protein
-
 An internationally renowned academic journal published the research result produced by a KAST research team on its cover.
Fc DAAP VEGF-Trap
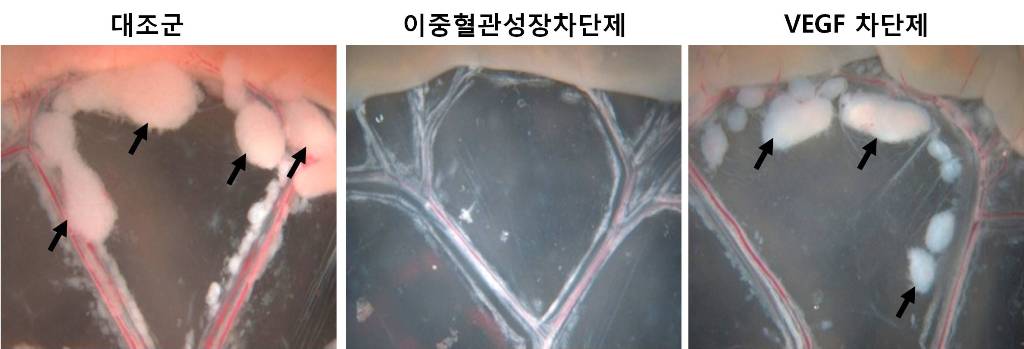
Photograph showing the gross features of tumor growth along the mesentery-intestinal border. T: tumor. Scale bars represent 5 mm.
Professor Gou-Young Koh of the Biological Sciences Department, KAIST, and his research team published their research result in Cancer Cell, a peer-review scientific journal, as a cover article dated August 17, 2010. It is the first time for the journal to pick up a paper written by a Korean research team and publish it as the cover.
It has been known that a vascular growth factor (VEGF) is closely related to the growth of a tumor. The research team recently discovered that in addition to VEGF, another growth factor, angiopoietin-2 (Ang2), is also engaged with the increase of tumors.
Professor Koh said, “VEGF and the angiopoietins play critical roles in tumor progression and metastasis, and a single inhibitor targeting both factors have not been available.”
The team led by Professor Koh has developed a double anti-angiogenic protein (DAAP) that can simultaneously bind VEGF-A and the angiopoietins and block their actions.
Professor Koh said in his paper, “DAAP is a highly effective molecule for regressing tumor angiogenesis and metastasis in implanted and spontaneous solid tumor; it can also effectively reduce ascites formation and vascular leakage in an ovarian carcinoma model. Thus, simultaneous blockade of VEGF-A and angiopoietins with DAAP is an effective therapeutic strategy for blocking tumor angiogenesis, metastasis, and vascular leakage.”
So far, cancer patients have received Avastin, anticancer drug, to inhibit VEGF, but the drug has not successfully restrained the growth of cancer tumors and brought to some of the patients with serious side effects instead.
Professor Koh said, “DAAP will be very effective to control the expansion of tumor growth factors, which will open up a new possibility for the development of more helpful cancer medicine with low side effects.”
2010.08.20 View 13164
An internationally renowned academic journal published the research result produced by a KAST research team on its cover.
Fc DAAP VEGF-Trap
Photograph showing the gross features of tumor growth along the mesentery-intestinal border. T: tumor. Scale bars represent 5 mm.
Professor Gou-Young Koh of the Biological Sciences Department, KAIST, and his research team published their research result in Cancer Cell, a peer-review scientific journal, as a cover article dated August 17, 2010. It is the first time for the journal to pick up a paper written by a Korean research team and publish it as the cover.
It has been known that a vascular growth factor (VEGF) is closely related to the growth of a tumor. The research team recently discovered that in addition to VEGF, another growth factor, angiopoietin-2 (Ang2), is also engaged with the increase of tumors.
Professor Koh said, “VEGF and the angiopoietins play critical roles in tumor progression and metastasis, and a single inhibitor targeting both factors have not been available.”
