. Perovskites are hybrid organic-inorganic halide-based photovoltaic materials, which are cheap to produce and easy to manufacture. They have recently received much attention as the efficiency of perovskite solar cells has rapidly increased to the level of silicon technologies in the past few years.
Using perovskites, a Korean research team led by Professor Seunghyup Yoo of the Electrical Engineering School at KAIST and Professor Nam-Gyu Park of the Chemical Engineering School at Sungkyunkwan University developed a semi-transparent solar cell that is highly efficient and, additionally, functions very effectively as a thermal-mirror.
The team has developed a top transparent electrode (TTE) that works well with perovskite solar cells. In most cases, a key to success in realizing semi-transparent solar cells is to find a TTE that is compatible with a given photoactive material system, which is also the case for perovskite solar cells. The proposed TTE is based on a multilayer stack consisting of a metal film sandwiched between a high refractive-index (high-index) layer and an interfacial buffer layer. This TTE, placed as a top-most layer, can be prepared without damaging ingredients used in perovskite solar cells. Unlike conventional transparent electrodes focusing only on transmitting visible light, the proposed TTE plays the dual role of passing through visible light while reflecting infrared rays. The semi-transparent solar cells made with the proposed TTEs exhibited average power conversion efficiency as high as 13.3% with 85.5% infrared rejection.
The team believes that if the semi-transparent perovskite solar cells are scaled up for practical applications, they can be used in solar windows for buildings and automobiles, which not only generate electrical energy but also enable the smart heat management for indoor environments, thereby utilizing solar energy more efficiently and effectively.
This result was published as a cover article in the July 20, 2016 issue of Advanced Energy Materials. The research paper is entitled “Empowering Semi-transparent Solar Cells with Thermal-mirror Functionality.” (DOI: 10.1002/aenm.201502466)
The team designed the transparent electrode (TE) stack in three layers: A thin-film of silver (Ag) is placed in between the bottom interfacial layer of molybdenum trioxide (MoO3) and the top high-index dielectric layer of zinc sulfide (ZnS). Such a tri-layer approach has been known as a means to increase the overall visible-light transmittance of metallic thin films via index matching technique, which is essentially the same technique used for anti-reflection coating of glasses except that the present case involves a metallic layer.
Traditionally, when a TE is based on a metal film, such as Ag, the film should be extremely thin, e.g., 7-12 nanometers (nm), to obtain transparency and, accordingly, to transmit visible light. However, the team took a different approach in this research. They made the Ag TE two or three times thicker (12-24 nm) than conventional metal films and, as a result, it reflected more infrared light. The high refractive index of the ZnS layer plays an essential role in keeping the visible light transmittance of the proposed TTE high even with the relatively thick Ag film when its thickness is carefully optimized for maximal destructive interference, leading to low reflectance (and thus high transmittance) within its visible light range.
The team confirmed the semi-transparent perovskite solar cell’s thermal-mirror function through an experiment in which a halogen lamp illuminated an object for five minutes through three mediums: a window of bare glass, automotive tinting film, and the proposed semi-transparent perovskite solar cell. An infrared (IR) camera took thermal images of the object as well as that of each window’s surface. The object’s temperature, when exposed through the glass window, rose to 36.8 Celsius degrees whereas both the tinting film and the cell allowed the object to remain below 27 Celsius degrees. The tinting film absorbs light to block solar energy, so the film’s surface became hot as it was continuously exposed to the lamp light, but the proposed semi-transparent solar cell stayed cool since it rejects solar heat energy by reflection, rather than by absorption. The total solar energy rejection (TSER) of the proposed cell was as high as 89.6%.
Professor Yoo of KAIST said, “The major contributions of this work are to find transparent electrode technology suitable for translucent perovskite cells and to provide a design approach to fully harness the potential it can further deliver as a heat mirror in addition to its main role as an electrode. The present work can be further fine-tuned to include colored solar cells and to incorporate flexible or rollable form factors, as they will allow for greater design freedom and thus offer more opportunities for them to be integrated into real-world objects and structures such as cars, buildings, and houses.”
The lead authors are Hoyeon Kim and Jaewon Ha, both Ph.D. candidates in the School of Electrical Engineering at KAIST, and Hui-Seon Kim, a student in the School of Chemical Engineering at Sungkyunkwan University. This research was supported mainly by the Climate Change Research Hub Program of KAIST.
Picture 1: Semi-transparent Perovskite Solar Cell
This picture shows a prototype of a semi-transparent perovskite solar cell with thermal-mirror functionality.
Picture 2: A Heat Rejection Performance Comparison Experiment
This picture presents thermal images taken by an infrared camera for comparing the heat rejection performance of bare glass, automotive tinting film, and a semi-transparent perovskite solar cell after being illuminated by a halogen lamp for five minutes.
2016.08.02 View 13870
KAIST Team Develops Semi-Transparent Solar Cells with Thermal Mirror Capability
A research team led by KAIST and Sungkyunkwan University professors has created semi-transparent perovskite solar cells that demonstrate high-power conversion efficiency and transmit visible light while blocking infrared light, making them great candidates for solar windows.
