Chem
-
 A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 12304
A Comprehensive Review of Biosynthesis of Inorganic Nanomaterials Using Microorganisms and Bacteriophages
There are diverse methods for producing numerous inorganic nanomaterials involving many experimental variables. Among the numerous possible matches, finding the best pair for synthesizing in an environmentally friendly way has been a longstanding challenge for researchers and industries.
A KAIST bioprocess engineering research team led by Distinguished Professor Sang Yup Lee conducted a summary of 146 biosynthesized single and multi-element inorganic nanomaterials covering 55 elements in the periodic table synthesized using wild-type and genetically engineered microorganisms. Their research highlights the diverse applications of biogenic nanomaterials and gives strategies for improving the biosynthesis of nanomaterials in terms of their producibility, crystallinity, size, and shape.
The research team described a 10-step flow chart for developing the biosynthesis of inorganic nanomaterials using microorganisms and bacteriophages. The research was published at Nature Review Chemistry as a cover and hero paper on December 3.
“We suggest general strategies for microbial nanomaterial biosynthesis via a step-by-step flow chart and give our perspectives on the future of nanomaterial biosynthesis and applications. This flow chart will serve as a general guide for those wishing to prepare biosynthetic inorganic nanomaterials using microbial cells,” explained Dr.Yoojin Choi, a co-author of this research.
Most inorganic nanomaterials are produced using physical and chemical methods and biological synthesis has been gaining more and more attention. However, conventional synthesis processes have drawbacks in terms of high energy consumption and non-environmentally friendly processes. Meanwhile, microorganisms such as microalgae, yeasts, fungi, bacteria, and even viruses can be utilized as biofactories to produce single and multi-element inorganic nanomaterials under mild conditions.
After conducting a massive survey, the research team summed up that the development of genetically engineered microorganisms with increased inorganic-ion-binding affinity, inorganic-ion-reduction ability, and nanomaterial biosynthetic efficiency has enabled the synthesis of many inorganic nanomaterials.
Among the strategies, the team introduced their analysis of a Pourbaix diagram for controlling the size and morphology of a product. The research team said this Pourbaix diagram analysis can be widely employed for biosynthesizing new nanomaterials with industrial applications.Professor Sang Yup Lee added, “This research provides extensive information and perspectives on the biosynthesis of diverse inorganic nanomaterials using microorganisms and bacteriophages and their applications. We expect that biosynthetic inorganic nanomaterials will find more diverse and innovative applications across diverse fields of science and technology.”
Dr. Choi started this research in 2018 and her interview about completing this extensive research was featured in an article at Nature Career article on December 4.
-ProfileDistinguished Professor Sang Yup Lee leesy@kaist.ac.krMetabolic &Biomolecular Engineering National Research Laboratoryhttp://mbel.kaist.ac.krDepartment of Chemical and Biomolecular EngineeringKAIST
2020.12.07 View 12304 -
 Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 10813
Three Professors Named to Highly Cited Researchers 2020 List
Distinguished Professor Sukbok Chang from the Department of Chemistry, Distinguished Professor Sang-Yup Lee from the Department of Chemical & Biomolecular Engineering, and Professor Jiyong Eom from the College of Business were named to Clarivate’s Highly Cited Researchers 2020 list.
Clarivate announced the researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. A total of 6,167 researchers from more than 60 countries were listed this year and 37 Korean scholars made the list.
The methodology that determines the “Who’s Who” of influential researchers draws on data and analyses performed by bibliometric experts and data scientists at the Institute for Scientific Information at Clarivate. It also uses the tallies to identify the countries and research institutions where these scientific elite are based. More than 6,000 researchers from 21 fields in the sciences, social sciences, and cross field categories were selected based on the number of highly cited papers they produced over an 11-year period from January 2009 to December 2019.
Professor Chang made the list six years in a row, while Professor Lee made it for four consecutive years, and Professor Eom for the last two years. Professor Chang’s group (http://sbchang.kaist.ac.kr) investigates catalytic hydrocarbon functionalization. Professor Lee (http://mbel.kaist.ac.kr) is a pioneering scholar in the field of metabolic engineering, systems, and synthetic biology. Professor Eom’s (https://kaistceps.quv.kr) research extends to energy and environmental economics and management, energy big data, and green information systems.
2020.11.30 View 10813 -
 Researchers Control Multiple Wavelengths of Light from a Single Source
KAIST researchers have synthesized a collection of nanoparticles, known as carbon dots, capable of emitting multiple wavelengths of light from a single particle. Additionally, the team discovered that the dispersion of the carbon dots, or the interparticle distance between each dot, influences the properties of the light the carbon dots emit. The discovery will allow researchers to understand how to control these carbon dots and create new, environmentally responsible displays, lighting, and sensing technology.
Research into nanoparticles capable of emitting light, such as quantum dots, has been an active area of interest for the last decade and a half. These particles, or phosphors, are nanoparticles made out of various materials that are capable of emitting light at specific wavelengths by leveraging quantum mechanical properties of the materials. This provides new ways to develop lighting and display solutions as well as more precise detection and sensing in instruments.
As technology becomes smaller and more sophisticated, the usage of fluorescent nanoparticles has seen a dramatic increase in many applications due to the purity of the colors emitting from the dots as well as their tunability to meet desired optical properties.
Carbon dots, a type of fluorescent nanoparticles, have seen an increase in interest from researchers as a candidate to replace non-carbon dots, the construction of which requires heavy metals that are toxic to the environment. Since they are made up of mostly carbon, the low toxicity is an extremely attractive quality when coupled with the tunability of their inherent optical properties.
Another striking feature of carbon dots is their capability to emit multiple wavelengths of light from a single nanoparticle. This multi-wavelength emission can be stimulated under a single excitation source, enabling the simple and robust generation of white light from a single particle by emitting multiple wavelengths simultaneously.
