research
-
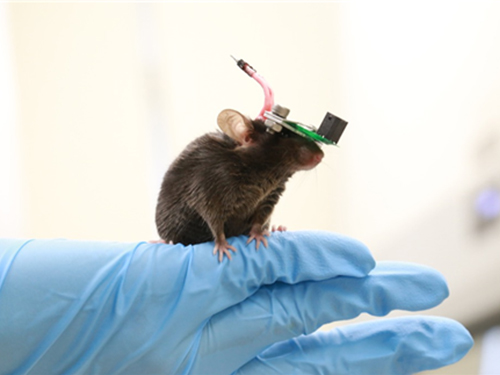 1g-Ultrasound System for the Brain Stimulation of a Freely-moving Mouse
A KAIST research team developed a light-weight capacitive micromachined ultrasonic transducer (CMUT) and succeeded in the ultrasound brain stimulation of a freely-moving mouse. With this lightweight and compact system, researchers can conduct a versatile set of in vivo experiments.
Conventional methods for stimulating a specific brain region, such as deep brain stimulation (DBS) and optogenetics technology, are highly invasive because they have to insert probes into a target brain, which makes them difficult to use for clinical application. While transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) are noninvasive, they have a wide range of stimulation and problems with in-depth stimulation, which makes them problematic for target-specific treatment.
Therefore, noninvasive and focused ultrasound stimulation technology is gaining a great deal of attention as a next-generation brain stimulation alternative. Since it is delivered noninvasively, it can be applied safely in humans as well as animal experiments. Focused ultrasound stimulation is more advantageous than conventional methods in terms of providing both local and deep stimulation.
Animal behavior experiments are essential for brain stimulation research; however, ultrasonic brain stimulation technology is currently in the early stages of development. So far, only research outcomes with fixed anesthetized mice have been studied because of the heavy ultrasonic device.
Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team reported a technology that can provide ultrasound stimulation to the brain of a freely-moving mouse through a microminiaturized ultrasound device.
The team studied miniaturization and ultra-lightweight CMUTs through microelectromechanical systems (MEMS) technology and designed a device suitable for behavior experiments. The device weighing less than 1g (around 0.05% of the mouse’s weight) has the center frequency, size, focal length, and ultrasonic intensity to fit a mouse’s dimensions.
To evaluate the performance of the ultrasonic device, the team stimulated the motor cortex of the mouse brain and observed the movement reaction of its forefoot. They also measured the electromyography (EMG) of the trapezius.
As a result, the team confirmed that their ultrasonic device can deliver ultrasound to a depth of 3-4mm in the mouse brain and stimulate an area of the mouse brain that represents 25% of its total size.
Based on this research, the team is investigating the effects of ultrasound on sleep by stimulating the brain of sleeping mice.
Professor Lee said, “Going beyond experimenting on fixed anesthetized mice, this research succeeded in the brain stimulation of a freely-moving mouse. We are planning to study mice with diseases, such as Parkinson’s disease, dementia, depression, and epilepsy. I believe that this basic research can contribute to treating human brain-related diseases through ultrasound brain stimulation.
This research, led by Masters candidates Hyunggug Kim and Seongyeon Kim, was published in Brain Stimulation (10.1016/j.brs.2018.11.007) on November 17, 2018.
Figure 1. The miniature transducer for the transcranial ultrasound of a freely-moving mouse
Figure 2. Its structure and simulated 2D beam profile in the axial ad radial directions
2019.03.13 View 9606
1g-Ultrasound System for the Brain Stimulation of a Freely-moving Mouse
A KAIST research team developed a light-weight capacitive micromachined ultrasonic transducer (CMUT) and succeeded in the ultrasound brain stimulation of a freely-moving mouse. With this lightweight and compact system, researchers can conduct a versatile set of in vivo experiments.
Conventional methods for stimulating a specific brain region, such as deep brain stimulation (DBS) and optogenetics technology, are highly invasive because they have to insert probes into a target brain, which makes them difficult to use for clinical application. While transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) are noninvasive, they have a wide range of stimulation and problems with in-depth stimulation, which makes them problematic for target-specific treatment.
Therefore, noninvasive and focused ultrasound stimulation technology is gaining a great deal of attention as a next-generation brain stimulation alternative. Since it is delivered noninvasively, it can be applied safely in humans as well as animal experiments. Focused ultrasound stimulation is more advantageous than conventional methods in terms of providing both local and deep stimulation.
Animal behavior experiments are essential for brain stimulation research; however, ultrasonic brain stimulation technology is currently in the early stages of development. So far, only research outcomes with fixed anesthetized mice have been studied because of the heavy ultrasonic device.
Professor Hyunjoo J. Lee from the School of Electrical Engineering and her team reported a technology that can provide ultrasound stimulation to the brain of a freely-moving mouse through a microminiaturized ultrasound device.
The team studied miniaturization and ultra-lightweight CMUTs through microelectromechanical systems (MEMS) technology and designed a device suitable for behavior experiments. The device weighing less than 1g (around 0.05% of the mouse’s weight) has the center frequency, size, focal length, and ultrasonic intensity to fit a mouse’s dimensions.
To evaluate the performance of the ultrasonic device, the team stimulated the motor cortex of the mouse brain and observed the movement reaction of its forefoot. They also measured the electromyography (EMG) of the trapezius.
As a result, the team confirmed that their ultrasonic device can deliver ultrasound to a depth of 3-4mm in the mouse brain and stimulate an area of the mouse brain that represents 25% of its total size.
Based on this research, the team is investigating the effects of ultrasound on sleep by stimulating the brain of sleeping mice.
Professor Lee said, “Going beyond experimenting on fixed anesthetized mice, this research succeeded in the brain stimulation of a freely-moving mouse. We are planning to study mice with diseases, such as Parkinson’s disease, dementia, depression, and epilepsy. I believe that this basic research can contribute to treating human brain-related diseases through ultrasound brain stimulation.
This research, led by Masters candidates Hyunggug Kim and Seongyeon Kim, was published in Brain Stimulation (10.1016/j.brs.2018.11.007) on November 17, 2018.
Figure 1. The miniature transducer for the transcranial ultrasound of a freely-moving mouse
Figure 2. Its structure and simulated 2D beam profile in the axial ad radial directions
2019.03.13 View 9606 -
 Blue-enriched White Light to Wake You Up in the Morning
(from left: Professor Hyun Jung Chung, Professor Hyeon-Jeong Suk, Taesu Kim and Professor Kyungah Choi)
Here is a good news for those of who have difficulty with morning alertness. A KAIST research team proposed that a blue-enriched LED light can effectively help people overcome morning drowsiness. This study will provide the basis for major changes in future lighting strategies and thereby help create better indoor environments.
Considerable research has been devoted to unmasking circadian rhythms. The 2017 Nobel Prize in Physiology or Medicine went to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for unveiling the molecular mechanisms that control circadian rhythms. In particular, the relationship between light and its physiological effects has been investigated since the discovery of a novel, third type of photoreceptor in the human retina in the early 2000s. Rods and cones regulate visual effects, while the third type, photosensitive retinal ganglion cells, regulate a large variety of biological and behavioral processes including melatonin and cortisol secretion, alertness, and functional magnetic resonance imaging (fMRI).