Modern architects prefer to build exteriors designed with glass mainly from artistic or cost perspectives. Scientists, however, go one step further and see opportunities from its potential ability to harness solar energy. Researchers have thus explored ways to make solar cells transparent or semi-transparent as a substitute material for glass, but this has proven to be a challenging task because solar cells need to absorb sunlight to generate electricity, and when they are transparent, it reduces their energy efficiency.
Typical solar cells today are made of crystalline silicon, but it is difficult to make them translucent. Semi-transparent solar cells under development use, for example, organic or dye-sensitized materials, but compared to crystalline silicon-based cells, their power-conversion efficiencies are relatively low. Perovskites are hybrid organic-inorganic halide-based photovoltaic materials, which are cheap to produce and easy to manufacture. They have recently received much attention as the efficiency of perovskite solar cells has rapidly increased to the level of silicon technologies in the past few years.
Using perovskites, a Korean research team led by Professor Seunghyup Yoo of the Electrical Engineering School at KAIST and Professor Nam-Gyu Park of the Chemical Engineering School at Sungkyunkwan University developed a semi-transparent solar cell that is highly efficient and, additionally, functions very effectively as a thermal-mirror.
The team has developed a top transparent electrode (TTE) that works well with perovskite solar cells. In most cases, a key to success in realizing semi-transparent solar cells is to find a TTE that is compatible with a given photoactive material system, which is also the case for perovskite solar cells. The proposed TTE is based on a multilayer stack consisting of a metal film sandwiched between a high refractive-index (high-index) layer and an interfacial buffer layer. This TTE, placed as a top-most layer, can be prepared without damaging ingredients used in perovskite solar cells. Unlike conventional transparent electrodes focusing only on transmitting visible light, the proposed TTE plays the dual role of passing through visible light while reflecting infrared rays. The semi-transparent solar cells made with the proposed TTEs exhibited average power conversion efficiency as high as 13.3% with 85.5% infrared rejection.
The team believes that if the semi-transparent perovskite solar cells are scaled up for practical applications, they can be used in solar windows for buildings and automobiles, which not only generate electrical energy but also enable the smart heat management for indoor environments, thereby utilizing solar energy more efficiently and effectively.
This result was published as a cover article in the July 20, 2016 issue of Advanced Energy Materials. The research paper is entitled “Empowering Semi-transparent Solar Cells with Thermal-mirror Functionality.” (DOI: 10.1002/aenm.201502466)
The team designed the transparent electrode (TE) stack in three layers: A thin-film of silver (Ag) is placed in between the bottom interfacial layer of molybdenum trioxide (MoO3) and the top high-index dielectric layer of zinc sulfide (ZnS). Such a tri-layer approach has been known as a means to increase the overall visible-light transmittance of metallic thin films via index matching technique, which is essentially the same technique used for anti-reflection coating of glasses except that the present case involves a metallic layer.
Traditionally, when a TE is based on a metal film, such as Ag, the film should be extremely thin, e.g., 7-12 nanometers (nm), to obtain transparency and, accordingly, to transmit visible light. However, the team took a different approach in this research. They made the Ag TE two or three times thicker (12-24 nm) than conventional metal films and, as a result, it reflected more infrared light. The high refractive index of the ZnS layer plays an essential role in keeping the visible light transmittance of the proposed TTE high even with the relatively thick Ag film when its thickness is carefully optimized for maximal destructive interference, leading to low reflectance (and thus high transmittance) within its visible light range.
The team confirmed the semi-transparent perovskite solar cell’s thermal-mirror function through an experiment in which a halogen lamp illuminated an object for five minutes through three mediums: a window of bare glass, automotive tinting film, and the proposed semi-transparent perovskite solar cell. An infrared (IR) camera took thermal images of the object as well as that of each window’s surface. The object’s temperature, when exposed through the glass window, rose to 36.8 Celsius degrees whereas both the tinting film and the cell allowed the object to remain below 27 Celsius degrees. The tinting film absorbs light to block solar energy, so the film’s surface became hot as it was continuously exposed to the lamp light, but the proposed semi-transparent solar cell stayed cool since it rejects solar heat energy by reflection, rather than by absorption. The total solar energy rejection (TSER) of the proposed cell was as high as 89.6%.
Professor Yoo of KAIST said, “The major contributions of this work are to find transparent electrode technology suitable for translucent perovskite cells and to provide a design approach to fully harness the potential it can further deliver as a heat mirror in addition to its main role as an electrode. The present work can be further fine-tuned to include colored solar cells and to incorporate flexible or rollable form factors, as they will allow for greater design freedom and thus offer more opportunities for them to be integrated into real-world objects and structures such as cars, buildings, and houses.”
The lead authors are Hoyeon Kim and Jaewon Ha, both Ph.D. candidates in the School of Electrical Engineering at KAIST, and Hui-Seon Kim, a student in the School of Chemical Engineering at Sungkyunkwan University. This research was supported mainly by the Climate Change Research Hub Program of KAIST.
Picture 1: Semi-transparent Perovskite Solar Cell
This picture shows a prototype of a semi-transparent perovskite solar cell with thermal-mirror functionality.
Picture 2: A Heat Rejection Performance Comparison Experiment
This picture presents thermal images taken by an infrared camera for comparing the heat rejection performance of bare glass, automotive tinting film, and a semi-transparent perovskite solar cell after being illuminated by a halogen lamp for five minutes.
2016.08.02 View 13870