Carbon dots also exhibit a concentration-dependent photoluminescence. In other words, the distance between individual carbon dots affects the light that the carbon dots subsequently emit under an excitation source. These combined properties make carbon dots a unique source that will result in extremely accurate detection and sensing.
This concentration-dependency, however, had not been fully understood. In order to fully utilize the capabilities of carbon dots, the mechanisms that govern the seemingly variable optical properties must first be uncovered. It was previously theorized that the concentration-dependency of carbon dots was due to a hydrogen bonding effect.
Now, a KAIST research team, led by Professor Do Hyun Kim of the Department of Chemical and Biomolecular Engineering has posited and demonstrated that the dual-color-emissiveness is instead due to the interparticle distances between each carbon dot. This study was made available online in June 2020 ahead of final publication in the 36th Issue of Physical Chemistry Chemical Physics on September 28, 2020.
First author of the paper, PhD candidate Hyo Jeong Yoo, along with Professor Kim and researcher Byeong Eun Kwak, examined how the relative light intensity of the red and blue colors changed when varying the interparticle distances, or concentration, of the carbon dots. They found that as the concentration was adjusted, the light emitted from the carbon dots would transform. By varying the concentration, the team was able to control the relative intensity of the colors, as well as emit them simultaneously to generate a white light from a single source (See Figure).
“The concentration-dependence of the photoluminescence of carbon dots on the change of the emissive origins for different interparticle distances has been overlooked in previous research. With the analysis of the dual-color-emission phenomenon of carbon dots, we believe that this result may provide a new perspective to investigate their photoluminescence mechanism,” Yoo explained.
The newly analyzed ability to control the photoluminescence of carbon dots will likely be heavily utilized in the continued development of solid-state lighting applications and sensing.
Publication:
Yoo, H. J., Kwak, B. E., and Kim. D. H. (2020) Interparticle distance as a key factor for controlling the dual-emission properties of carbon dots. Physical Chemistry Chemical Physics, Issue 36, Pages 20227-20237. Available online at https://doi.org/10.1039/d0cp02120b
Profile:
Do Hyun Kim, Sc.D.
Professor
dokim@kaist.ac.kr
http://procal.kaist.ac.kr/
Process Analysis Laboratory
Department of Chemical and Biomolecular Engineering
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2020.11.23 View 11869
Researchers Control Multiple Wavelengths of Light from a Single Source
KAIST researchers have synthesized a collection of nanoparticles, known as carbon dots, capable of emitting multiple wavelengths of light from a single particle. Additionally, the team discovered that the dispersion of the carbon dots, or the interparticle distance between each dot, influences the properties of the light the carbon dots emit. The discovery will allow researchers to understand how to control these carbon dots and create new, environmentally responsible displays, lighting, and sensing technology.
Research into nanoparticles capable of emitting light, such as quantum dots, has been an active area of interest for the last decade and a half. These particles, or phosphors, are nanoparticles made out of various materials that are capable of emitting light at specific wavelengths by leveraging quantum mechanical properties of the materials. This provides new ways to develop lighting and display solutions as well as more precise detection and sensing in instruments.
As technology becomes smaller and more sophisticated, the usage of fluorescent nanoparticles has seen a dramatic increase in many applications due to the purity of the colors emitting from the dots as well as their tunability to meet desired optical properties.
Carbon dots, a type of fluorescent nanoparticles, have seen an increase in interest from researchers as a candidate to replace non-carbon dots, the construction of which requires heavy metals that are toxic to the environment. Since they are made up of mostly carbon, the low toxicity is an extremely attractive quality when coupled with the tunability of their inherent optical properties.
Another striking feature of carbon dots is their capability to emit multiple wavelengths of light from a single nanoparticle. This multi-wavelength emission can be stimulated under a single excitation source, enabling the simple and robust generation of white light from a single particle by emitting multiple wavelengths simultaneously.
Carbon dots also exhibit a concentration-dependent photoluminescence. In other words, the distance between individual carbon dots affects the light that the carbon dots subsequently emit under an excitation source. These combined properties make carbon dots a unique source that will result in extremely accurate detection and sensing.
This concentration-dependency, however, had not been fully understood. In order to fully utilize the capabilities of carbon dots, the mechanisms that govern the seemingly variable optical properties must first be uncovered. It was previously theorized that the concentration-dependency of carbon dots was due to a hydrogen bonding effect.
Now, a KAIST research team, led by Professor Do Hyun Kim of the Department of Chemical and Biomolecular Engineering has posited and demonstrated that the dual-color-emissiveness is instead due to the interparticle distances between each carbon dot. This study was made available online in June 2020 ahead of final publication in the 36th Issue of Physical Chemistry Chemical Physics on September 28, 2020.
First author of the paper, PhD candidate Hyo Jeong Yoo, along with Professor Kim and researcher Byeong Eun Kwak, examined how the relative light intensity of the red and blue colors changed when varying the interparticle distances, or concentration, of the carbon dots. They found that as the concentration was adjusted, the light emitted from the carbon dots would transform. By varying the concentration, the team was able to control the relative intensity of the colors, as well as emit them simultaneously to generate a white light from a single source (See Figure).
“The concentration-dependence of the photoluminescence of carbon dots on the change of the emissive origins for different interparticle distances has been overlooked in previous research. With the analysis of the dual-color-emission phenomenon of carbon dots, we believe that this result may provide a new perspective to investigate their photoluminescence mechanism,” Yoo explained.
The newly analyzed ability to control the photoluminescence of carbon dots will likely be heavily utilized in the continued development of solid-state lighting applications and sensing.
Publication:
Yoo, H. J., Kwak, B. E., and Kim. D. H. (2020) Interparticle distance as a key factor for controlling the dual-emission properties of carbon dots. Physical Chemistry Chemical Physics, Issue 36, Pages 20227-20237. Available online at https://doi.org/10.1039/d0cp02120b
Profile:
Do Hyun Kim, Sc.D.