Initial studies on light sources have shown that blue monochromatic, fully saturated lights are effective for stimulating physiological responses, but the relative effectiveness of commercially available white light sources is less well understood. Moreover, the research was more focused on the negative effects of blue light; for instance, when people are exposed to blue light at night, they have trouble achieving deep sleep because the light restrains melatonin secretion.
However, Professor Hyeon-Jeong Suk and Professor Kyungah Choi from the Department of Industrial Design and their team argue that the effects of blue-enriched morning light on physiological responses are time dependent, and that it has positive effects on melatonin levels and the subjective perception of alertness, mood, and visual comfort compared with warm white light.
The team conducted an experiment with 15 university students. They investigated whether an hour of morning light exposure with different chromaticity would affect their physiological and subjective responses differently. The decline of melatonin levels was significantly greater after the exposure to blue-enriched white light in comparison with warm white light.
Professor Suk said, “Light takes a huge part of our lives since we spend most of our time indoors. Light is one of the most powerful tools to affect changes in how we perceive and experience the environment around us.”
Professor Choi added, “When we investigate all of the psychological and physiological effects of light, we see there is much more to light than just efficient quantities. I believe that human-centric lighting strategies could be applied to a variety of environments, including residential areas, learning environments, and working spaces to improve our everyday lives.”
This research was collaborated with Professor Hyun Jung Chung from the Graduate School of Nanoscience and Technology and was published in Scientific Reports (10.1038/s41598-018-36791-5) on January 23, 2019.
Figure 1. Changes in melatonin secretion during day and night time
2019.03.06 View 11883
Blue-enriched White Light to Wake You Up in the Morning
(from left: Professor Hyun Jung Chung, Professor Hyeon-Jeong Suk, Taesu Kim and Professor Kyungah Choi)
Here is a good news for those of who have difficulty with morning alertness. A KAIST research team proposed that a blue-enriched LED light can effectively help people overcome morning drowsiness. This study will provide the basis for major changes in future lighting strategies and thereby help create better indoor environments.
Considerable research has been devoted to unmasking circadian rhythms. The 2017 Nobel Prize in Physiology or Medicine went to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for unveiling the molecular mechanisms that control circadian rhythms. In particular, the relationship between light and its physiological effects has been investigated since the discovery of a novel, third type of photoreceptor in the human retina in the early 2000s. Rods and cones regulate visual effects, while the third type, photosensitive retinal ganglion cells, regulate a large variety of biological and behavioral processes including melatonin and cortisol secretion, alertness, and functional magnetic resonance imaging (fMRI).
Initial studies on light sources have shown that blue monochromatic, fully saturated lights are effective for stimulating physiological responses, but the relative effectiveness of commercially available white light sources is less well understood. Moreover, the research was more focused on the negative effects of blue light; for instance, when people are exposed to blue light at night, they have trouble achieving deep sleep because the light restrains melatonin secretion.
However, Professor Hyeon-Jeong Suk and Professor Kyungah Choi from the Department of Industrial Design and their team argue that the effects of blue-enriched morning light on physiological responses are time dependent, and that it has positive effects on melatonin levels and the subjective perception of alertness, mood, and visual comfort compared with warm white light.
The team conducted an experiment with 15 university students. They investigated whether an hour of morning light exposure with different chromaticity would affect their physiological and subjective responses differently. The decline of melatonin levels was significantly greater after the exposure to blue-enriched white light in comparison with warm white light.
Professor Suk said, “Light takes a huge part of our lives since we spend most of our time indoors. Light is one of the most powerful tools to affect changes in how we perceive and experience the environment around us.”
Professor Choi added, “When we investigate all of the psychological and physiological effects of light, we see there is much more to light than just efficient quantities. I believe that human-centric lighting strategies could be applied to a variety of environments, including residential areas, learning environments, and working spaces to improve our everyday lives.”
This research was collaborated with Professor Hyun Jung Chung from the Graduate School of Nanoscience and Technology and was published in Scientific Reports (10.1038/s41598-018-36791-5) on January 23, 2019.
Figure 1. Changes in melatonin secretion during day and night time
2019.03.06 View 11883 -
 New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7659
New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry)
Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties.
Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry developed a new catalyst capable of selectively synthesizing only one of the two enantiomers. Using this catalyst, the have succeeded in manufacturing the chiral lactam, an essential ingredient in pharmaceuticals, from a hydrocarbon compound.
Enantiomerism or chirality is considered very important for drug development. Biomaterials, such as DNAs and proteins also have chiral properties, but they exhibit different physiological activities depending on the types of drugs. One type of the enantiomer could be useful while the other is toxic. Hence, the technology for selective synthesizing (i.e. asymmetric synthesis) is required, but it is still regarded as a great challenge faced by modern chemistry to date.
The researchers solved this problem by developing a new catalyst. Earlier they presented their research on developing an iridium catalyst that converts hydrocarbons into high value γ-lactam compounds, and published it in Science in March 2018. However, the developed catalyst still had a limitation that both types of enantiomers are obtained without selectivity.
In this study, they found that among dozens of other catalyst candidates, iridium catalysts with chiral diamine scaffolds were able to select the correct enantiomer with a selectivity of 99% or more. This novel catalyst can be used to synthesize the various chiral γ-lactam as required. A left-handed γ-lactam and a right-handed γ-lactam can be produced using a left-handed iridium catalyst and a right-handed iridium catalyst, respectively.
They analyzed the reason for the high selectivity through computational chemistry simulations. They identified that temporal hydrogen bonding occurred between the chiral diamine catalysts and the hydrocarbon compound during the reaction. As a result of the hydrogen bonding, the formation of the left-handed lactam was boosted.
With their new catalyst, they also succeeded in synthesizing chiral lactam compounds with different structures. By using inexpensive and readily available feedstock hydrocarbons, the researchers produced a group of chiral lactams in different shapes. As their chirality and diverse structures enable lactams to function as an active compound in the body for antibiotic, anti-inflammatory, or anti-tumoral functions, this study may facilitate the development of potential drugs in a more efficient and cheaper way.
Professor Chang said, “We hope that our research on selectively producing core units of effective drugs will lead to developing new drugs that demonstrate fewer side-effects and higher efficacy. There are also economic advantages of this research because it uses hydrocarbon compounds, which can be abundantly found in nature, to produce high-value raw materials.
This research was published in Nature Catalysis(10.1038/s41929-019-0230-x) on February 19, 2019.
Figure 1. Asymmetric formation of chiral γ-lactam
Figure 2. Outline of research outcome
2019.03.05 View 7659 -
 KAIST Develops Analog Memristive Synapses for Neuromorphic Chips
(Professor Sung-Yool Choi from the School of Electrical Engineering)
A KAIST research team developed a technology that makes a transition of the operation mode of flexible memristors to synaptic analog switching by reducing the size of the formed filament. Through this technology, memristors can extend their role to memristive synapses for neuromorphic chips, which will lead to developing soft neuromorphic intelligent systems.