The team led by Professor Koh has developed a double anti-angiogenic protein (DAAP) that can simultaneously bind VEGF-A and the angiopoietins and block their actions.
Professor Koh said in his paper, “DAAP is a highly effective molecule for regressing tumor angiogenesis and metastasis in implanted and spontaneous solid tumor; it can also effectively reduce ascites formation and vascular leakage in an ovarian carcinoma model. Thus, simultaneous blockade of VEGF-A and angiopoietins with DAAP is an effective therapeutic strategy for blocking tumor angiogenesis, metastasis, and vascular leakage.”
So far, cancer patients have received Avastin, anticancer drug, to inhibit VEGF, but the drug has not successfully restrained the growth of cancer tumors and brought to some of the patients with serious side effects instead.
Professor Koh said, “DAAP will be very effective to control the expansion of tumor growth factors, which will open up a new possibility for the development of more helpful cancer medicine with low side effects.”
2010.08.20 View 13164 -
 Native-like Spider Silk Produced in Metabolically Engineered Bacterium
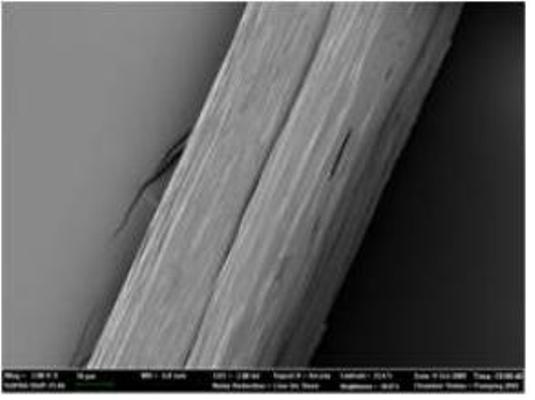
Microscopic picture of 285 kilodalton recombinant spider silk fiber
Researchers have long envied spiders’ ability to manufacture silk that is light-weighted while as strong and tough as steel or Kevlar. Indeed, finer than human hair, five times stronger by weight than steel, and three times tougher than the top quality man-made fiber Kevlar, spider dragline silk is an ideal material for numerous applications. Suggested industrial applications have ranged from parachute cords and protective clothing to composite materials in aircrafts. Also, many biomedical applications are envisioned due to its biocompatibility and biodegradability.
Unfortunately, natural dragline silk cannot be conveniently obtained by farming spiders because they are highly territorial and aggressive. To develop a more sustainable process, can scientists mass-produce artificial silk while maintaining the amazing properties of native silk? That is something Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) in Daejeon, the Republic of Korea, and his collaborators, Professor Young Hwan Park at Seoul National University and Professor David Kaplan at Tufts University, wanted to figure out. Their method is very similar to what spiders essentially do: first, expression of recombinant silk proteins; second, making the soluble silk proteins into water-insoluble fibers through spinning.
For the successful expression of high molecular weight spider silk protein, Professor Lee and his colleagues pieced together the silk gene from chemically synthesized oligonucleotides, and then inserted it into the expression host (in this case, an industrially safe bacterium Escherichia coli which is normally found in our gut). Initially, the bacterium refused to the challenging task of producing high molecular weight spider silk protein due to the unique characteristics of the protein, such as extremely large size, repetitive nature of the protein structure, and biased abundance of a particular amino acid glycine. “To make E. coli synthesize this ultra high molecular weight (as big as 285 kilodalton) spider silk protein having highly repetitive amino acid sequence, we helped E. coli overcome the difficulties by systems metabolic engineering,” says Sang Yup Lee, Distinguished Professor of KAIST, who led this project. His team boosted the pool of glycyl-tRNA, the major building block of spider silk protein synthesis. “We could obtain appreciable expression of the 285 kilodalton spider silk protein, which is the largest recombinant silk protein ever produced in E. coli. That was really incredible.” says Dr. Xia.
