Graduate
-
 Thinking Out of the Box: KAIST Silicon Valley Innovation Platform
KAIST established a liaison office in San Jose, California, to support the entrepreneurship of KAIST graduates, students, and faculty who aspire to transform their innovative ideas into business.
The office, KAIST Silicon Valley Innovation Platform (SVIP), is located within the Korea Trade-Investment Promotion Agency (KOTRA) IT Center on North First Street in San Jose.
SVIP collects information and analyzes trends on emerging technologies; provides various educational programs on entrepreneurship and technology translation; offers opportunities to prospective entrepreneurs to engage with industry and research and government organizations; and assists Korean startups in accessing the US and North American market.
President Steve Kang attended the opening ceremony of the office on June 14th and encouraged KAIST alumni living in the US to share their ideas and technology innovations and transform them into business opportunities.
For more information, please contact Professor Soung-Hie Kim (seekim@business.kaist.ac.kr) from the Graduate School of Information and Media Management, KAIST.
2013.07.04 View 9478
Thinking Out of the Box: KAIST Silicon Valley Innovation Platform
KAIST established a liaison office in San Jose, California, to support the entrepreneurship of KAIST graduates, students, and faculty who aspire to transform their innovative ideas into business.
The office, KAIST Silicon Valley Innovation Platform (SVIP), is located within the Korea Trade-Investment Promotion Agency (KOTRA) IT Center on North First Street in San Jose.
SVIP collects information and analyzes trends on emerging technologies; provides various educational programs on entrepreneurship and technology translation; offers opportunities to prospective entrepreneurs to engage with industry and research and government organizations; and assists Korean startups in accessing the US and North American market.
President Steve Kang attended the opening ceremony of the office on June 14th and encouraged KAIST alumni living in the US to share their ideas and technology innovations and transform them into business opportunities.
For more information, please contact Professor Soung-Hie Kim (seekim@business.kaist.ac.kr) from the Graduate School of Information and Media Management, KAIST.
2013.07.04 View 9478 -
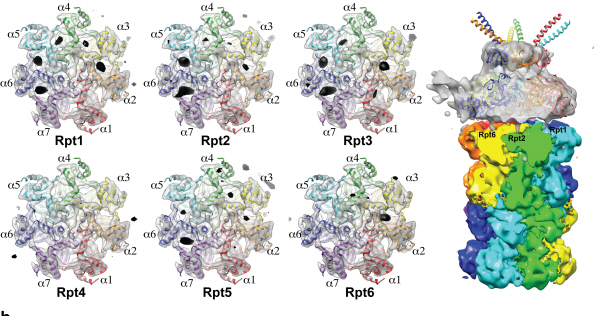 Complex responsible for protein breakdown in cells identified using Bio TEM
Professor Ho-Min Kim
- High resolution 3D structure analysis success using Bio Transmission Electron Microscopy (TEM), a giant step towards new anticancer treatment development
- Published in Nature on May 5th
Using TEM to observe protein molecules and analysing its high resolution 3D structure is now possible. KAIST Biomedical Science and Engineering Department’s Professor Ho-Min Kim has identified the high resolution structure of proteasome complexes, which is responsible for protein breakdown in cells, using Bio TEM.
This research has been published on the world"s most prestigious journal, Nature, online on May 5th. Our body controls many cellular processes through production and degradation of proteins to maintain homeostasis. A proteasome complex acts as a garbage disposal system and degrades cellular proteins when needed for regulation, which is one of the central roles of the body.
However, a mutation in proteasome complex leads to diseases such as cancer, degenerative brain diseases, and autoimmune diseases.
Currently, the anticancer drug Velcade is used to decrease proteasome function to treat Multiple Myeloma, a form of blood cancer. Research concerning proteasome complexes for more effective anticancer drugs and treatments with fewer side effects has been taking place for more than 20 years. There have been many difficulties in understanding proteasome function through 3D structure analysis since a proteasome complex, consisting of around 30 different proteins, has a great size and complexity.
The research team used Bio TEM instead of conventionally used protein crystallography technique. The protein sample was inserted into Bio TEM, hundreds of photographs were taken from various angles, and then a high–performance computer was used to analyse its structure. Bio TEM requires a smaller sample and can analyse the complexes of great size of proteins.