Brain-inspired neuromorphic chips have been gaining a great deal of attention for reducing the power consumption and integrating data processing, compared to conventional semiconductor chips. Similarly, memristors are known to be the most suitable candidate for making a crossbar array which is the most efficient architecture for realizing hardware-based artificial neural network (ANN) inside a neuromorphic chip.
A hardware-based ANN consists of a neuron circuit and synapse elements, the connecting pieces. In the neuromorphic system, the synaptic weight, which represents the connection strength between neurons, should be stored and updated as the type of analog data at each synapse.
However, most memristors have digital characteristics suitable for nonvolatile memory. These characteristics put a limitation on the analog operation of the memristors, which makes it difficult to apply them to synaptic devices.
Professor Sung-Yool Choi from the School of Electrical Engineering and his team fabricated a flexible polymer memristor on a plastic substrate, and found that changing the size of the conductive metal filaments formed inside the device on the scale of metal atoms can make a transition of the memristor behavior from digital to analog.
Using this phenomenon, the team developed flexible memristor-based electronic synapses, which can continuously and linearly update synaptic weight, and operate under mechanical deformations such as bending.
The team confirmed that the ANN based on these memristor synapses can effectively classify person’s facial images even when they were damaged. This research demonstrated the possibility of a neuromorphic chip that can efficiently recognize faces, numbers, and objects.
Professor Choi said, “We found the principles underlying the transition from digital to analog operation of the memristors. I believe that this research paves the way for applying various memristors to either digital memory or electronic synapses, and will accelerate the development of a high-performing neuromorphic chip.”
In a joint research project with Professor Sung Gap Im (KAIST) and Professor V. P. Dravid (Northwestern University), this study was led by Dr. Byung Chul Jang (Samsung Electronics), Dr. Sungkyu Kim (Northwestern University) and Dr. Sang Yoon Yang (KAIST), and was published online in Nano Letters (10.1021/acs.nanolett.8b04023) on January 4, 2019.
Figure 1. a) Schematic illustration of a flexible pV3D3 memristor-based electronic synapse array. b) Cross-sectional TEM image of the flexible pV3D3 memristor
2019.02.28 View 9767
KAIST Develops Analog Memristive Synapses for Neuromorphic Chips
(Professor Sung-Yool Choi from the School of Electrical Engineering)
A KAIST research team developed a technology that makes a transition of the operation mode of flexible memristors to synaptic analog switching by reducing the size of the formed filament. Through this technology, memristors can extend their role to memristive synapses for neuromorphic chips, which will lead to developing soft neuromorphic intelligent systems.
Brain-inspired neuromorphic chips have been gaining a great deal of attention for reducing the power consumption and integrating data processing, compared to conventional semiconductor chips. Similarly, memristors are known to be the most suitable candidate for making a crossbar array which is the most efficient architecture for realizing hardware-based artificial neural network (ANN) inside a neuromorphic chip.
A hardware-based ANN consists of a neuron circuit and synapse elements, the connecting pieces. In the neuromorphic system, the synaptic weight, which represents the connection strength between neurons, should be stored and updated as the type of analog data at each synapse.
However, most memristors have digital characteristics suitable for nonvolatile memory. These characteristics put a limitation on the analog operation of the memristors, which makes it difficult to apply them to synaptic devices.
Professor Sung-Yool Choi from the School of Electrical Engineering and his team fabricated a flexible polymer memristor on a plastic substrate, and found that changing the size of the conductive metal filaments formed inside the device on the scale of metal atoms can make a transition of the memristor behavior from digital to analog.
Using this phenomenon, the team developed flexible memristor-based electronic synapses, which can continuously and linearly update synaptic weight, and operate under mechanical deformations such as bending.
The team confirmed that the ANN based on these memristor synapses can effectively classify person’s facial images even when they were damaged. This research demonstrated the possibility of a neuromorphic chip that can efficiently recognize faces, numbers, and objects.
Professor Choi said, “We found the principles underlying the transition from digital to analog operation of the memristors. I believe that this research paves the way for applying various memristors to either digital memory or electronic synapses, and will accelerate the development of a high-performing neuromorphic chip.”
In a joint research project with Professor Sung Gap Im (KAIST) and Professor V. P. Dravid (Northwestern University), this study was led by Dr. Byung Chul Jang (Samsung Electronics), Dr. Sungkyu Kim (Northwestern University) and Dr. Sang Yoon Yang (KAIST), and was published online in Nano Letters (10.1021/acs.nanolett.8b04023) on January 4, 2019.
Figure 1. a) Schematic illustration of a flexible pV3D3 memristor-based electronic synapse array. b) Cross-sectional TEM image of the flexible pV3D3 memristor
2019.02.28 View 9767 -
 Novel Material Properties of Hybrid Perovskite Nanostructures for Next-generation Non-linear Electronic Devices
(from left: Juho Lee, Dr. Muhammad Ejaz Khan and Professor Yong-Hoon Kim)
A KAIST research team reported a novel non-linear device with the founding property coming from perovskite nanowires. They showed that hybrid perovskite-derived, inorganic-framework nanowires can acquire semi-metallicity, and proposed negative differential resistance (NDR) devices with excellent NDR characteristics that resulted from a novel quantum-hybridization NDR mechanism, implying the potential of perovskite nanowires to be realized in next-generation electronic devices.
Organic-inorganic hybrid halide perovskites have recently emerged as prominent candidates for photonic applications due to their excellent optoelectronic properties as well as their low cost and facile synthesis processes. Prominent progresses have been already made for devices including solar cells, light-emitting diodes, lasers and photodetectors.
However, research on electronic devices based on hybrid halide perovskites has not been actively pursued compared with their photonic device counterparts.
Professor Yong-Hoon Kim from the School of Electrical Engineering and his team took a closer look at low-dimensional organic-inorganic halide perovskite materials, which have enhanced quantum confinement effects, and particularly focused on the recently synthesized trimethylsulfonium (TMS) lead triiodide (CH3)3SPbI3.
Using supercomputer simulations, the team first showed that stripping the (CH3)3S or TMS organic ligands from the TMS PbI3 perovskite nanowires results in semi-metallic PbI3 columns, which contradicts the conventional assumption of the semiconducting or insulating characteristics of the inorganic perovskite framework.
Utilizing the semi-metallic PbI3 inorganic framework as the electrode, the team designed a tunneling junction device from perovskite nanowires and found that they exhibit excellent nonlinear negative differential resistance (NDR) behavior. The NDR property is a key to realizing next-generation, ultra-low-power, and multivalued non-linear devices. Furthermore, the team found that this NDR originates from a novel mechanism that involves the quantum-mechanical hybridization between channel and electrode states.
Professor Kim said, “This research demonstrates the potential of quantum mechanics-based computer simulations to lead developments in advanced nanomaterials and nanodevices. In particular, this research proposes a new direction in the development of a quantum mechanical tunneling device, which was the topic for which the Nobel Laureate in Physics in 1973 was awarded to Dr. Leo Esaki.