Professor
dokim@kaist.ac.kr
http://procal.kaist.ac.kr/
Process Analysis Laboratory
Department of Chemical and Biomolecular Engineering
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
(END)
2020.11.23 View 11869 -
 Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 18155
Biomarker Predicts Who Will Have Severe COVID-19
- Airway cell analyses showing an activated immune axis could pinpoint the COVID-19 patients who will most benefit from targeted therapies.-
KAIST researchers have identified key markers that could help pinpoint patients who are bound to get a severe reaction to COVID-19 infection. This would help doctors provide the right treatments at the right time, potentially saving lives. The findings were published in the journal Frontiers in Immunology on August 28.
People’s immune systems react differently to infection with SARS-CoV-2, the virus that causes COVID-19, ranging from mild to severe, life-threatening responses.
To understand the differences in responses, Professor Heung Kyu Lee and PhD candidate Jang Hyun Park from the Graduate School of Medical Science and Engineering at KAIST analysed ribonucleic acid (RNA) sequencing data extracted from individual airway cells of healthy controls and of mildly and severely ill patients with COVID-19. The data was available in a public database previously published by a group of Chinese researchers.
“Our analyses identified an association between immune cells called neutrophils and special cell receptors that bind to the steroid hormone glucocorticoid,” Professor Lee explained. “This finding could be used as a biomarker for predicting disease severity in patients and thus selecting a targeted therapy that can help treat them at an appropriate time,” he added.
Severe illness in COVID-19 is associated with an exaggerated immune response that leads to excessive airway-damaging inflammation. This condition, known as acute respiratory distress syndrome (ARDS), accounts for 70% of deaths in fatal COVID-19 infections.
Scientists already know that this excessive inflammation involves heightened neutrophil recruitment to the airways, but the detailed mechanisms of this reaction are still unclear.
Lee and Park’s analyses found that a group of immune cells called myeloid cells produced excess amounts of neutrophil-recruiting chemicals in severely ill patients, including a cytokine called tumour necrosis factor (TNF) and a chemokine called CXCL8.
Further RNA analyses of neutrophils in severely ill patients showed they were less able to recruit very important T cells needed for attacking the virus. At the same time, the neutrophils produced too many extracellular molecules that normally trap pathogens, but damage airway cells when produced in excess.
The researchers additionally found that the airway cells in severely ill patients were not expressing enough glucocorticoid receptors. This was correlated with increased CXCL8 expression and neutrophil recruitment.
Glucocorticoids, like the well-known drug dexamethasone, are anti-inflammatory agents that could play a role in treating COVID-19. However, using them in early or mild forms of the infection could suppress the necessary immune reactions to combat the virus. But if airway damage has already happened in more severe cases, glucocorticoid treatment would be ineffective.
Knowing who to give this treatment to and when is really important. COVID-19 patients showing reduced glucocorticoid receptor expression, increased CXCL8 expression, and excess neutrophil recruitment to the airways could benefit from treatment with glucocorticoids to prevent airway damage. Further research is needed, however, to confirm the relationship between glucocorticoids and neutrophil inflammation at the protein level.
“Our study could serve as a springboard towards more accurate and reliable COVID-19 treatments,” Professor Lee said.
This work was supported by the National Research Foundation of Korea, and Mobile Clinic Module Project funded by KAIST.
Figure. Low glucocorticoid receptor (GR) expression led to excessive inflammation and lung damage by neutrophils through enhancing the expression of CXCL8 and other cytokines.
Image credit: Professor Heung Kyu Lee, KAIST. Created with Biorender.com.
Image usage restrictions: News organizations may use or redistribute these figures and image, with proper attribution, as part of news coverage of this paper only.
-Publication:
Jang Hyun Park, and Heung Kyu Lee. (2020). Re-analysis of Single Cell Transcriptome Reveals That the NR3C1-CXCL8-Neutrophil Axis Determines the Severity of COVID-19. Frontiers in Immunology, Available online at https://doi.org/10.3389/fimmu.2020.02145
-Profile: Heung Kyu Lee
Associate Professor
heungkyu.lee@kaist.ac.kr
https://www.heungkyulee.kaist.ac.kr/
Laboratory of Host Defenses
Graduate School of Medical Science and Engineering (GSMSE)
The Center for Epidemic Preparedness at KAIST Institute
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile: Jang Hyun Park
PhD Candidate
janghyun.park@kaist.ac.kr
GSMSE, KAIST
2020.09.17 View 18155 -
 Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 19753
Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 19753 -
 A New Strategy for Early Evaluations of CO2 Utilization Technologies
- A three-step evaluation procedure based on technology readiness levels helps find the most efficient technology before allocating R&D manpower and investments in CO2 utilization technologies. -
Researchers presented a unified framework for early-stage evaluations of a variety of emerging CO2 utilization (CU) technologies. The three-step procedure allows a large number of potential CU technologies to be screened in order to identify the most promising ones, including those at low level of technical maturity, before allocating R&D manpower and investments.
When evaluating new technology, various aspects of the new technology should be considered. Its feasibility, efficiency, economic competitiveness, and environmental friendliness are crucial, and its level of technical maturity is also an important component for further consideration. However, most technology evaluation procedures are data-driven, and the amount of reliable data in the early stages of technology development has been often limited.
A research team led by Professor Jay Hyung Lee from the Department of Chemical and Biomolecular Engineering at KAIST proposed a new procedure for evaluating the early development stages of emerging CU technologies which are applicable at various technology readiness levels (TRLs).
The procedure obtains performance indicators via primary data preparation, secondary data calculation, and performance indicator calculation, and the lead author of the study Dr. Kosan Roh and his colleagues presented a number of databases, methods, and computer-aided tools that can effectively facilitate the procedure.