Professor Ho-Min Kim said, “Identifying proteasome complex assembly process and 3D structure will increase our understanding of cellular protein degradation process and hence assist in new drug development using this knowledge.” He added, “High resolution protein structure analysis using Bio TEM, used for the first time in Korea, will enable us to observe structure analysis of large protein complexes that were difficult to approach using protein crystallography.” Professor Kim continued, “If protein crystallography technology and Bio TEM could be used together to complement one another, it would bring a great synergetic effect to protein complex 3D structure analysis research in the future.”
Professor Ho-Min Kim has conducted this research since his post-doctorate at the University of California, San Francisco, under the advice of Professor Yifan Cheng; in co-operation with Harvard University and Colorado University.
Figure 1: A picture taken by Bio TEM of open state protein sample (proteasome complex)
Figure 2: Bio TEM image analysis showing protein 3D structure
2013.05.25 View 11651
Complex responsible for protein breakdown in cells identified using Bio TEM
Professor Ho-Min Kim
- High resolution 3D structure analysis success using Bio Transmission Electron Microscopy (TEM), a giant step towards new anticancer treatment development
- Published in Nature on May 5th
Using TEM to observe protein molecules and analysing its high resolution 3D structure is now possible. KAIST Biomedical Science and Engineering Department’s Professor Ho-Min Kim has identified the high resolution structure of proteasome complexes, which is responsible for protein breakdown in cells, using Bio TEM.
This research has been published on the world"s most prestigious journal, Nature, online on May 5th. Our body controls many cellular processes through production and degradation of proteins to maintain homeostasis. A proteasome complex acts as a garbage disposal system and degrades cellular proteins when needed for regulation, which is one of the central roles of the body.
However, a mutation in proteasome complex leads to diseases such as cancer, degenerative brain diseases, and autoimmune diseases.
Currently, the anticancer drug Velcade is used to decrease proteasome function to treat Multiple Myeloma, a form of blood cancer. Research concerning proteasome complexes for more effective anticancer drugs and treatments with fewer side effects has been taking place for more than 20 years. There have been many difficulties in understanding proteasome function through 3D structure analysis since a proteasome complex, consisting of around 30 different proteins, has a great size and complexity.
The research team used Bio TEM instead of conventionally used protein crystallography technique. The protein sample was inserted into Bio TEM, hundreds of photographs were taken from various angles, and then a high–performance computer was used to analyse its structure. Bio TEM requires a smaller sample and can analyse the complexes of great size of proteins.
Professor Ho-Min Kim said, “Identifying proteasome complex assembly process and 3D structure will increase our understanding of cellular protein degradation process and hence assist in new drug development using this knowledge.” He added, “High resolution protein structure analysis using Bio TEM, used for the first time in Korea, will enable us to observe structure analysis of large protein complexes that were difficult to approach using protein crystallography.” Professor Kim continued, “If protein crystallography technology and Bio TEM could be used together to complement one another, it would bring a great synergetic effect to protein complex 3D structure analysis research in the future.”
Professor Ho-Min Kim has conducted this research since his post-doctorate at the University of California, San Francisco, under the advice of Professor Yifan Cheng; in co-operation with Harvard University and Colorado University.
Figure 1: A picture taken by Bio TEM of open state protein sample (proteasome complex)
Figure 2: Bio TEM image analysis showing protein 3D structure
2013.05.25 View 11651 -
 KAIST Holds Robot Taekwondo Competition Recognized by the World Taekwondo Federation
KAIST will host the 12th Intelligent System-on-Chip (SoC) Robot War in October 2013, a robot competition. The event will have two entries: robot Taekwondo contest and HURO competition.
The World Taekwondo Federation has decided to offer an honorary Taekwondo degree to the winner of SoC Taekwondo Robot competition.
The Intelligent SoC Robot War was created in 2002 by KAIST’s Professor Hoi-Jun Yoo in the Department of Electrical Engineering.
For SoC Taekwondo Robot event, two robots compete in the form of Taekwondo, traditional Korean martial arts. The robots competing in this event have a camera and semiconductor chips on board, and therefore they have the brain-like functions to identify an object and control movements on their own. The robots have 21 joints with humanoid robot technology on their body for the techniques needed to compete in a typical Taekwondo match. They employ moves such as front kicks, side kicks, and upper punches.
In particular, KAIST’s System Design Innovation & Application Research Center, the organizer of this competition, has operated a team to demonstrate robot Taekwondo since last year with the purpose of displaying the basic movements of Taekwondo.