This research, led by Dr. Muhammad Ejaz Khan and PhD candidate Juho Lee, was published online in Advanced Functional Materials (10.1002/adfm.201807620) on January 7, 2019.
Figure. The draft version of the cover page of 'Advanced Functional Materials'
2019.02.22 View 7428
Novel Material Properties of Hybrid Perovskite Nanostructures for Next-generation Non-linear Electronic Devices
(from left: Juho Lee, Dr. Muhammad Ejaz Khan and Professor Yong-Hoon Kim)
A KAIST research team reported a novel non-linear device with the founding property coming from perovskite nanowires. They showed that hybrid perovskite-derived, inorganic-framework nanowires can acquire semi-metallicity, and proposed negative differential resistance (NDR) devices with excellent NDR characteristics that resulted from a novel quantum-hybridization NDR mechanism, implying the potential of perovskite nanowires to be realized in next-generation electronic devices.
Organic-inorganic hybrid halide perovskites have recently emerged as prominent candidates for photonic applications due to their excellent optoelectronic properties as well as their low cost and facile synthesis processes. Prominent progresses have been already made for devices including solar cells, light-emitting diodes, lasers and photodetectors.
However, research on electronic devices based on hybrid halide perovskites has not been actively pursued compared with their photonic device counterparts.
Professor Yong-Hoon Kim from the School of Electrical Engineering and his team took a closer look at low-dimensional organic-inorganic halide perovskite materials, which have enhanced quantum confinement effects, and particularly focused on the recently synthesized trimethylsulfonium (TMS) lead triiodide (CH3)3SPbI3.
Using supercomputer simulations, the team first showed that stripping the (CH3)3S or TMS organic ligands from the TMS PbI3 perovskite nanowires results in semi-metallic PbI3 columns, which contradicts the conventional assumption of the semiconducting or insulating characteristics of the inorganic perovskite framework.
Utilizing the semi-metallic PbI3 inorganic framework as the electrode, the team designed a tunneling junction device from perovskite nanowires and found that they exhibit excellent nonlinear negative differential resistance (NDR) behavior. The NDR property is a key to realizing next-generation, ultra-low-power, and multivalued non-linear devices. Furthermore, the team found that this NDR originates from a novel mechanism that involves the quantum-mechanical hybridization between channel and electrode states.
Professor Kim said, “This research demonstrates the potential of quantum mechanics-based computer simulations to lead developments in advanced nanomaterials and nanodevices. In particular, this research proposes a new direction in the development of a quantum mechanical tunneling device, which was the topic for which the Nobel Laureate in Physics in 1973 was awarded to Dr. Leo Esaki.
This research, led by Dr. Muhammad Ejaz Khan and PhD candidate Juho Lee, was published online in Advanced Functional Materials (10.1002/adfm.201807620) on January 7, 2019.
Figure. The draft version of the cover page of 'Advanced Functional Materials'
2019.02.22 View 7428 -
 New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 8197
New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu)
A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2.
LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way.
However, LSBs face the obstacle of having a capacity reaching its theoretical maximum because they are prone to uncontrolled growth of lithium sulfide on the electrodes, which leads to blocking electron transfer.
To address the problem of electrode passivation, researchers introduced additional conductive agent into the electrode; however, it drastically lowered the energy density of LSBs, making it difficult to exceed 70% of the theoretical capacity.
Professor Hee-Tak Kim from the Department of Chemical and Biomolecular Engineering and his team replaced the lithium salt anions used in conventional LSB electrolytes with anions with a high donor number. The team successfully induced the three-dimensional growth of lithium sulfide on electrode surfaces and efficiently delayed the electrode passivation.
Based on this electrolyte design, the research team achieved 92% of the theoretical capacity with their high-capacity sulfur electrode (4mAh/cm2), which is equivalent to that of conventional LIB cathode. Furthermore, they were able to form a stable passivation film on the surface of the lithium anode and exhibited stable operation over 100 cycles.
This technology, which can be flexibly used with various types of sulfur electrodes, can mark a new milestone in the battery industry.
Professor Kim said, “We proposed a new physiochemical principle to overcome the limitations of conventional LSBs. I believe our achievement of obtaining 90% of the LBSs’ theoretical capacity without any capacity loss after 100 cycles will become a new milestone.”
This research, first-authored by Hyunwon Chu and Hyungjun Noh, was published in Nature Communications on January 14, 2019. It was also selected in the editor’s highlight for its outstanding achievements.
Figure 1. Lithium sulfur growth and its deposition mechanism for different sulfide growth behaviors
Figure 2. Capacity and cycle life characteristics of the LSBs
2019.02.11 View 8197 -
 KAIST Develops Core Technology for Ultra-small 3D Image Sensor
(from left: Dr. Jong-Bum Yo, PhD candidate Seong-Hwan Kimand Professor Hyo-Hoon Park)
A KAIST research team developed a silicon optical phased array (OPA) chip, which can be a core component for three-dimensional image sensors. This research was co-led by PhD candidate Seong-Hwan Kim and Dr. Jong-Bum You from the National Nanofab Center (NNFC).
A 3D image sensor adds distance information to a two-dimensional image, such as a photo, to recognize it as a 3D image. It plays a vital role in various electronics including autonomous vehicles, drones, robots, and facial recognition systems, which require accurate measurement of the distance from objects.
Many automobile and drone companies are focusing on developing 3D image sensor systems, based on mechanical light detection and ranging (LiDAR) systems. However, it can only get as small as the size of a fist and has a high possibility of malfunctioning because it employs a mechanical method for laser beam-steering.
OPAs have gained a great attention as a key component to implement solid-state LiDAR because it can control the light direction electronically without moving parts. Silicon-based OPAs are small, durable, and can be mass-produced through conventional Si-CMOS processes.
However, in the development of OPAs, a big issue has been raised about how to achieve wide beam-steering in transversal and longitudinal directions. In the transversal direction, a wide beam-steering has been implemented, relatively easily, through a thermo-optic or electro-optic control of the phase shifters integrated with a 1D array. But the longitudinal beam-steering has been remaining as a technical challenge since only a narrow steering was possible with the same 1D array by changing the wavelengths of light, which is hard to implement in semiconductor processes.
If a light wavelength is changed, characteristics of element devices consisting the OPA can vary, which makes it difficult to control the light direction with reliability as well as to integrate a wavelength-tunable laser on a silicon-based chip. Therefore, it is essential to devise a new structure that can easily adjust the radiated light in both transversal and longitudinal directions.
By integrating tunable radiator, instead of tunable laser in a conventional OPA, Professor Hyo-Hoon Park from the School of Electrical Engineering and his team developed an ultra-small, low-power OPA chip that facilitates a wide 2D beam-steering with a monochromatic light source.
This OPA structure allows the minimizing of the 3D image sensors, as small as a dragonfly’s eye.
According to the team, the OPA can function as a 3D image sensor and also as a wireless transmitter sending the image data to a desired direction, enabling high-quality image data to be freely communicated between electronic devices.