The research team demonstrated the procedure through four case studies involving novel CU technologies of different types and at various TRLs. They confirmed the electrochemical CO2 reduction for the production of ten chemicals, the co-electrolysis of CO2 and water for ethylene production, the direct oxidation of CO2 -based methanol for oxymethylene dimethyl production, and the microalgal biomass co-firing for power generation.
The expected range of the performance indicators for low TRL technologies is broader than that for high TRL technologies, however, it is not the case for high TRL technologies as they are already at an optimized state. The research team believes that low TRL technologies will be significantly improved through future R&D until they are commercialized. “We plan to develop a systematic approach for such a comparison to help avoid misguided decision-making,” Professor Lee explained.
Professor Lee added, “This procedure allows us to conduct a comprehensive and systematic evaluation of new technology. On top of that, it helps make efficient and reliable assessment possible.”
The research team collaborated with Professor Alexander Mitsos, Professor André Bardow, and Professor Matthias Wessling at RWTH Aachen University in Germany. Their findings were reported in Green Chemistry on May 21. This work was supported by the Korea Carbon Capture and Sequestration R&D Center (KCRC).
Publications:
Roh, K., et al. (2020) ‘Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels’ Green Chemistry. Available online at https://doi.org/10.1039/c9gc04440j
Profile: Jay Hyung Lee, Ph.D.
Professor
jayhlee@kaist.ac.kr
http://lense.kaist.ac.kr/
Laboratory for Energy System Engineering (LENSE)
Department of Chemical and Biomolecular Engineering
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.22 View 11619
A New Strategy for Early Evaluations of CO2 Utilization Technologies
- A three-step evaluation procedure based on technology readiness levels helps find the most efficient technology before allocating R&D manpower and investments in CO2 utilization technologies. -
Researchers presented a unified framework for early-stage evaluations of a variety of emerging CO2 utilization (CU) technologies. The three-step procedure allows a large number of potential CU technologies to be screened in order to identify the most promising ones, including those at low level of technical maturity, before allocating R&D manpower and investments.
When evaluating new technology, various aspects of the new technology should be considered. Its feasibility, efficiency, economic competitiveness, and environmental friendliness are crucial, and its level of technical maturity is also an important component for further consideration. However, most technology evaluation procedures are data-driven, and the amount of reliable data in the early stages of technology development has been often limited.
A research team led by Professor Jay Hyung Lee from the Department of Chemical and Biomolecular Engineering at KAIST proposed a new procedure for evaluating the early development stages of emerging CU technologies which are applicable at various technology readiness levels (TRLs).
The procedure obtains performance indicators via primary data preparation, secondary data calculation, and performance indicator calculation, and the lead author of the study Dr. Kosan Roh and his colleagues presented a number of databases, methods, and computer-aided tools that can effectively facilitate the procedure.
The research team demonstrated the procedure through four case studies involving novel CU technologies of different types and at various TRLs. They confirmed the electrochemical CO2 reduction for the production of ten chemicals, the co-electrolysis of CO2 and water for ethylene production, the direct oxidation of CO2 -based methanol for oxymethylene dimethyl production, and the microalgal biomass co-firing for power generation.
The expected range of the performance indicators for low TRL technologies is broader than that for high TRL technologies, however, it is not the case for high TRL technologies as they are already at an optimized state. The research team believes that low TRL technologies will be significantly improved through future R&D until they are commercialized. “We plan to develop a systematic approach for such a comparison to help avoid misguided decision-making,” Professor Lee explained.
Professor Lee added, “This procedure allows us to conduct a comprehensive and systematic evaluation of new technology. On top of that, it helps make efficient and reliable assessment possible.”
The research team collaborated with Professor Alexander Mitsos, Professor André Bardow, and Professor Matthias Wessling at RWTH Aachen University in Germany. Their findings were reported in Green Chemistry on May 21. This work was supported by the Korea Carbon Capture and Sequestration R&D Center (KCRC).
Publications:
Roh, K., et al. (2020) ‘Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels’ Green Chemistry. Available online at https://doi.org/10.1039/c9gc04440j
Profile: Jay Hyung Lee, Ph.D.
Professor
jayhlee@kaist.ac.kr
http://lense.kaist.ac.kr/
Laboratory for Energy System Engineering (LENSE)
Department of Chemical and Biomolecular Engineering
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.22 View 11619 -
 Visualization of Functional Components to Characterize Optimal Composite Electrodes
Researchers have developed a visualization method that will determine the distribution of components in battery electrodes using atomic force microscopy. The method provides insights into the optimal conditions of composite electrodes and takes us one step closer to being able to manufacture next-generation all-solid-state batteries.
Lithium-ion batteries are widely used in smart devices and vehicles. However, their flammability makes them a safety concern, arising from potential leakage of liquid electrolytes.
All-solid-state lithium ion batteries have emerged as an alternative because of their better safety and wider electrochemical stability. Despite their advantages, all-solid-state lithium ion batteries still have drawbacks such as limited ion conductivity, insufficient contact areas, and high interfacial resistance between the electrode and solid electrolyte.
To solve these issues, studies have been conducted on composite electrodes in which lithium ion conducting additives are dispersed as a medium to provide ion conductive paths at the interface and increase the overall ionic conductivity.
It is very important to identify the shape and distribution of the components used in active materials, ion conductors, binders, and conductive additives on a microscopic scale for significantly improving the battery operation performance.
The developed method is able to distinguish regions of each component based on detected signal sensitivity, by using various modes of atomic force microscopy on a multiscale basis, including electrochemical strain microscopy and lateral force microscopy.
For this research project, both conventional electrodes and composite electrodes were tested, and the results were compared. Individual regions were distinguished and nanoscale correlation between ion reactivity distribution and friction force distribution within a single region was determined to examine the effect of the distribution of binder on the electrochemical strain.