“Robots received attention as the source of growth in the near future. We have been developing robotics technology, and as part of our endeavor, preparing the Taekwondo demonstration team since 2012 to exhibit Korea’s robot technology and introduce our traditional martial arts,” said Professor Hoi-Jun Yoo. “We will continue to develop various capabilities for Taekwondo robots in cooperation with the World Taekwondo Federation.”
In HURO-Competition, robots compete for crossing the finishing line first by completing various missions, such as putting in a golf ball or overcoming obstacles while avoiding unexpected accidents. The winning team is awarded with a Presidential Award of Korea.
The 12th Intelligent SoC Robot War Competition is open to all graduate or undergraduate students. For details, visit the homepage at http://www.socrobotwar.org/.
2013.05.06 View 11452
KAIST Holds Robot Taekwondo Competition Recognized by the World Taekwondo Federation
KAIST will host the 12th Intelligent System-on-Chip (SoC) Robot War in October 2013, a robot competition. The event will have two entries: robot Taekwondo contest and HURO competition.
The World Taekwondo Federation has decided to offer an honorary Taekwondo degree to the winner of SoC Taekwondo Robot competition.
The Intelligent SoC Robot War was created in 2002 by KAIST’s Professor Hoi-Jun Yoo in the Department of Electrical Engineering.
For SoC Taekwondo Robot event, two robots compete in the form of Taekwondo, traditional Korean martial arts. The robots competing in this event have a camera and semiconductor chips on board, and therefore they have the brain-like functions to identify an object and control movements on their own. The robots have 21 joints with humanoid robot technology on their body for the techniques needed to compete in a typical Taekwondo match. They employ moves such as front kicks, side kicks, and upper punches.
In particular, KAIST’s System Design Innovation & Application Research Center, the organizer of this competition, has operated a team to demonstrate robot Taekwondo since last year with the purpose of displaying the basic movements of Taekwondo.
“Robots received attention as the source of growth in the near future. We have been developing robotics technology, and as part of our endeavor, preparing the Taekwondo demonstration team since 2012 to exhibit Korea’s robot technology and introduce our traditional martial arts,” said Professor Hoi-Jun Yoo. “We will continue to develop various capabilities for Taekwondo robots in cooperation with the World Taekwondo Federation.”
In HURO-Competition, robots compete for crossing the finishing line first by completing various missions, such as putting in a golf ball or overcoming obstacles while avoiding unexpected accidents. The winning team is awarded with a Presidential Award of Korea.
The 12th Intelligent SoC Robot War Competition is open to all graduate or undergraduate students. For details, visit the homepage at http://www.socrobotwar.org/.
2013.05.06 View 11452 -
 Op-Ed by Professor David Helfman: Global Science and Education in Korea for the 21st Century
Professor David Helfman from the Department of Biological Sciences and Graduate School of Nanoscience and Technology contributed an op-ed, “Global Science and Education in Korea for the 21st Century, to the Korea Herald on February 20, 2013. For the article, please click the link below:
http://www.koreaherald.com/view.php?ud=20130220000623.
2013.02.26 View 11345
Op-Ed by Professor David Helfman: Global Science and Education in Korea for the 21st Century
Professor David Helfman from the Department of Biological Sciences and Graduate School of Nanoscience and Technology contributed an op-ed, “Global Science and Education in Korea for the 21st Century, to the Korea Herald on February 20, 2013. For the article, please click the link below:
http://www.koreaherald.com/view.php?ud=20130220000623.
2013.02.26 View 11345 -
 2013 Graduation Ceremony Held on February 22
KAIST held a graduation ceremony for the year 2013 at Ryu Keun-Chul Sports Complex on February 22nd.
A total of 2,475 academic degrees were awarded this day, including 482 doctoral degrees, 1,153 master’s degrees, 838 bachelor’s degrees, and two honorary doctorates to Dr. Han Seung-Soo, a former prime minister of South Korea, and Lee Soo-young, the chairwoman of Kwang Won Industrial Co. Ltd. This commencement made KAIST to have turned out overall 46,117 talented graduates – 9,383 doctorates, 23,941 master’s degrees, and 12,793 bachelor’s degrees – to the fields of science and technology since its establishment in 1971.