Kim said, “It’s not an easy task to integrate a tunable light source in the OPA structures of previous works. We hope our research proposing a tunable radiator makes a big step towards commercializing OPAs.”
Dr. You added, “We will be able to support application researches of 3D image sensors, especially for facial recognition with smartphones and augmented reality services. We will try to prepare a processing platform in NNFC that provides core technologies of the 3D image sensor fabrication.”
This research was published in Optics Letters on January 15.
Figure 1.The manufactured OPA chip
Figure 2. Schematic feature showing an application of the OPA to a 3D image sensor
2019.02.08 View 7168
KAIST Develops Core Technology for Ultra-small 3D Image Sensor
(from left: Dr. Jong-Bum Yo, PhD candidate Seong-Hwan Kimand Professor Hyo-Hoon Park)
A KAIST research team developed a silicon optical phased array (OPA) chip, which can be a core component for three-dimensional image sensors. This research was co-led by PhD candidate Seong-Hwan Kim and Dr. Jong-Bum You from the National Nanofab Center (NNFC).
A 3D image sensor adds distance information to a two-dimensional image, such as a photo, to recognize it as a 3D image. It plays a vital role in various electronics including autonomous vehicles, drones, robots, and facial recognition systems, which require accurate measurement of the distance from objects.
Many automobile and drone companies are focusing on developing 3D image sensor systems, based on mechanical light detection and ranging (LiDAR) systems. However, it can only get as small as the size of a fist and has a high possibility of malfunctioning because it employs a mechanical method for laser beam-steering.
OPAs have gained a great attention as a key component to implement solid-state LiDAR because it can control the light direction electronically without moving parts. Silicon-based OPAs are small, durable, and can be mass-produced through conventional Si-CMOS processes.
However, in the development of OPAs, a big issue has been raised about how to achieve wide beam-steering in transversal and longitudinal directions. In the transversal direction, a wide beam-steering has been implemented, relatively easily, through a thermo-optic or electro-optic control of the phase shifters integrated with a 1D array. But the longitudinal beam-steering has been remaining as a technical challenge since only a narrow steering was possible with the same 1D array by changing the wavelengths of light, which is hard to implement in semiconductor processes.
If a light wavelength is changed, characteristics of element devices consisting the OPA can vary, which makes it difficult to control the light direction with reliability as well as to integrate a wavelength-tunable laser on a silicon-based chip. Therefore, it is essential to devise a new structure that can easily adjust the radiated light in both transversal and longitudinal directions.
By integrating tunable radiator, instead of tunable laser in a conventional OPA, Professor Hyo-Hoon Park from the School of Electrical Engineering and his team developed an ultra-small, low-power OPA chip that facilitates a wide 2D beam-steering with a monochromatic light source.
This OPA structure allows the minimizing of the 3D image sensors, as small as a dragonfly’s eye.
According to the team, the OPA can function as a 3D image sensor and also as a wireless transmitter sending the image data to a desired direction, enabling high-quality image data to be freely communicated between electronic devices.
Kim said, “It’s not an easy task to integrate a tunable light source in the OPA structures of previous works. We hope our research proposing a tunable radiator makes a big step towards commercializing OPAs.”
Dr. You added, “We will be able to support application researches of 3D image sensors, especially for facial recognition with smartphones and augmented reality services. We will try to prepare a processing platform in NNFC that provides core technologies of the 3D image sensor fabrication.”
This research was published in Optics Letters on January 15.
Figure 1.The manufactured OPA chip
Figure 2. Schematic feature showing an application of the OPA to a 3D image sensor
2019.02.08 View 7168 -
 Stretchable Multi-functional Fiber for Energy Harvesting and Strain Sensing
(from left: Professor Steve Park, Jeongjae Ryu and Professor Seungbum Hong)
Fiber-based electronics are expected to play a vital role in next-generation wearable electronics. Woven into textiles, they can provide higher durability, comfort, and integrated multi-functionality. A KAIST team has developed a stretchable multi-functional fiber (SMF) that can harvest energy and detect strain, which can be applied to future wearable electronics.
With wearable electronics, health and physical conditions can be assessed by analyzing biological signals from the human body, such as pulse and muscle movements. Fibers are highly suitable for future wearable electronics because they can be easily integrated into textiles, which are designed to be conformable to curvilinear surfaces and comfortable to wear. Moreover, their weave structures offer support that makes them resistant to fatigue. Many research groups have developed fiber-based strain sensors to sense external biological signals. However, their sensitivities were relatively low.
The applicability of wearable devices is currently limited by their power source, as the size, weight, and lifetime of the battery lessens their versatility. Harvesting mechanical energy from the human body is a promising solution to overcome such limitations by utilizing various types of motions like bending, stretching, and pressing. However, previously reported, fiber-based energy harvesters were not stretchable and could not fully harvest the available mechanical energy.
Professor Seungbum Hong and Professor Steve Park from the Department of Materials Science and Engineering and their team fabricated a stretchable fiber by using a ferroelectric layer composed of P(VDF-TrFE)/PDMS sandwiched between stretchable electrodes composed of a composite of multi-walled carbon nanotubes (MWCNT) and poly 3,4-ethylenedioxythiophene polystyrenesulfonate (PEDOT:PSS).
Cracks formed in MWCNT/PEDOT:PSS layer help the fiber show high sensitivity compared to the previously reported fiber strain sensors. Furthermore, the new fiber can harvest mechanical energy under various mechanical stimuli such as stretching, tapping, and injecting water into the fiber using the piezoelectric effect of the P(VDF-TrFE)/PDMS layer.
Professor Hong said, “This new fiber has various functionalities and makes the device simple and compact. It is a core technology for developing wearable devices with energy harvesting and strain sensing capabilities.”
This article, led by PhD candidate Jeongjae Ryu, was published in the January 2019 issue of Nano Energy.
Figure 1.Schematic illustration of an SMF fiber and its piezoelectric voltage output and response to strain.
Figure 2. Photographs of a stretchable multi-functional fiber being stretched by 100%, bent, and twisted.
2019.01.31 View 9027
Stretchable Multi-functional Fiber for Energy Harvesting and Strain Sensing
(from left: Professor Steve Park, Jeongjae Ryu and Professor Seungbum Hong)
Fiber-based electronics are expected to play a vital role in next-generation wearable electronics. Woven into textiles, they can provide higher durability, comfort, and integrated multi-functionality. A KAIST team has developed a stretchable multi-functional fiber (SMF) that can harvest energy and detect strain, which can be applied to future wearable electronics.
With wearable electronics, health and physical conditions can be assessed by analyzing biological signals from the human body, such as pulse and muscle movements. Fibers are highly suitable for future wearable electronics because they can be easily integrated into textiles, which are designed to be conformable to curvilinear surfaces and comfortable to wear. Moreover, their weave structures offer support that makes them resistant to fatigue. Many research groups have developed fiber-based strain sensors to sense external biological signals. However, their sensitivities were relatively low.
