research
-
 New Insights into How the Human Brain Solves Complex Decision-Making Problems
A new study on meta reinforcement learning algorithms helps us understand how the human brain learns to adapt to complexity and uncertainty when learning and making decisions. A research team, led by Professor Sang Wan Lee at KAIST jointly with John O’Doherty at Caltech, succeeded in discovering both a computational and neural mechanism for human meta reinforcement learning, opening up the possibility of porting key elements of human intelligence into artificial intelligence algorithms. This study provides a glimpse into how it might ultimately use computational models to reverse engineer human reinforcement learning.
This work was published on Dec 16, 2019 in the journal Nature Communications. The title of the paper is “Task complexity interacts with state-space uncertainty in the arbitration between model-based and model-free learning.”
Human reinforcement learning is an inherently complex and dynamic process, involving goal setting, strategy choice, action selection, strategy modification, cognitive resource allocation etc. This a very challenging problem for humans to solve owing to the rapidly changing and multifaced environment in which humans have to operate. To make matters worse, humans often need to often rapidly make important decisions even before getting the opportunity to collect a lot of information, unlike the case when using deep learning methods to model learning and decision-making in artificial intelligence applications.
In order to solve this problem, the research team used a technique called 'reinforcement learning theory-based experiment design' to optimize the three variables of the two-stage Markov decision task - goal, task complexity, and task uncertainty. This experimental design technique allowed the team not only to control confounding factors, but also to create a situation similar to that which occurs in actual human problem solving.
Secondly, the team used a technique called ‘model-based neuroimaging analysis.’ Based on the acquired behavior and fMRI data, more than 100 different types of meta reinforcement learning algorithms were pitted against each other to find a computational model that can explain both behavioral and neural data. Thirdly, for the sake of a more rigorous verification, the team applied an analytical method called ‘parameter recovery analysis,’ which involves high-precision behavioral profiling of both human subjects and computational models.
In this way, the team was able to accurately identify a computational model of meta reinforcement learning, ensuring not only that the model’s apparent behavior is similar to that of humans, but also that the model solves the problem in the same way as humans do.
The team found that people tended to increase planning-based reinforcement learning (called model-based control), in response to increasing task complexity. However, they resorted to a simpler, more resource efficient strategy called model-free control, when both uncertainty and task complexity were high. This suggests that both the task uncertainty and the task complexity interact during the meta control of reinforcement learning. Computational fMRI analyses revealed that task complexity interacts with neural representations of the reliability of the learning strategies in the inferior prefrontal cortex.
These findings significantly advance understanding of the nature of the computations being implemented in the inferior prefrontal cortex during meta reinforcement learning as well as providing insight into the more general question of how the brain resolves uncertainty and complexity in a dynamically changing environment. Identifying the key computational variables that drive prefrontal meta reinforcement learning, can also inform understanding of how this process might be vulnerable to break down in certain psychiatric disorders such as depression and OCD. Furthermore, gaining a computational understanding of how this process can sometimes lead to increased model-free control, can provide insights into how under some situations task performance might break down under conditions of high cognitive load.
Professor Lee said, “This study will be of enormous interest to researchers in both the artificial intelligence and human/computer interaction fields since this holds significant potential for applying core insights gleaned into how human intelligence works with AI algorithms.”
This work was funded by the National Institute on Drug Abuse, the National Research Foundation of Korea, the Ministry of Science and ICT, Samsung Research Funding Center of Samsung Electronics.
Figure 1 (modified from the figures of the original paper doi:10.1038/s41467-019-13632-1). Computations implemented in the inferior prefrontal cortex during meta reinforcement learning. (A) Computational model of human prefrontal meta reinforcement learning (left) and the brain areas whose neural activity patterns are explained by the latent variables of the model. (B) Examples of behavioral profiles. Shown on the left is choice bias for different goal types and on the right is choice optimality for task complexity and uncertainty. (C) Parameter recoverability analysis. Compared are the effect of task uncertainty (left) and task complexity (right) on choice optimality.
-Profile
Professor Sang Wan Lee
sangwan@kaist.ac.kr
Department of Bio and Brain Engineering
Director, KAIST Center for Neuroscience-inspired AI
KAIST Institute for Artificial Intelligence (http://aibrain.kaist.ac.kr)
KAIST Institute for Health, Science, and Technology
KAIST (https://www.kaist.ac.kr)
2020.01.31 View 6178
New Insights into How the Human Brain Solves Complex Decision-Making Problems
A new study on meta reinforcement learning algorithms helps us understand how the human brain learns to adapt to complexity and uncertainty when learning and making decisions. A research team, led by Professor Sang Wan Lee at KAIST jointly with John O’Doherty at Caltech, succeeded in discovering both a computational and neural mechanism for human meta reinforcement learning, opening up the possibility of porting key elements of human intelligence into artificial intelligence algorithms. This study provides a glimpse into how it might ultimately use computational models to reverse engineer human reinforcement learning.
This work was published on Dec 16, 2019 in the journal Nature Communications. The title of the paper is “Task complexity interacts with state-space uncertainty in the arbitration between model-based and model-free learning.”
Human reinforcement learning is an inherently complex and dynamic process, involving goal setting, strategy choice, action selection, strategy modification, cognitive resource allocation etc. This a very challenging problem for humans to solve owing to the rapidly changing and multifaced environment in which humans have to operate. To make matters worse, humans often need to often rapidly make important decisions even before getting the opportunity to collect a lot of information, unlike the case when using deep learning methods to model learning and decision-making in artificial intelligence applications.