The Minister of Education and Science Technology Award, which is for the student receiving bachelor’s degree with the highest academic performance, was given to Seung-Uk Jang from the Department of Mathematical Sciences. In addition, the Chairman of the KAIST Board of Trustees Award was given to Chi-Heon Kwon from the Department of Chemistry, KAIST Presidential Award to Yong-Jin Park from the Department of Chemical and Biomolecular Engineering, President of Alumni Association Award to Bong-Soo Choi from the Department Electrical Engineering, and School Supporting Association’s Award to Bo-Kyung Kim from the Bio and Brain Engineering Department.
“Climate changes due to humanity’s economic activities are threatening crucial resources such as water, food, and energy security,” said Former Prime Minister Han Seung-Soo, who received an honorary doctorate at the commencement ceremony. “Please try to solve the greatest issues that human society is facing,” he entreated in his congratulatory message.
“Use the excellent education that you have received at KAIST wisely with good purpose and ethics,” also congratulated President Suh Nam-Pyo. “I hope the graduating students of KAIST to become global leaders in the near future,” he said to the graduates entering the society.
“It was a great honor to contribute as the president of KAIST for almost 7 years, which has been the most challenging and worthwhile time in my life,” he delivered words of gratitude to all members of KAIST. “I appreciate everyone’s efforts for KAIST to develop so far.”
President Suh completed his duty as the fourteenth president of KAIST with the ceremony and returned to the United States on the 25th.
2013.02.26 View 9876
2013 Graduation Ceremony Held on February 22
KAIST held a graduation ceremony for the year 2013 at Ryu Keun-Chul Sports Complex on February 22nd.
A total of 2,475 academic degrees were awarded this day, including 482 doctoral degrees, 1,153 master’s degrees, 838 bachelor’s degrees, and two honorary doctorates to Dr. Han Seung-Soo, a former prime minister of South Korea, and Lee Soo-young, the chairwoman of Kwang Won Industrial Co. Ltd. This commencement made KAIST to have turned out overall 46,117 talented graduates – 9,383 doctorates, 23,941 master’s degrees, and 12,793 bachelor’s degrees – to the fields of science and technology since its establishment in 1971.
The Minister of Education and Science Technology Award, which is for the student receiving bachelor’s degree with the highest academic performance, was given to Seung-Uk Jang from the Department of Mathematical Sciences. In addition, the Chairman of the KAIST Board of Trustees Award was given to Chi-Heon Kwon from the Department of Chemistry, KAIST Presidential Award to Yong-Jin Park from the Department of Chemical and Biomolecular Engineering, President of Alumni Association Award to Bong-Soo Choi from the Department Electrical Engineering, and School Supporting Association’s Award to Bo-Kyung Kim from the Bio and Brain Engineering Department.
“Climate changes due to humanity’s economic activities are threatening crucial resources such as water, food, and energy security,” said Former Prime Minister Han Seung-Soo, who received an honorary doctorate at the commencement ceremony. “Please try to solve the greatest issues that human society is facing,” he entreated in his congratulatory message.
“Use the excellent education that you have received at KAIST wisely with good purpose and ethics,” also congratulated President Suh Nam-Pyo. “I hope the graduating students of KAIST to become global leaders in the near future,” he said to the graduates entering the society.
“It was a great honor to contribute as the president of KAIST for almost 7 years, which has been the most challenging and worthwhile time in my life,” he delivered words of gratitude to all members of KAIST. “I appreciate everyone’s efforts for KAIST to develop so far.”
President Suh completed his duty as the fourteenth president of KAIST with the ceremony and returned to the United States on the 25th.
2013.02.26 View 9876 -
 Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12220
Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12220 -
 A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13248
A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13248 -
 Op-Ed by Prof. David Helfman: Global Science and Education in the 21st Century
Professor David Helfman from the Department of Biological Sciences and Graduate School of Nanoscience and Technology(https://sites.google.com/site/cellsignalinglaboratory/home) recently wrote an Op-Ed in the January 2013 issue of Journal of Happy Scientists and Engineers that ispublished by the Ministry of Science, Education and Technology, the Republic of Korea. In the article entitled “Global Science and Education in the 21st Century,” Professor Helfman addressed three important issues in science and education, which will have a great impact for the development of world-leading universities in Korea. For the article, please see the attachment.