The research team explored the electrochemical strain microscopy amplitude/phase and lateral force microscopy friction force dependence on the AC drive voltage and the tip loading force, and used their sensitivities as markers for each component in the composite anode.
This method allows for direct multiscale observation of the composite electrode in ambient condition, distinguishing various components and measuring their properties simultaneously.
Lead author Dr. Hongjun Kim said, “It is easy to prepare the test sample for observation while providing much higher spatial resolution and intensity resolution for detected signals.” He added, “The method also has the advantage of providing 3D surface morphology information for the observed specimens.”
Professor Seungbum Hong from the Department of Material Sciences and Engineering said, “This analytical technique using atomic force microscopy will be useful for quantitatively understanding what role each component of a composite material plays in the final properties.”
“Our method not only will suggest the new direction for next-generation all-solid-state battery design on a multiscale basis but also lay the groundwork for innovation in the manufacturing process of other electrochemical materials.”
This study is published in ACS Applied Energy Materials and supported by the Big Science Research and Development Project under the Ministry of Science and ICT and the National Research Foundation of Korea, the Basic Research Project under the Wearable Platform Materials Technology Center, and KAIST Global Singularity Research Program for 2019 and 2020.
Publication:Kim, H, et al. (2020) ‘Visualization of Functional Components in a Lithium Silicon Titanium Phosphate-Natural Graphite Composite Anode’. ACS Applied Energy Materials, Volume 3, Issue 4, pp. 3253-3261. Available online at https://doi.org/10.1021/acsaem.9b02045
Profile:
Seungbum Hong
Professor
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Materials Imaging and Integration Laboratory
Department of Material Sciences and Engineering
KAIST
2020.05.22 View 11219
Visualization of Functional Components to Characterize Optimal Composite Electrodes
Researchers have developed a visualization method that will determine the distribution of components in battery electrodes using atomic force microscopy. The method provides insights into the optimal conditions of composite electrodes and takes us one step closer to being able to manufacture next-generation all-solid-state batteries.
Lithium-ion batteries are widely used in smart devices and vehicles. However, their flammability makes them a safety concern, arising from potential leakage of liquid electrolytes.
All-solid-state lithium ion batteries have emerged as an alternative because of their better safety and wider electrochemical stability. Despite their advantages, all-solid-state lithium ion batteries still have drawbacks such as limited ion conductivity, insufficient contact areas, and high interfacial resistance between the electrode and solid electrolyte.
To solve these issues, studies have been conducted on composite electrodes in which lithium ion conducting additives are dispersed as a medium to provide ion conductive paths at the interface and increase the overall ionic conductivity.
It is very important to identify the shape and distribution of the components used in active materials, ion conductors, binders, and conductive additives on a microscopic scale for significantly improving the battery operation performance.
The developed method is able to distinguish regions of each component based on detected signal sensitivity, by using various modes of atomic force microscopy on a multiscale basis, including electrochemical strain microscopy and lateral force microscopy.
For this research project, both conventional electrodes and composite electrodes were tested, and the results were compared. Individual regions were distinguished and nanoscale correlation between ion reactivity distribution and friction force distribution within a single region was determined to examine the effect of the distribution of binder on the electrochemical strain.
The research team explored the electrochemical strain microscopy amplitude/phase and lateral force microscopy friction force dependence on the AC drive voltage and the tip loading force, and used their sensitivities as markers for each component in the composite anode.
This method allows for direct multiscale observation of the composite electrode in ambient condition, distinguishing various components and measuring their properties simultaneously.
Lead author Dr. Hongjun Kim said, “It is easy to prepare the test sample for observation while providing much higher spatial resolution and intensity resolution for detected signals.” He added, “The method also has the advantage of providing 3D surface morphology information for the observed specimens.”
Professor Seungbum Hong from the Department of Material Sciences and Engineering said, “This analytical technique using atomic force microscopy will be useful for quantitatively understanding what role each component of a composite material plays in the final properties.”
“Our method not only will suggest the new direction for next-generation all-solid-state battery design on a multiscale basis but also lay the groundwork for innovation in the manufacturing process of other electrochemical materials.”
This study is published in ACS Applied Energy Materials and supported by the Big Science Research and Development Project under the Ministry of Science and ICT and the National Research Foundation of Korea, the Basic Research Project under the Wearable Platform Materials Technology Center, and KAIST Global Singularity Research Program for 2019 and 2020.
Publication:Kim, H, et al. (2020) ‘Visualization of Functional Components in a Lithium Silicon Titanium Phosphate-Natural Graphite Composite Anode’. ACS Applied Energy Materials, Volume 3, Issue 4, pp. 3253-3261. Available online at https://doi.org/10.1021/acsaem.9b02045
Profile:
Seungbum Hong
Professor
seungbum@kaist.ac.kr
http://mii.kaist.ac.kr/
Materials Imaging and Integration Laboratory
Department of Material Sciences and Engineering
KAIST
2020.05.22 View 11219 -
 Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17583
Simple Molecular Reagents to Treat Alzheimer’s Disease
- Researchers report minimalistic principles for designing small molecules with multiple reactivities against dementia. -
Sometimes the most complex problems actually have very simple solutions. A group of South Korean researchers reported an efficient and effective redox-based strategy for incorporating multiple functions into simple molecular reagents against neurodegenerative disorders. The team developed redox-active aromatic molecular reagents with a simple structural composition that can simultaneously target and modulate various pathogenic factors in complex neurodegenerative disorders such as Alzheimer’s disease.
Alzheimer’s disease is one of the most prevalent neurodegenerative disorders, affecting one in ten people over the age of 65. Early-onset dementia also increasingly affects younger people.