But this was only step one. The KAIST team performed high-cell-density cultures for mass production of the recombinant spider silk protein. Then, the team developed a simple, easy to scale-up purification process for the recombinant spider silk protein. The purified spider silk protein could be spun into beautiful silk fiber. To study the mechanical properties of the artificial spider silk, the researchers determined tenacity, elongation, and Young’s modulus, the three critical mechanical parameters that represent a fiber’s strength, extensibility, and stiffness. Importantly, the artificial fiber displayed the tenacity, elongation, and Young’s modulus of 508 MPa, 15%, and 21 GPa, respectively, which are comparable to those of the native spider silk.
“We have offered an overall platform for mass production of native-like spider dragline silk. This platform would enable us to have broader industrial and biomedical applications for spider silk. Moreover, many other silk-like biomaterials such as elastin, collagen, byssus, resilin, and other repetitive proteins have similar features to spider silk protein. Thus, our platform should also be useful for their efficient bio-based production and applications,” concludes Professor Lee.
This work is published on July 26 in the Proceedings of the National Academy of Sciences (PNAS) online.
2010.07.28 View 19366
Native-like Spider Silk Produced in Metabolically Engineered Bacterium
Microscopic picture of 285 kilodalton recombinant spider silk fiber
Researchers have long envied spiders’ ability to manufacture silk that is light-weighted while as strong and tough as steel or Kevlar. Indeed, finer than human hair, five times stronger by weight than steel, and three times tougher than the top quality man-made fiber Kevlar, spider dragline silk is an ideal material for numerous applications. Suggested industrial applications have ranged from parachute cords and protective clothing to composite materials in aircrafts. Also, many biomedical applications are envisioned due to its biocompatibility and biodegradability.
Unfortunately, natural dragline silk cannot be conveniently obtained by farming spiders because they are highly territorial and aggressive. To develop a more sustainable process, can scientists mass-produce artificial silk while maintaining the amazing properties of native silk? That is something Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) in Daejeon, the Republic of Korea, and his collaborators, Professor Young Hwan Park at Seoul National University and Professor David Kaplan at Tufts University, wanted to figure out. Their method is very similar to what spiders essentially do: first, expression of recombinant silk proteins; second, making the soluble silk proteins into water-insoluble fibers through spinning.
For the successful expression of high molecular weight spider silk protein, Professor Lee and his colleagues pieced together the silk gene from chemically synthesized oligonucleotides, and then inserted it into the expression host (in this case, an industrially safe bacterium Escherichia coli which is normally found in our gut). Initially, the bacterium refused to the challenging task of producing high molecular weight spider silk protein due to the unique characteristics of the protein, such as extremely large size, repetitive nature of the protein structure, and biased abundance of a particular amino acid glycine. “To make E. coli synthesize this ultra high molecular weight (as big as 285 kilodalton) spider silk protein having highly repetitive amino acid sequence, we helped E. coli overcome the difficulties by systems metabolic engineering,” says Sang Yup Lee, Distinguished Professor of KAIST, who led this project. His team boosted the pool of glycyl-tRNA, the major building block of spider silk protein synthesis. “We could obtain appreciable expression of the 285 kilodalton spider silk protein, which is the largest recombinant silk protein ever produced in E. coli. That was really incredible.” says Dr. Xia.
But this was only step one. The KAIST team performed high-cell-density cultures for mass production of the recombinant spider silk protein. Then, the team developed a simple, easy to scale-up purification process for the recombinant spider silk protein. The purified spider silk protein could be spun into beautiful silk fiber. To study the mechanical properties of the artificial spider silk, the researchers determined tenacity, elongation, and Young’s modulus, the three critical mechanical parameters that represent a fiber’s strength, extensibility, and stiffness. Importantly, the artificial fiber displayed the tenacity, elongation, and Young’s modulus of 508 MPa, 15%, and 21 GPa, respectively, which are comparable to those of the native spider silk.