2013.01.22 View 13267
Op-Ed by Prof. David Helfman: Global Science and Education in the 21st Century
Professor David Helfman from the Department of Biological Sciences and Graduate School of Nanoscience and Technology(https://sites.google.com/site/cellsignalinglaboratory/home) recently wrote an Op-Ed in the January 2013 issue of Journal of Happy Scientists and Engineers that ispublished by the Ministry of Science, Education and Technology, the Republic of Korea. In the article entitled “Global Science and Education in the 21st Century,” Professor Helfman addressed three important issues in science and education, which will have a great impact for the development of world-leading universities in Korea. For the article, please see the attachment.
2013.01.22 View 13267 -
 KAIST Alumni Association Selects 'Proud Alums'
KAIST Alumni Association selected ‘Proud Alums’ who have contributed to the development of Korea and society and brought honor to KAIST.
The Alums selected were: CEO of Hyundai Heavy Industry Lee Jae Seong, Vice President of SK Hynix Park Sang Hoon, President of Samsung Display Kim Ki Nam, Director of Korea Research Institute of Standards and Science Kang Dae Lim, and President of Dawonsys Park Sun Soon.
Lee Jae Song (Department of Industrial and Systems Engineering, M.S. 3rd) has led Hyundai Heavy Industries through innovation and had contributed in the development of Korea and oversaw the growth of Hyundai Heavy Industries to number 1 in Shipbuilding.
Park Sang Hoon (Biological and Chemical Engineering, M.S. 5th) has led SK Hynix in the fields of energy, chemical and biological medicine and oversaw the development of world class R&D and production technologies to aid the development of Korea.
Kim Ki Nam (Electrical and Electronic Engineering, M.S. 9th) has led the development of innovative semiconductor technologies thereby helping strengthening the competitiveness of Korean semiconductor industry.
Kang Dae Lim (Mechanical Engineering, Ph.D. 1994 graduate) has helped in the development of Korean science and technology by leading the field of measurement standardization as Chairman of International Measurement Confederation and Chairman of Korea Association of Standards & Testing Organizations.
Park Sun Soon (Electrical and Electronic Engineering, M.S. 12th) has succeeded in advancing the field of electronics by pioneering the field of creative technology.
2013.01.22 View 10828
KAIST Alumni Association Selects 'Proud Alums'
KAIST Alumni Association selected ‘Proud Alums’ who have contributed to the development of Korea and society and brought honor to KAIST.
The Alums selected were: CEO of Hyundai Heavy Industry Lee Jae Seong, Vice President of SK Hynix Park Sang Hoon, President of Samsung Display Kim Ki Nam, Director of Korea Research Institute of Standards and Science Kang Dae Lim, and President of Dawonsys Park Sun Soon.
Lee Jae Song (Department of Industrial and Systems Engineering, M.S. 3rd) has led Hyundai Heavy Industries through innovation and had contributed in the development of Korea and oversaw the growth of Hyundai Heavy Industries to number 1 in Shipbuilding.
Park Sang Hoon (Biological and Chemical Engineering, M.S. 5th) has led SK Hynix in the fields of energy, chemical and biological medicine and oversaw the development of world class R&D and production technologies to aid the development of Korea.
Kim Ki Nam (Electrical and Electronic Engineering, M.S. 9th) has led the development of innovative semiconductor technologies thereby helping strengthening the competitiveness of Korean semiconductor industry.
Kang Dae Lim (Mechanical Engineering, Ph.D. 1994 graduate) has helped in the development of Korean science and technology by leading the field of measurement standardization as Chairman of International Measurement Confederation and Chairman of Korea Association of Standards & Testing Organizations.
Park Sun Soon (Electrical and Electronic Engineering, M.S. 12th) has succeeded in advancing the field of electronics by pioneering the field of creative technology.
2013.01.22 View 10828 -
 Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10297
Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10297 -
 Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9508
Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9508 -
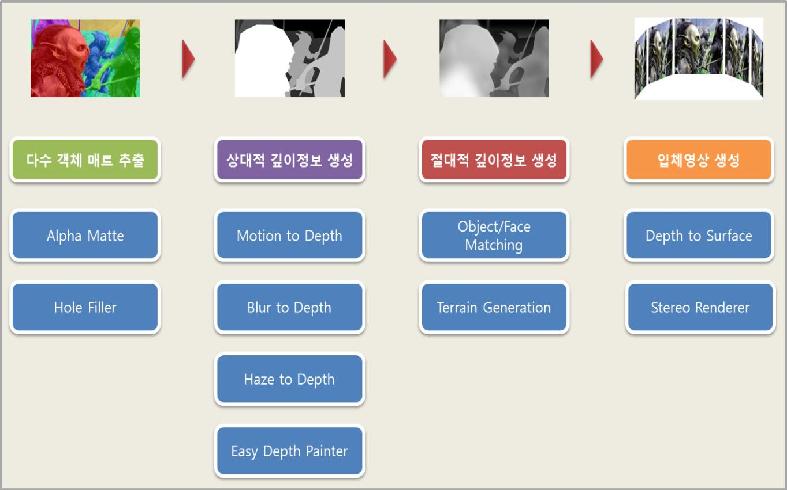 3D contents using our technology
Professor Noh Jun Yong’s research team from KAIST Graduate School of Culture Technology has successfully developed a software program that improves the semiautomatic conversation rate efficiency of 3D stereoscopic images by 3 times.