The applicability of wearable devices is currently limited by their power source, as the size, weight, and lifetime of the battery lessens their versatility. Harvesting mechanical energy from the human body is a promising solution to overcome such limitations by utilizing various types of motions like bending, stretching, and pressing. However, previously reported, fiber-based energy harvesters were not stretchable and could not fully harvest the available mechanical energy.
Professor Seungbum Hong and Professor Steve Park from the Department of Materials Science and Engineering and their team fabricated a stretchable fiber by using a ferroelectric layer composed of P(VDF-TrFE)/PDMS sandwiched between stretchable electrodes composed of a composite of multi-walled carbon nanotubes (MWCNT) and poly 3,4-ethylenedioxythiophene polystyrenesulfonate (PEDOT:PSS).
Cracks formed in MWCNT/PEDOT:PSS layer help the fiber show high sensitivity compared to the previously reported fiber strain sensors. Furthermore, the new fiber can harvest mechanical energy under various mechanical stimuli such as stretching, tapping, and injecting water into the fiber using the piezoelectric effect of the P(VDF-TrFE)/PDMS layer.
Professor Hong said, “This new fiber has various functionalities and makes the device simple and compact. It is a core technology for developing wearable devices with energy harvesting and strain sensing capabilities.”
This article, led by PhD candidate Jeongjae Ryu, was published in the January 2019 issue of Nano Energy.
Figure 1.Schematic illustration of an SMF fiber and its piezoelectric voltage output and response to strain.
Figure 2. Photographs of a stretchable multi-functional fiber being stretched by 100%, bent, and twisted.
2019.01.31 View 9027 -
 Hierarchical Porous Titanium Nitride Synthesized by Multiscale Phase Separation for LSBs
(from left: Professor Jinwoo Lee and PhD candidate Won-Gwang Lim)
A KAIST research team developed ultra-stable, high-rate lithium-sulfur batteries (LSBs) by using hierarchical porous titanium nitride as a sulfur host, and achieved superior cycle stability and high rate performance for LSBs.
The control of large amounts of energy is required for use in an electric vehicle or smart grid system. In this sense, the development of next-generation secondary batteries is in high demand. Theoretically, LSBs have an energy density seven times higher than commercial lithium ion batteries (LIBs). Also, their production cost can be reduced dramatically since sulfur can be obtained at a low price.
Despite these positive aspects, there have been several issues impeding the commercialization of LSBs, such as the low electric conductivity of sulfur, the dissolution of active materials during operation, and sluggish conversion reactions. These issues decrease the cycle stability and rate capability of batteries.
To tackle those issues, Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering and his team synthesized a well-developed hierarchical macro/mesoporous titanium nitride as a host material for sulfur. The titanium nitride has a high chemical affinity for sulfur and high electrical conductivity. As a result, it prevents the dissolution of active materials and facilitates the charge transfer. Moreover, the synergistic effect of macropore and mesopore structures allows the stable accommodation of large amounts of sulfur and facilitates the electrolyte penetration.
Previously reported polar inorganic materials have a high affinity for sulfur, but it was challenging to control the porous architecture suitable to the sulfur host. This work breaks such limitations by developing a synthetic route to easily control the porous architecture of inorganic materials, which led to obtaining superior cycle stability and high rate capabilities.
Professor Lee said, “Some problems still remain in commercializing LSBs as next-generation batteries. Hence, there should be a continued research on this matter to solve the issues. Through this research, we secured a key technology for ultrastable, high-rate LSBs.”
This research was led by PhD candidate Won-Gwang Lim and collaborated on by Jeong Woo Han from POSTECH. It was chosen as the cover article of Advanced Materials on January 15, 2019.
Figure 1. Schematic illustration for the synthetic route of co-continuous h-TiN
Figure 2. The hierarchical multiscale porous structure is still retained without any collapse after the conversion to h-TiN. The good retention of the porous structure is attributed to the thick pore wall of the h-TiO₂derived from the block copolymer self-assembly
Figure 3. The cover page of Advanced Materials
2019.01.28 View 7457
Hierarchical Porous Titanium Nitride Synthesized by Multiscale Phase Separation for LSBs
(from left: Professor Jinwoo Lee and PhD candidate Won-Gwang Lim)
A KAIST research team developed ultra-stable, high-rate lithium-sulfur batteries (LSBs) by using hierarchical porous titanium nitride as a sulfur host, and achieved superior cycle stability and high rate performance for LSBs.
The control of large amounts of energy is required for use in an electric vehicle or smart grid system. In this sense, the development of next-generation secondary batteries is in high demand. Theoretically, LSBs have an energy density seven times higher than commercial lithium ion batteries (LIBs). Also, their production cost can be reduced dramatically since sulfur can be obtained at a low price.
Despite these positive aspects, there have been several issues impeding the commercialization of LSBs, such as the low electric conductivity of sulfur, the dissolution of active materials during operation, and sluggish conversion reactions. These issues decrease the cycle stability and rate capability of batteries.
To tackle those issues, Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering and his team synthesized a well-developed hierarchical macro/mesoporous titanium nitride as a host material for sulfur. The titanium nitride has a high chemical affinity for sulfur and high electrical conductivity. As a result, it prevents the dissolution of active materials and facilitates the charge transfer. Moreover, the synergistic effect of macropore and mesopore structures allows the stable accommodation of large amounts of sulfur and facilitates the electrolyte penetration.
Previously reported polar inorganic materials have a high affinity for sulfur, but it was challenging to control the porous architecture suitable to the sulfur host. This work breaks such limitations by developing a synthetic route to easily control the porous architecture of inorganic materials, which led to obtaining superior cycle stability and high rate capabilities.
Professor Lee said, “Some problems still remain in commercializing LSBs as next-generation batteries. Hence, there should be a continued research on this matter to solve the issues. Through this research, we secured a key technology for ultrastable, high-rate LSBs.”
This research was led by PhD candidate Won-Gwang Lim and collaborated on by Jeong Woo Han from POSTECH. It was chosen as the cover article of Advanced Materials on January 15, 2019.
Figure 1. Schematic illustration for the synthetic route of co-continuous h-TiN
Figure 2. The hierarchical multiscale porous structure is still retained without any collapse after the conversion to h-TiN. The good retention of the porous structure is attributed to the thick pore wall of the h-TiO₂derived from the block copolymer self-assembly
Figure 3. The cover page of Advanced Materials
2019.01.28 View 7457 -
 A Novel Material for Transparent and Flexible Displays
(Research team led by Professor Sang Youl Kim from the Department of Chemistry)
The next generation of flexible and transparent displays will require a high-performing and flexible polymeric material that has the optical and thermal properties of glass. The material must be transparent to visible light and have a low coefficient of thermal expansion (CTE). Unfortunately, such a polymeric material has not been available. A KAIST research team has succeeded in making a new polymeric material with an exceptionally low CTE value while retaining high transparency and excellent thermal and mechanical properties. The method developed for amorphous polymers with a controlled CTE can be applied to control the thermal expansion of organic materials as well.