“We have offered an overall platform for mass production of native-like spider dragline silk. This platform would enable us to have broader industrial and biomedical applications for spider silk. Moreover, many other silk-like biomaterials such as elastin, collagen, byssus, resilin, and other repetitive proteins have similar features to spider silk protein. Thus, our platform should also be useful for their efficient bio-based production and applications,” concludes Professor Lee.
This work is published on July 26 in the Proceedings of the National Academy of Sciences (PNAS) online.
2010.07.28 View 19366 -
 The thermal fluctuation and elasticity of cell membranes, lipid vesicles, interacting with pore-forming peptides were reported by a research team at KAIST.
A research team from KAIST, consisted of Sung-Min Choi, Professor of Nuclear and Quantum Engineering Department, and Ji-Hwan Lee, a doctoral student in the Department, published a paper on the “thermal fluctuation and elasticity of lipid vesicles interacting with pore-forming peptides.” The paper was carried by Physical Review Letters, an internationally renowned peer-review journal on physics on July 16, 2010.
Cell membranes, which consist of lipid bilayers, play important roles in cells as barriers to maintain concentrations and matrices to host membrane proteins. During cellular processes such as cell fission and fusion, the cell membranes undergo various morphological changes governed by the interplay between protein and lipid membranes. There have been many theoretical and experimental approaches to understand cellular processes driven by protein-lipid membrane interactions. However, it is not fully established how the membrane elastic properties, which play an important role in membrane deformation, are affected by the protein-membrane interactions.
Antimicrobial peptides are one of the most common examples of proteins that modify membrane morphology. While the pore-forming mechanisms of antimicrobial peptides in lipid bilayers have been widely investigated, there have been only a few attempts to understand the mechanisms in terms of membrane elastic properties. In particular, the effects of pore formation on the membrane fluctuation and elastic properties, which provide key information to understand the mechanism of antimicrobial peptide activity, have not been reported yet. The research team reports the thermal fluctuation and elasticity of lipid vesicles interacting with pore-forming peptides, which were measured by neutron spin-echo spectroscopy.
The results of this study are expected to pay an important role in understanding the elastic behavior and morphological changes of cell membranes induced by protein-membrane interactions, and may provide new insights for developing new theoretical models for membrane fluctuations which include the membrane mediated interaction between protein patches.
(a) (b)
Figure
(a) Schematics for bound melittin and pores in lipid bilayers
(b) P NMR signal ratio (with/without Mn2+) of DOPC LUV-melittin vs P/L at 30˚C. The dashed line is a guide for eyes.
2010.07.23 View 13118
The thermal fluctuation and elasticity of cell membranes, lipid vesicles, interacting with pore-forming peptides were reported by a research team at KAIST.
A research team from KAIST, consisted of Sung-Min Choi, Professor of Nuclear and Quantum Engineering Department, and Ji-Hwan Lee, a doctoral student in the Department, published a paper on the “thermal fluctuation and elasticity of lipid vesicles interacting with pore-forming peptides.” The paper was carried by Physical Review Letters, an internationally renowned peer-review journal on physics on July 16, 2010.
Cell membranes, which consist of lipid bilayers, play important roles in cells as barriers to maintain concentrations and matrices to host membrane proteins. During cellular processes such as cell fission and fusion, the cell membranes undergo various morphological changes governed by the interplay between protein and lipid membranes. There have been many theoretical and experimental approaches to understand cellular processes driven by protein-lipid membrane interactions. However, it is not fully established how the membrane elastic properties, which play an important role in membrane deformation, are affected by the protein-membrane interactions.