This software, named ‘NAKiD’, was first presented at the renowned Computer Graphics conference/exhibition ‘Siggraph 2012’ in August and received intense interest from the participants.
The ‘NAKiD’ technology is forecasted to replace the expensive imported equipment and technology used in 3D filming.
For multi-viewpoint no-glasses 3D stereopsis, two cameras are needed to film the image. However, ‘NAKiD’ can easily convert images from a single camera into a 3D image, greatly decreasing the problems in the film production process as well as its cost.
There are 2 methods commonly used in the production of 3D stereoscopic images; filming using two cameras and the 3D conversion using computer software.
The use of two cameras requires expensive equipment and the filmed images need further processing after production. On the other hand, 3D conversion technology does not require extra devices in the production process and can also convert the existing 2D contents into 3D, a main reason why many countries are focusing on the development of stereoscopic technology.
Stereoscopic conversion is largely divided in to 3 steps; object separation, formation of depth information and stereo rendering. Professor Noh’s teams focused on the optimization of each step to increase the efficiency of the conversion system.
Professor Noh’s research team first increased the separation accuracy to the degree of a single hair and created an algorithm that automatically fills in the background originally covered by the separated object.
The team succeeded in the automatic formation of depth information using the geographic or architectural characteristic and vanishing points. For the stereo rendering process, the team decreased the rendering time by reusing the rendered information of one side, rather than the traditional method of rendering the left and right images separately.
Professor Noh said that ‘although 3D TVs are becoming more and more commercialized, there are not enough programs that can be watched in 3D’ and that ‘stereoscopic conversion technology is receiving high praise in the field of graphics because it allows the easy production of 3D contents with small cost’.
2012.10.20 View 10795
3D contents using our technology
Professor Noh Jun Yong’s research team from KAIST Graduate School of Culture Technology has successfully developed a software program that improves the semiautomatic conversation rate efficiency of 3D stereoscopic images by 3 times.
This software, named ‘NAKiD’, was first presented at the renowned Computer Graphics conference/exhibition ‘Siggraph 2012’ in August and received intense interest from the participants.
The ‘NAKiD’ technology is forecasted to replace the expensive imported equipment and technology used in 3D filming.
For multi-viewpoint no-glasses 3D stereopsis, two cameras are needed to film the image. However, ‘NAKiD’ can easily convert images from a single camera into a 3D image, greatly decreasing the problems in the film production process as well as its cost.
There are 2 methods commonly used in the production of 3D stereoscopic images; filming using two cameras and the 3D conversion using computer software.
The use of two cameras requires expensive equipment and the filmed images need further processing after production. On the other hand, 3D conversion technology does not require extra devices in the production process and can also convert the existing 2D contents into 3D, a main reason why many countries are focusing on the development of stereoscopic technology.
Stereoscopic conversion is largely divided in to 3 steps; object separation, formation of depth information and stereo rendering. Professor Noh’s teams focused on the optimization of each step to increase the efficiency of the conversion system.
Professor Noh’s research team first increased the separation accuracy to the degree of a single hair and created an algorithm that automatically fills in the background originally covered by the separated object.
The team succeeded in the automatic formation of depth information using the geographic or architectural characteristic and vanishing points. For the stereo rendering process, the team decreased the rendering time by reusing the rendered information of one side, rather than the traditional method of rendering the left and right images separately.
Professor Noh said that ‘although 3D TVs are becoming more and more commercialized, there are not enough programs that can be watched in 3D’ and that ‘stereoscopic conversion technology is receiving high praise in the field of graphics because it allows the easy production of 3D contents with small cost’.
2012.10.20 View 10795