A number of pathogenic elements such as reactive oxygen species, amyloid-beta, and metal ions have been suggested as potential causes of Alzheimer’s disease. Each element itself can lead to Alzheimer’s disease, but interactions between them may also aggravate the patient’s condition or interfere with the appropriate clinical care.
For example, when interacting with amyloid-beta, metal ions foster the aggregation and accumulation of amyloid-beta peptides that can induce oxidative stress and toxicity in the brain and lead to neurodegeneration.
Because these pathogenic factors of Alzheimer’s disease are intertwined, developing therapeutic agents that are capable of simultaneously regulating metal ion dyshomeostasis, amyloid-beta agglutination, and oxidative stress responses remains a key to halting the progression of the disease.
A research team led by Professor Mi Hee Lim from the Department of Chemistry at KAIST demonstrated the feasibility of structure-mechanism-based molecular design for controlling a molecule’s chemical reactivity toward the various pathological factors of Alzheimer’s disease by tuning the redox properties of the molecule.
This study, featured as the ‘ACS Editors’ Choice’ in the May 6th issue of the Journal of the American Chemical Society (JACS), was conducted in conjunction with KAIST Professor Mu-Hyun Baik’s group and Professor Joo-Young Lee’s group at the Asan Medical Center.
Professor Lim and her collaborators rationally designed and generated 10 compact aromatic molecules presenting a range of redox potentials by adjusting the electronic distribution of the phenyl, phenylene, or pyridyl moiety to impart redox-dependent reactivities against the multiple pathogenic factors in Alzheimer’s disease.
During the team’s biochemical and biophysical studies, these designed molecular reagents displayed redox-dependent reactivities against numerous desirable targets that are associated with Alzheimer’s disease such as free radicals, metal-free amyloid-beta, and metal-bound amyloid-beta.
Further mechanistic results revealed that the redox properties of these designed molecular reagents were essential for their function. The team demonstrated that these reagents engaged in oxidative reactions with metal-free and metal-bound amyloid-beta and led to chemical modifications. The products of such oxidative transformations were observed to form covalent adducts with amyloid-beta and alter its aggregation.
Moreover, the administration of the most promising candidate molecule significantly attenuated the amyloid pathology in the brains of Alzheimer’s disease transgenic mice and improved their cognitive defects.
Professor Lim said, “This strategy is straightforward, time-saving, and cost-effective, and its effect is significant. We are excited to help enable the advancement of new therapeutic agents for neurodegenerative disorders, which can improve the lives of so many patients.”
This work was supported by the National Research Foundation (NRF) of Korea, the Institute for Basic Science (IBS), and the Asan Institute for Life Sciences.
Image credit: Professor Mi Hee Lim, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of the news coverage of this paper only.
Publication:
Kim, M. et al. (2020) ‘Minimalistic Principles for Designing Small Molecules with Multiple Reactivities against Pathological Factors in Dementia.’ Journal of the American Chemical Society (JACS), Volume 142, Issue 18, pp.8183-8193. Available online at https://doi.org/10.1021/jacs.9b13100
Profile:
Mi Hee Lim
Professor
miheelim@kaist.ac.kr
http://sites.google.com/site/miheelimlab
Lim Laboratory
Department of Chemistry
KAIST
Profile:
Mu-Hyun Baik
Professor
mbaik2805@kaist.ac.kr
https://baik-laboratory.com/
Baik Laboratory
Department of Chemistry
KAIST
Profile:
Joo-Yong Lee
Professor
jlee@amc.seoul.kr
Asan Institute for Life Sciences
Asan Medical Center
(END)
2020.05.11 View 17583 -
 Researchers Present a Microbial Strain Capable of Massive Succinic Acid Production
A research team led by Distinguished Professor Sang Yup Lee reported the production of a microbial strain capable of the massive production of succinic acid with the highest production efficiency to date. This strategy of integrating systems metabolic engineering with enzyme engineering will be useful for the production of industrially competitive bio-based chemicals. Their strategy was described in Nature Communications on April 23.
The bio-based production of industrial chemicals from renewable non-food biomass has become increasingly important as a sustainable substitute for conventional petroleum-based production processes relying on fossil resources. Here, systems metabolic engineering, which is the key component for biorefinery technology, is utilized to effectively engineer the complex metabolic pathways of microorganisms to enable the efficient production of industrial chemicals.
Succinic acid, a four-carbon dicarboxylic acid, is one of the most promising platform chemicals serving as a precursor for industrially important chemicals. Among microorganisms producing succinic acid, Mannheimia succiniciproducens has been proven to be one of the best strains for succinic acid production.
The research team has developed a bio-based succinic acid production technology using the M. succiniciproducens strain isolated from the rumen of Korean cow for over 20 years and succeeded in developing a strain capable of producing succinic acid with the highest production efficiency.
They carried out systems metabolic engineering to optimize the succinic acid production pathway of the M. succiniciproducens strain by determining the crystal structure of key enzymes important for succinic acid production and performing protein engineering to develop enzymes with better catalytic performance.
As a result, 134 g per liter of succinic acid was produced from the fermentation of an engineered strain using glucose, glycerol, and carbon dioxide. They were able to achieve 21 g per liter per hour of succinic acid production, which is one of the key factors determining the economic feasibility of the overall production process. This is the world’s best succinic acid production efficiency reported to date. Previous production methods averaged 1~3 g per liter per hour.
Distinguished professor Sang Yup Lee explained that his team’s work will significantly contribute to transforming the current petrochemical-based industry into an eco-friendly bio-based one.
“Our research on the highly efficient bio-based production of succinic acid from renewable non-food resources and carbon dioxide has provided a basis for reducing our strong dependence on fossil resources, which is the main cause of the environmental crisis,” Professor Lee said.
This work was supported by the Technology Development Program to Solve Climate Changes via Systems Metabolic Engineering for Biorefineries and the C1 Gas Refinery Program from the Ministry of Science and ICT through the National Research Foundation of Korea.