Antimicrobial peptides are one of the most common examples of proteins that modify membrane morphology. While the pore-forming mechanisms of antimicrobial peptides in lipid bilayers have been widely investigated, there have been only a few attempts to understand the mechanisms in terms of membrane elastic properties. In particular, the effects of pore formation on the membrane fluctuation and elastic properties, which provide key information to understand the mechanism of antimicrobial peptide activity, have not been reported yet. The research team reports the thermal fluctuation and elasticity of lipid vesicles interacting with pore-forming peptides, which were measured by neutron spin-echo spectroscopy.
The results of this study are expected to pay an important role in understanding the elastic behavior and morphological changes of cell membranes induced by protein-membrane interactions, and may provide new insights for developing new theoretical models for membrane fluctuations which include the membrane mediated interaction between protein patches.
(a) (b)
Figure
(a) Schematics for bound melittin and pores in lipid bilayers
(b) P NMR signal ratio (with/without Mn2+) of DOPC LUV-melittin vs P/L at 30˚C. The dashed line is a guide for eyes.
2010.07.23 View 13118 -
 KAIST Professor Exposes Structural Dynamics of Protein in Solution
-- Dr. Hyot-Cherl Ihee"s 3-Year Research Is Valuable in Pharmaceutical Application
Prof. Hyot-Cherl Ihee and his team at the Department of Chemistry, KAIST, has successfully unveiled the structural dynamics of protein in solution as a result of more than three years" research work.
Nature Methods, a sister publication of the authoritative science magazine Nature, published the treatise, titled "Tracking the structural dynamics of proteins in solution using time-resolved wide-angle X-ray scattering" in its Sept. 22 online edition. The research paper will be carried in the magazine"s printed version in its October edition, according to Dr. Lee who is its correspondence author.
In May 2005, Prof. Ihee successfully photographed the structural dynamics of protein in solid state and his findings were published in the Proceedings of National Academy of Science of the United States. As protein normally exists in human body in solution, not in solid state, he directed his research to developing the technology to capture protein"s dynamics in resolved state.
In July that year, Prof. Ihee succeeded in measuring the structural changes of simple organic molecules in real time. He further developed the technology to uncover the structural dynamics of hemoglobin, myoglobin and cytochrome C.
Prof. Ihee"s research, helped with the Education-Science-Technology Ministry"s Creative Research Promotion Fund, can be applied to new pharmaceutical development projects as well as nanotechnology development, according to KAIST officials.
Prof. Ihee who earned his doctorate at California Institute of Technology in 1994 began teaching at KAIST in 2003. He won the Young Scientist Award given by the Korean government in 2006.
2008.09.22 View 14627
KAIST Professor Exposes Structural Dynamics of Protein in Solution
-- Dr. Hyot-Cherl Ihee"s 3-Year Research Is Valuable in Pharmaceutical Application
Prof. Hyot-Cherl Ihee and his team at the Department of Chemistry, KAIST, has successfully unveiled the structural dynamics of protein in solution as a result of more than three years" research work.
Nature Methods, a sister publication of the authoritative science magazine Nature, published the treatise, titled "Tracking the structural dynamics of proteins in solution using time-resolved wide-angle X-ray scattering" in its Sept. 22 online edition. The research paper will be carried in the magazine"s printed version in its October edition, according to Dr. Lee who is its correspondence author.
In May 2005, Prof. Ihee successfully photographed the structural dynamics of protein in solid state and his findings were published in the Proceedings of National Academy of Science of the United States. As protein normally exists in human body in solution, not in solid state, he directed his research to developing the technology to capture protein"s dynamics in resolved state.
In July that year, Prof. Ihee succeeded in measuring the structural changes of simple organic molecules in real time. He further developed the technology to uncover the structural dynamics of hemoglobin, myoglobin and cytochrome C.
Prof. Ihee"s research, helped with the Education-Science-Technology Ministry"s Creative Research Promotion Fund, can be applied to new pharmaceutical development projects as well as nanotechnology development, according to KAIST officials.
Prof. Ihee who earned his doctorate at California Institute of Technology in 1994 began teaching at KAIST in 2003. He won the Young Scientist Award given by the Korean government in 2006.