Most of objects expands upon heating and shrinks by cooling, and organic polymers have a relatively large CTE compared to that of ceramics or metals. Thin, light-weight planar substrates for semiconductor devices should have a similar CTE of ceramics. Otherwise, the device can be cracked due to the stress caused by thermal expansion and contraction. Therefore, matching the CTE of the semiconductor device and the substrate is crucial for successful manufacturing of display devices. Forming a network structure by connecting polymer chains is a well-known method of reducing the CTE of amorphous polymers. However, polymers with a network structure eventually lose their flexibility and becomes brittle.
As an alternative method, Professor Sang Youl Kim from the Department of Chemistry and his team chose to adjust the distance and interaction between polymer chains. Thermal expansion and contraction of polymer films can be minimized by introducing interaction forces between the polymer chains and by arranging the direction of the force perpendicularly. The team successfully implemented this approach by appropriately designing the chemical structure of a transparent polymeric material. It is called poly (amide-imide) film, which is a transparent, flexible, and high-performing polymeric material. It is thermally stable enough to be used in the AMOLED (active-matrix organic light-emitting diode) fabrication process (stable at >400℃) with a low CTE (4ppm/℃).
The team made IGZO TFT (Indium Gallium Zinc Oxide Thin Film Transistor) devices on the newly synthesized transparent poly(amide-imide) film, and confirmed that the device could indeed operate normally even when it is folded down to a radius of 1mm.
Professor Kim said, “Our results suggest a way of controlling the thermal expansion of amorphous polymers similar to a level of glass without chemical cross-linking, which has long been regarded as a challenging problem. At the same time, we succeeded in making the polymer transparent and flexible. We expect that it can be applied to controlling the thermal expansion of various organic materials.”
This research, led by researchers Sun Dal Kim and Byungyoung Lee, was published in Science Advances on October 26. (DOI: 10.1126/sciadv.aau1956v)
2019.01.24 View 6707
A Novel Material for Transparent and Flexible Displays
(Research team led by Professor Sang Youl Kim from the Department of Chemistry)
The next generation of flexible and transparent displays will require a high-performing and flexible polymeric material that has the optical and thermal properties of glass. The material must be transparent to visible light and have a low coefficient of thermal expansion (CTE). Unfortunately, such a polymeric material has not been available. A KAIST research team has succeeded in making a new polymeric material with an exceptionally low CTE value while retaining high transparency and excellent thermal and mechanical properties. The method developed for amorphous polymers with a controlled CTE can be applied to control the thermal expansion of organic materials as well.
Most of objects expands upon heating and shrinks by cooling, and organic polymers have a relatively large CTE compared to that of ceramics or metals. Thin, light-weight planar substrates for semiconductor devices should have a similar CTE of ceramics. Otherwise, the device can be cracked due to the stress caused by thermal expansion and contraction. Therefore, matching the CTE of the semiconductor device and the substrate is crucial for successful manufacturing of display devices. Forming a network structure by connecting polymer chains is a well-known method of reducing the CTE of amorphous polymers. However, polymers with a network structure eventually lose their flexibility and becomes brittle.
As an alternative method, Professor Sang Youl Kim from the Department of Chemistry and his team chose to adjust the distance and interaction between polymer chains. Thermal expansion and contraction of polymer films can be minimized by introducing interaction forces between the polymer chains and by arranging the direction of the force perpendicularly. The team successfully implemented this approach by appropriately designing the chemical structure of a transparent polymeric material. It is called poly (amide-imide) film, which is a transparent, flexible, and high-performing polymeric material. It is thermally stable enough to be used in the AMOLED (active-matrix organic light-emitting diode) fabrication process (stable at >400℃) with a low CTE (4ppm/℃).
The team made IGZO TFT (Indium Gallium Zinc Oxide Thin Film Transistor) devices on the newly synthesized transparent poly(amide-imide) film, and confirmed that the device could indeed operate normally even when it is folded down to a radius of 1mm.
Professor Kim said, “Our results suggest a way of controlling the thermal expansion of amorphous polymers similar to a level of glass without chemical cross-linking, which has long been regarded as a challenging problem. At the same time, we succeeded in making the polymer transparent and flexible. We expect that it can be applied to controlling the thermal expansion of various organic materials.”
This research, led by researchers Sun Dal Kim and Byungyoung Lee, was published in Science Advances on October 26. (DOI: 10.1126/sciadv.aau1956v)
2019.01.24 View 6707 -
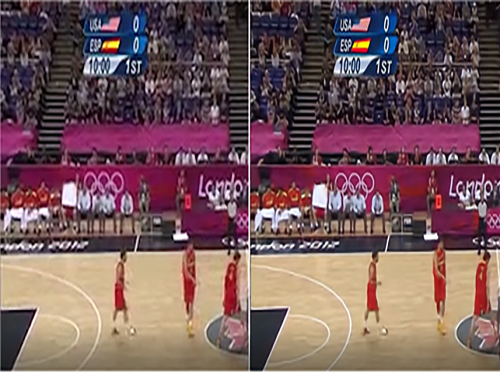 Enhanced Video Quality despite Poor Network Conditions
(from left: Jaehong Kim, Youngmok Jung, Hyunho Yeo, Professor Dongsu Han and Professor Jinwoo Shin)
Professor Jinwoo Shin and Professor Dongsu Han from the School of Electrical Engineering developed neural adaptive content-aware internet video delivery. This technology is a novel method that combines adaptive streaming over HTTP, the video transmission system adopted by YouTube and Netflix, with a deep learning model.
This technology is expected to create an internet environment where users can enjoy watching 4K and AV/VR videos with high-quality and high-definition (HD) videos even with weak internet connections.
Thanks to video streaming services, internet video has experienced remarkable growth; nevertheless, users often suffer from low video quality due to unfavorable network conditions. Currently, existing adaptive streaming systems adjust the quality of the video in real time, accommodating the continuously changing internet bandwidth. Various algorithms are being researched for adaptive streaming systems, but there is an inherent limitation; that is, high-quality videos cannot be streamed in poor network environments regardless of which algorithm is used.
By incorporating super-resolution in adaptive streaming, the team overcame the limit of existing content distribution networks, of which their quality relies too much on the bandwidth. In the conventional method, the server that provides the video splits a video into certain lengths of time in advance. But the novel system introduced by the team allows the downloading of neural network segments. To facilitate this method, the video server needs to provide deep neural networks for each video segment as well as sizes of Deep Neural Networks (DNN) according to the specifications of the user’s computing capacity.
The largest neural network size is two megabytes, which is considerably smaller than video. When downloading the neural network from the user’s video player, it is split into several segments. Even its partial download is sufficient for a slightly comprised super-resolution.
While playing the video, the system converts the low quality video to a high-quality version by employing super-resolution based on deep convolution neural networks (CNN). The entire process is done in real time, and users can enjoy the high-definition video.
Even with a 17% smaller bandwidth, the system can provide the Quality of Experience equivalent to the latest adaptive streaming service. At a given internet bandwidth, it can provide 43% higher average QoE than the latest service.