2020.05.06 View 10784
Researchers Present a Microbial Strain Capable of Massive Succinic Acid Production
A research team led by Distinguished Professor Sang Yup Lee reported the production of a microbial strain capable of the massive production of succinic acid with the highest production efficiency to date. This strategy of integrating systems metabolic engineering with enzyme engineering will be useful for the production of industrially competitive bio-based chemicals. Their strategy was described in Nature Communications on April 23.
The bio-based production of industrial chemicals from renewable non-food biomass has become increasingly important as a sustainable substitute for conventional petroleum-based production processes relying on fossil resources. Here, systems metabolic engineering, which is the key component for biorefinery technology, is utilized to effectively engineer the complex metabolic pathways of microorganisms to enable the efficient production of industrial chemicals.
Succinic acid, a four-carbon dicarboxylic acid, is one of the most promising platform chemicals serving as a precursor for industrially important chemicals. Among microorganisms producing succinic acid, Mannheimia succiniciproducens has been proven to be one of the best strains for succinic acid production.
The research team has developed a bio-based succinic acid production technology using the M. succiniciproducens strain isolated from the rumen of Korean cow for over 20 years and succeeded in developing a strain capable of producing succinic acid with the highest production efficiency.
They carried out systems metabolic engineering to optimize the succinic acid production pathway of the M. succiniciproducens strain by determining the crystal structure of key enzymes important for succinic acid production and performing protein engineering to develop enzymes with better catalytic performance.
As a result, 134 g per liter of succinic acid was produced from the fermentation of an engineered strain using glucose, glycerol, and carbon dioxide. They were able to achieve 21 g per liter per hour of succinic acid production, which is one of the key factors determining the economic feasibility of the overall production process. This is the world’s best succinic acid production efficiency reported to date. Previous production methods averaged 1~3 g per liter per hour.
Distinguished professor Sang Yup Lee explained that his team’s work will significantly contribute to transforming the current petrochemical-based industry into an eco-friendly bio-based one.
“Our research on the highly efficient bio-based production of succinic acid from renewable non-food resources and carbon dioxide has provided a basis for reducing our strong dependence on fossil resources, which is the main cause of the environmental crisis,” Professor Lee said.
This work was supported by the Technology Development Program to Solve Climate Changes via Systems Metabolic Engineering for Biorefineries and the C1 Gas Refinery Program from the Ministry of Science and ICT through the National Research Foundation of Korea.
2020.05.06 View 10784 -
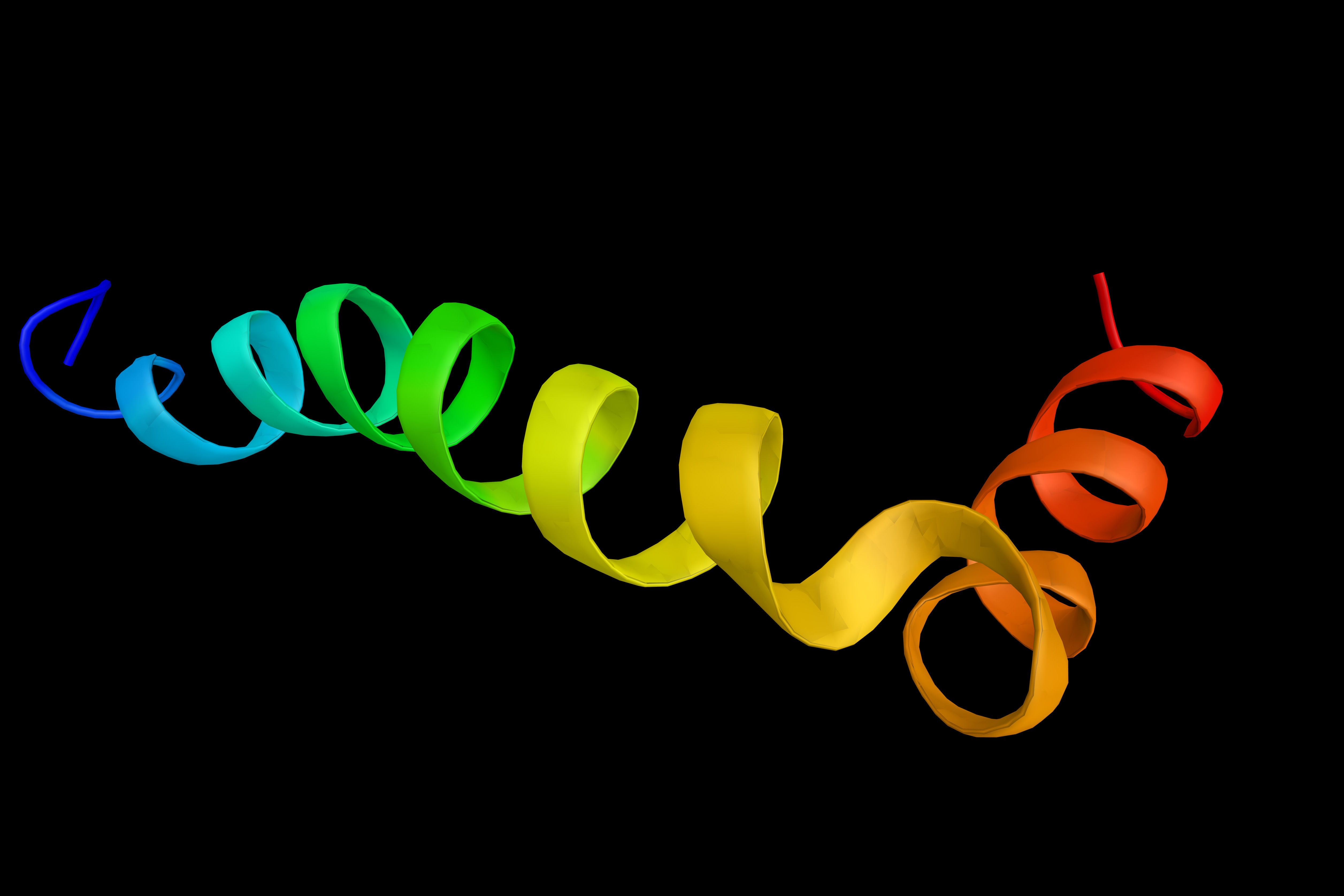 Coordination Chemistry and Alzheimer’s Disease
It has become evident recently that the interactions between copper and amyloid-b neurotoxically impact the brain of patients with Alzheimer’s disease. KAIST researchers have reported a new strategy to alter the neurotoxicity in Alzheimer’s disease by using a rationally designed chemical reagent.