2008.09.22 View 14627 -
 Prof. Chung Named Winner of 2008 KAIST Scientific Award
Professor Chung Jong-Kyeong of the Department of Biological Sciences was named the winner of the 2008 KAIST Scientific Award.
The prize was awarded by KAIST President Suh Nam-Pyo during the 37th KAIST anniversary ceremony on Feb. 16.
Chung was cited for disclosing the new anti-cancer aspect of adenosine monophosphate-activated protein kinase (AMPK). His papers, published in the science magazine Nature in 2006 and again in 2007, revealed that the protein could be used to treat certain forms of cancer, as well as prevent malignant growths.
2008.02.28 View 13942
Prof. Chung Named Winner of 2008 KAIST Scientific Award
Professor Chung Jong-Kyeong of the Department of Biological Sciences was named the winner of the 2008 KAIST Scientific Award.
The prize was awarded by KAIST President Suh Nam-Pyo during the 37th KAIST anniversary ceremony on Feb. 16.
Chung was cited for disclosing the new anti-cancer aspect of adenosine monophosphate-activated protein kinase (AMPK). His papers, published in the science magazine Nature in 2006 and again in 2007, revealed that the protein could be used to treat certain forms of cancer, as well as prevent malignant growths.
2008.02.28 View 13942 -
 Professor Jie-Oh Lee of the Department of Chemistry of KAIST
Professor Jie-Oh Lee of the Department of Chemistry of KAIST was selected as the "KAIST Man of the Year."
Lee was cited for his successful identifying of the three-dimensional structure of protein that causes sepsis. His research is expected to contribute greatly to the development of medicines for immune system treatment.
The prize was given by KAIST President Suh Nam Pyo at the New Year"s ceremony on Jan. 2, 2008 at the KAIST auditorium.
Professor Lee published a series of research papers in Science, one of the world"s most prestigious scientific journals. Most recently, Lee was awarded the "Scientist of the Year" prize by the Korean Science Reporters Association.
2008.01.02 View 15156
Professor Jie-Oh Lee of the Department of Chemistry of KAIST
Professor Jie-Oh Lee of the Department of Chemistry of KAIST was selected as the "KAIST Man of the Year."
Lee was cited for his successful identifying of the three-dimensional structure of protein that causes sepsis. His research is expected to contribute greatly to the development of medicines for immune system treatment.
The prize was given by KAIST President Suh Nam Pyo at the New Year"s ceremony on Jan. 2, 2008 at the KAIST auditorium.
Professor Lee published a series of research papers in Science, one of the world"s most prestigious scientific journals. Most recently, Lee was awarded the "Scientist of the Year" prize by the Korean Science Reporters Association.
2008.01.02 View 15156 -
 Professor Jie-Oh Lee awarded 'Scientist of the Year'
Professor Jie-Oh Lee of the Department of Chemistry was awarded the ‘Scientist of the Year’ prize for identifying the three-dimensional structure of protein that causes sepsis, and it was announced by the Korean Science Reporters Association (KOSRA) on November 26th.“Humans have about 30,000 different kinds of proteins, and they all have different structures, just like our faces,” said Professor Lee. “It is extremely helpful to know the three-dimensional shape of proteins when you are trying to understand what their functions in an organism are and trying to develop medicine for them.”
When looking for the three-dimensional structure, protein must first be crystallized and radiated with x-ray, so that reflected x-ray can be interpreted. The three-dimensional structure of sepsis immunity proteins TLR1-TLR2 and TLR4-MD2 could not be found until now because they would not even crystallize.
“I began to doubt if it was even possible to crystallize them because we went through so many failures,” reflected Professor Lee.
In August of last year, after about three years of research, the team finally came up with a new idea. The team decided to ‘stick’ the sepsis immunity protein to protein that easily crystallizes. If the combined structure of sepsis immunity protein and the known protein could be identified, the structure of sepsis immunity protein would be a combined structure subtracted by the known structure.