Using a deep learning method allows this system to achieve a higher level of compression than the existing video compression methods. Their technology was recognized as a next-generation internet video system that applies super-resolution based on a deep convolution neural network to online videos.
Professor Han said, “So far, it has only been implemented on desktops, but we will further develop applications that work in mobile devices as well. This technology has been applied to the same video transmission systems used by streaming channels such as YouTube and Netflix, and thus shows good signs for practicability.”
This research, led by Hyunho Yeo, Youngmok Jung and Jaehong Kim, was presented at the 13th UNSENIX OSDI conference on October 10 2018 and completed for filing international patent application.
For further information, please click here.
Figure 1. Image quality before (left) and after (right) the technology application
Figure 2. The technology Concept
Figure 3. A transition from low-quality to high quality video after video transmission from the video server
2019.01.22 View 7008
Enhanced Video Quality despite Poor Network Conditions
(from left: Jaehong Kim, Youngmok Jung, Hyunho Yeo, Professor Dongsu Han and Professor Jinwoo Shin)
Professor Jinwoo Shin and Professor Dongsu Han from the School of Electrical Engineering developed neural adaptive content-aware internet video delivery. This technology is a novel method that combines adaptive streaming over HTTP, the video transmission system adopted by YouTube and Netflix, with a deep learning model.
This technology is expected to create an internet environment where users can enjoy watching 4K and AV/VR videos with high-quality and high-definition (HD) videos even with weak internet connections.
Thanks to video streaming services, internet video has experienced remarkable growth; nevertheless, users often suffer from low video quality due to unfavorable network conditions. Currently, existing adaptive streaming systems adjust the quality of the video in real time, accommodating the continuously changing internet bandwidth. Various algorithms are being researched for adaptive streaming systems, but there is an inherent limitation; that is, high-quality videos cannot be streamed in poor network environments regardless of which algorithm is used.
By incorporating super-resolution in adaptive streaming, the team overcame the limit of existing content distribution networks, of which their quality relies too much on the bandwidth. In the conventional method, the server that provides the video splits a video into certain lengths of time in advance. But the novel system introduced by the team allows the downloading of neural network segments. To facilitate this method, the video server needs to provide deep neural networks for each video segment as well as sizes of Deep Neural Networks (DNN) according to the specifications of the user’s computing capacity.
The largest neural network size is two megabytes, which is considerably smaller than video. When downloading the neural network from the user’s video player, it is split into several segments. Even its partial download is sufficient for a slightly comprised super-resolution.
While playing the video, the system converts the low quality video to a high-quality version by employing super-resolution based on deep convolution neural networks (CNN). The entire process is done in real time, and users can enjoy the high-definition video.
Even with a 17% smaller bandwidth, the system can provide the Quality of Experience equivalent to the latest adaptive streaming service. At a given internet bandwidth, it can provide 43% higher average QoE than the latest service.
Using a deep learning method allows this system to achieve a higher level of compression than the existing video compression methods. Their technology was recognized as a next-generation internet video system that applies super-resolution based on a deep convolution neural network to online videos.
Professor Han said, “So far, it has only been implemented on desktops, but we will further develop applications that work in mobile devices as well. This technology has been applied to the same video transmission systems used by streaming channels such as YouTube and Netflix, and thus shows good signs for practicability.”
This research, led by Hyunho Yeo, Youngmok Jung and Jaehong Kim, was presented at the 13th UNSENIX OSDI conference on October 10 2018 and completed for filing international patent application.
For further information, please click here.
Figure 1. Image quality before (left) and after (right) the technology application
Figure 2. The technology Concept
Figure 3. A transition from low-quality to high quality video after video transmission from the video server
2019.01.22 View 7008 -
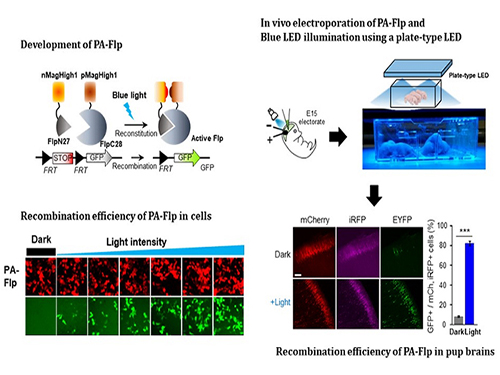 Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7409
Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neuroscience research.
Spatiotemporal control of gene expression has been acclaimed as a valuable strategy for identifying functions of genes with complex neural circuits. Studies of complex brain functions require highly sophisticated and robust technologies that enable specific labeling and rapid genetic modification in live animals. A number of approaches for controlling the activity of proteins or expression of genes in a spatiotemporal manner using light, small molecules, hormones, and peptides have been developed for manipulating intact circuits or functions.
Among them, recombination-employing, chemically inducible systems are the most commonly used in vivo gene-modification systems. Other approaches include selective or conditional Cre-activation systems within subsets of green fluorescent protein-expressing cells or dual-promoter-driven intersectional populations of cells.
However, these methods are limited by the considerable time and effort required to establish knock-in mouse lines and by constraints on spatiotemporal control, which relies on a limited set of available genetic promoters and transgenic mouse resources.
Beyond these constraints, optogenetic approaches allow the activity of genetically defined neurons in the mouse brain to be controlled with high spatiotemporal resolution. However, an optogenetic module for gene-manipulation capable of revealing the spatiotemporal functions of specific target genes in the mouse brain has remained a challenge.
In the study published at Nature Communication on Jan. 18, the team featured photoactivatable Flp recombinase by searching out split sites of Flp recombinase that were not previously identified, being capable of reconstitution to be active. The team validated the highly light-sensitive, efficient performance of photoactivatable Flp recombinase through precise light targeting by showing transgene expression within anatomically confined mouse brain regions.
The concept of local genetic labeling presented here suggests a new approach for genetically identifying subpopulations of cells defined by the spatial and temporal characteristics of light delivery. To date, an optogenetic module for gene-manipulation capable of revealing spatiotemporal functions of specific target genes in the mouse brain has remained out of reach and no such light-inducible Flp system has been developed. Accordingly, the team sought to develop a photoactivatable Flp recombinase that takes full advantage of the high spatiotemporal control offered by light stimulation.
This activation through noninvasive light illumination deep inside the brain is advantageous in that it avoids chemical or optic fiber implantation-mediated side effects, such as off-target cytotoxicity or physical lesions that might influence animal physiology or behaviors. The technique provides expandable utilities for transgene expression systems upon Flp recombinase activity in vivo, by designing a viral vector for minimal leaky expression influenced by viral nascent promoters.
The team demonstrated the utility of PA-Flp as a noninvasive in vivo optogenetic manipulation tool for use in the mouse brain, even applicable for deep brain structures as it can reach the hippocampus or medial septum using external LED light illumination.
The study is the result of five years of research by Professor Heo, who has led the bio-imaging and optogenetics fields by developing his own bio-imaging and optogenetics technologies. “It will be a great advantage to control specific gene expression desired by LEDs with little physical and chemical stimulation that can affect the physiological phenomenon in living animals,” he explained.
2019.01.22 View 7409