This strategy, developed by Professor Mi Hee Lim from the Department of Chemistry, can modify the coordination sphere of copper bound to amyloid-b, effectively inhibiting copper’s binding to amyloid-b and altering its aggregation and toxicity. Their study was featured in PNAS last month.
The researchers developed a small molecule that is able to directly interact with the coordination sphere of copper–amyloid-b complexes followed by modifications via either covalent conjugation, oxidation, or both under aerobic conditions. The research team simply utilized copper–dioxygen chemistry to design a chemical reagent.
Answering how peptide modifications by a small molecule occur remains very challenging. The system includes transition metals and amyloidogenic proteins and is quite heterogeneous, since they are continuously being changed. It is critical to carefully check the multiple variables such as the presence of dioxygen and the type of transition metal ions and amyloidogenic proteins in order to identify the underlying mechanisms and target specificity of the chemical reagent.
The research team employed various biophysical and biochemical methods to determine the mechanisms for modifications on the coordination sphere of copper–Aꞵ complexes. Among them, peptide modifications were mainly analyzed using electrospray ionization-mass spectrometry.
Mass spectrometry (MS) has been applied to verify such peptide modifications by calculating the shift in exact mass. The research team also performed collision-induced dissociation (CID) of the target ion detected by MS to pinpoint which amino acid residue is specifically modified. The CID fragmentizes the amide bond located between the amino acid residues. This fragmental analysis allows us to identify the specific sites of peptide modifications.
The copper and amyloid-b complexes represent a pathological connection between metal ions and amyloid-b in Alzheimer’s disease. Recent findings indicate that copper and amyloid-b can directly contribute toward neurodegeneration by producing toxic amyloid-b oligomers and reactive oxygen species.
Professor Lim said, “This study illustrates the first experimental evidence that the 14th histidine residue in copper–amyloid-b complexes can be specifically modified through either covalent conjugation, oxidation, or both. Considering the neurotoxic implications of the interactions between copper and amyloid-b, such modifications at the coordination sphere of copper in amyloid-b could effectively alter its properties and toxicity.”
“This multidisciplinary study with an emphasis on approaches, reactivities, and mechanisms looks forward to opening a new way to develop candidates of anti-neurodegenerative diseases,” she added. The National Research Foundation of Korea funded this research.
2020.03.03 View 8472
Coordination Chemistry and Alzheimer’s Disease
It has become evident recently that the interactions between copper and amyloid-b neurotoxically impact the brain of patients with Alzheimer’s disease. KAIST researchers have reported a new strategy to alter the neurotoxicity in Alzheimer’s disease by using a rationally designed chemical reagent.
This strategy, developed by Professor Mi Hee Lim from the Department of Chemistry, can modify the coordination sphere of copper bound to amyloid-b, effectively inhibiting copper’s binding to amyloid-b and altering its aggregation and toxicity. Their study was featured in PNAS last month.
The researchers developed a small molecule that is able to directly interact with the coordination sphere of copper–amyloid-b complexes followed by modifications via either covalent conjugation, oxidation, or both under aerobic conditions. The research team simply utilized copper–dioxygen chemistry to design a chemical reagent.
Answering how peptide modifications by a small molecule occur remains very challenging. The system includes transition metals and amyloidogenic proteins and is quite heterogeneous, since they are continuously being changed. It is critical to carefully check the multiple variables such as the presence of dioxygen and the type of transition metal ions and amyloidogenic proteins in order to identify the underlying mechanisms and target specificity of the chemical reagent.
The research team employed various biophysical and biochemical methods to determine the mechanisms for modifications on the coordination sphere of copper–Aꞵ complexes. Among them, peptide modifications were mainly analyzed using electrospray ionization-mass spectrometry.
Mass spectrometry (MS) has been applied to verify such peptide modifications by calculating the shift in exact mass. The research team also performed collision-induced dissociation (CID) of the target ion detected by MS to pinpoint which amino acid residue is specifically modified. The CID fragmentizes the amide bond located between the amino acid residues. This fragmental analysis allows us to identify the specific sites of peptide modifications.
The copper and amyloid-b complexes represent a pathological connection between metal ions and amyloid-b in Alzheimer’s disease. Recent findings indicate that copper and amyloid-b can directly contribute toward neurodegeneration by producing toxic amyloid-b oligomers and reactive oxygen species.
Professor Lim said, “This study illustrates the first experimental evidence that the 14th histidine residue in copper–amyloid-b complexes can be specifically modified through either covalent conjugation, oxidation, or both. Considering the neurotoxic implications of the interactions between copper and amyloid-b, such modifications at the coordination sphere of copper in amyloid-b could effectively alter its properties and toxicity.”
“This multidisciplinary study with an emphasis on approaches, reactivities, and mechanisms looks forward to opening a new way to develop candidates of anti-neurodegenerative diseases,” she added. The National Research Foundation of Korea funded this research.
2020.03.03 View 8472 -
 New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19126
New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19126 -
 Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 22965
Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 22965