The three-dimensional structure was obtained with x-ray radiation from combined protein crystal. The combined protein was derived from an insect cell with altered DNA.
“This method seems very simple but no one ever tried it or no one ever succeeded in it,” said Professor Lee.
The result was a horseshoe shaped protein structure. The research team also expects the new protein-combining technology to contribute to the development of a new immune system treatment medicine.
The prize-awarding ceremony was held on November 26th in an event hosted by the Korean Hospital Association.
Also, Professor Ryong Ryoo of the Department of Chemistry was selected as the National Scientist last month.By KAIST Herald on November, 2007
2007.12.21 View 14606
Professor Jie-Oh Lee awarded 'Scientist of the Year'
Professor Jie-Oh Lee of the Department of Chemistry was awarded the ‘Scientist of the Year’ prize for identifying the three-dimensional structure of protein that causes sepsis, and it was announced by the Korean Science Reporters Association (KOSRA) on November 26th.“Humans have about 30,000 different kinds of proteins, and they all have different structures, just like our faces,” said Professor Lee. “It is extremely helpful to know the three-dimensional shape of proteins when you are trying to understand what their functions in an organism are and trying to develop medicine for them.”
When looking for the three-dimensional structure, protein must first be crystallized and radiated with x-ray, so that reflected x-ray can be interpreted. The three-dimensional structure of sepsis immunity proteins TLR1-TLR2 and TLR4-MD2 could not be found until now because they would not even crystallize.
“I began to doubt if it was even possible to crystallize them because we went through so many failures,” reflected Professor Lee.
In August of last year, after about three years of research, the team finally came up with a new idea. The team decided to ‘stick’ the sepsis immunity protein to protein that easily crystallizes. If the combined structure of sepsis immunity protein and the known protein could be identified, the structure of sepsis immunity protein would be a combined structure subtracted by the known structure.
The three-dimensional structure was obtained with x-ray radiation from combined protein crystal. The combined protein was derived from an insect cell with altered DNA.
“This method seems very simple but no one ever tried it or no one ever succeeded in it,” said Professor Lee.
The result was a horseshoe shaped protein structure. The research team also expects the new protein-combining technology to contribute to the development of a new immune system treatment medicine.
The prize-awarding ceremony was held on November 26th in an event hosted by the Korean Hospital Association.
Also, Professor Ryong Ryoo of the Department of Chemistry was selected as the National Scientist last month.By KAIST Herald on November, 2007
2007.12.21 View 14606 -
 Professor Eunjoon Kim's team finds synapse-forming protein
Professor Eunjoon Kim’s team finds synapse-forming protein
- discover a new protein ‘NGL’ that promotes the formation of neuronal synapses
- can presume the cause of various brain disorders including schizophrenia
- will be published at Nature Neuroscience Vol. 9 in September
A new protein that promotes the formation of synapses in human brains was discovered by a Korean research team.
The team led by Eunjoon Kim, Professor of Department of Biological Sciences and Head of Creative Research Group of Synapse Formation), announced that it had discovered a new fact that NGL protein promotes the formation of neuronal synapses and this fact would be published in Nature Neuroscience Vol. 9 on September 18.
Professor Kim’s team discovered that a membrane protein named ‘NGL’ located at post synapse links with other membrane protein named netrin-G in pre synapse, acting as crosslink, and promotes the formation of a new synapse.
‘NGL’ is the second protein found to crosslink synapse, following neuoroligin. With the discovery of this new protein, the principle of synapse formation and the causes of various brain disorders can be presumed.
In the human brain, about more than 100 billion neuron cells and about 10,000 synapses compose neural circuit. A synapse is the place where innervation occurs between neuron cells. The formation of synapse induces the formation of neural circuit, and neural circuit is deeply related with various brain disorders as well as normal development of brains or brain functions.
“As netrin-G linked with NGL is related with schizonphrenia and neuoroligin and synapse crosslinking protein having a similar function with NGL is deeply related with mental retardation and autism, I think NGL is related with various brain disorders including schizophrenia.”
<Explanation of attached photos>
■ Photo1: Experiment for confirming NGL’s ability to form synapse No. 1
Mix ordinary cell (green) revealing NGL at its surface and neuron cell. Axon grows toward NGL (ordinary cell) located in the middle of ten o’clock direction and meets NGL, where NGL induces the formation of pre synapse (red) in the contacting axon.
Whether pre synapse has been formed can be told by the fluorescent dying (red) of pre synapse protein named Synapsin.
- Figure a-b: formation of synapse by NGL
- Figure c-d: transformed NGL losing synapse forming ability cannot form synapse
■ Photo 2: Experiment for confirming NGL’s ability to form synapse No. 2
When beads coated with NGL are scattered on neuron cell, the beads contact with the axon of the neuron cell (the beads are clearly visible at the phase differentiation image in the middle panel). At this time, NGL induces the formation of pre synapse (red) in the axon. Whether pre synapse has been formed can be told by the fluorescent dying (red) of pre synapse protein named SynPhy (panel a) or VGlut1 (panel b).
2006.09.21 View 16794
Professor Eunjoon Kim's team finds synapse-forming protein
Professor Eunjoon Kim’s team finds synapse-forming protein
- discover a new protein ‘NGL’ that promotes the formation of neuronal synapses
- can presume the cause of various brain disorders including schizophrenia
- will be published at Nature Neuroscience Vol. 9 in September
A new protein that promotes the formation of synapses in human brains was discovered by a Korean research team.
The team led by Eunjoon Kim, Professor of Department of Biological Sciences and Head of Creative Research Group of Synapse Formation), announced that it had discovered a new fact that NGL protein promotes the formation of neuronal synapses and this fact would be published in Nature Neuroscience Vol. 9 on September 18.
Professor Kim’s team discovered that a membrane protein named ‘NGL’ located at post synapse links with other membrane protein named netrin-G in pre synapse, acting as crosslink, and promotes the formation of a new synapse.
‘NGL’ is the second protein found to crosslink synapse, following neuoroligin. With the discovery of this new protein, the principle of synapse formation and the causes of various brain disorders can be presumed.
In the human brain, about more than 100 billion neuron cells and about 10,000 synapses compose neural circuit. A synapse is the place where innervation occurs between neuron cells. The formation of synapse induces the formation of neural circuit, and neural circuit is deeply related with various brain disorders as well as normal development of brains or brain functions.
“As netrin-G linked with NGL is related with schizonphrenia and neuoroligin and synapse crosslinking protein having a similar function with NGL is deeply related with mental retardation and autism, I think NGL is related with various brain disorders including schizophrenia.”
<Explanation of attached photos>
■ Photo1: Experiment for confirming NGL’s ability to form synapse No. 1
Mix ordinary cell (green) revealing NGL at its surface and neuron cell. Axon grows toward NGL (ordinary cell) located in the middle of ten o’clock direction and meets NGL, where NGL induces the formation of pre synapse (red) in the contacting axon.
Whether pre synapse has been formed can be told by the fluorescent dying (red) of pre synapse protein named Synapsin.
- Figure a-b: formation of synapse by NGL
- Figure c-d: transformed NGL losing synapse forming ability cannot form synapse
■ Photo 2: Experiment for confirming NGL’s ability to form synapse No. 2
When beads coated with NGL are scattered on neuron cell, the beads contact with the axon of the neuron cell (the beads are clearly visible at the phase differentiation image in the middle panel). At this time, NGL induces the formation of pre synapse (red) in the axon. Whether pre synapse has been formed can be told by the fluorescent dying (red) of pre synapse protein named SynPhy (panel a) or VGlut1 (panel b).
2006.09.21 View 16794