In order to solve this problem, the research team used a technique called 'reinforcement learning theory-based experiment design' to optimize the three variables of the two-stage Markov decision task - goal, task complexity, and task uncertainty. This experimental design technique allowed the team not only to control confounding factors, but also to create a situation similar to that which occurs in actual human problem solving.
Secondly, the team used a technique called ‘model-based neuroimaging analysis.’ Based on the acquired behavior and fMRI data, more than 100 different types of meta reinforcement learning algorithms were pitted against each other to find a computational model that can explain both behavioral and neural data. Thirdly, for the sake of a more rigorous verification, the team applied an analytical method called ‘parameter recovery analysis,’ which involves high-precision behavioral profiling of both human subjects and computational models.
In this way, the team was able to accurately identify a computational model of meta reinforcement learning, ensuring not only that the model’s apparent behavior is similar to that of humans, but also that the model solves the problem in the same way as humans do.
The team found that people tended to increase planning-based reinforcement learning (called model-based control), in response to increasing task complexity. However, they resorted to a simpler, more resource efficient strategy called model-free control, when both uncertainty and task complexity were high. This suggests that both the task uncertainty and the task complexity interact during the meta control of reinforcement learning. Computational fMRI analyses revealed that task complexity interacts with neural representations of the reliability of the learning strategies in the inferior prefrontal cortex.
These findings significantly advance understanding of the nature of the computations being implemented in the inferior prefrontal cortex during meta reinforcement learning as well as providing insight into the more general question of how the brain resolves uncertainty and complexity in a dynamically changing environment. Identifying the key computational variables that drive prefrontal meta reinforcement learning, can also inform understanding of how this process might be vulnerable to break down in certain psychiatric disorders such as depression and OCD. Furthermore, gaining a computational understanding of how this process can sometimes lead to increased model-free control, can provide insights into how under some situations task performance might break down under conditions of high cognitive load.
Professor Lee said, “This study will be of enormous interest to researchers in both the artificial intelligence and human/computer interaction fields since this holds significant potential for applying core insights gleaned into how human intelligence works with AI algorithms.”
This work was funded by the National Institute on Drug Abuse, the National Research Foundation of Korea, the Ministry of Science and ICT, Samsung Research Funding Center of Samsung Electronics.
Figure 1 (modified from the figures of the original paper doi:10.1038/s41467-019-13632-1). Computations implemented in the inferior prefrontal cortex during meta reinforcement learning. (A) Computational model of human prefrontal meta reinforcement learning (left) and the brain areas whose neural activity patterns are explained by the latent variables of the model. (B) Examples of behavioral profiles. Shown on the left is choice bias for different goal types and on the right is choice optimality for task complexity and uncertainty. (C) Parameter recoverability analysis. Compared are the effect of task uncertainty (left) and task complexity (right) on choice optimality.
-Profile
Professor Sang Wan Lee
sangwan@kaist.ac.kr
Department of Bio and Brain Engineering
Director, KAIST Center for Neuroscience-inspired AI
KAIST Institute for Artificial Intelligence (http://aibrain.kaist.ac.kr)
KAIST Institute for Health, Science, and Technology
KAIST (https://www.kaist.ac.kr)
2020.01.31 View 6178 -
 KAIST Vaccine for Tick-Borne Disease ‘SFTS’ Protects Against Lethal Infection
A KAIST research team reported the development of a DNA vaccine for Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) which completely protects against lethal infection in ferrets. The team confirmed that ferrets immunized with DNA vaccines encoding all SFTSV proteins showed 100% survival rate without detectable viremia and did not develop any clinical symptoms. This study was published in Nature Communications on August 23.
Severe Fever with Thrombocytopenia Syndrome (SFTS) is a newly emerging tick-borne infectious disease. The disease causes fever, severe thrombocytopenia, leukocytopenia as well as vomiting and diarrhea. Severe cases end up with organ system failure often accompanied by hemorrhages, and its mortality rate stands at 10–20%.
The viral disease has been endemic to East Asia but the spread of the tick vector to North America increases the likelihood of potential outbreak beyond the Far East Asia. The World Health Organization (WHO) has also put SFTSV into the priority pathogen requiring urgent attention category. Currently, no vaccine has been available to prevent SFTS.
The research team led by Professor Su-Hyung Park noted that DNA vaccines induce broader immunity to multiple antigens than traditional ones. Moreover, DNA vaccines stimulate both T cell and antibody immunity, which make them suitable for vaccine development.
They constructed DNA vaccines that encode full-length Gn, Gc, N, NS, and RNA polymerase genes based on common sequences of 31 SFTSV strains isolated from patients. Their vaccine candidates induced both neutralizing antibody response and multifunctional SFTSV-specific T cell response in mice and ferrets.
To investigate the vaccine’s efficacy in vivo, the research team applied a recently developed ferret model that recapitulates fatal clinical symptoms in SFTSV infection in humans. Vaccinated ferrets were completely protected from lethal SFTSV challenge without SFTSV detection in their blood, whereas all control ferrets died within 10 days’ post-infection.
The KAIST team found that anti-envelope antibodies play an important role in protective immunity, suggesting that envelope glycoproteins of SFTSV may be the most effective antigens for inducing protective immunity. Moreover, the study revealed that T cell responses specific to non-envelope proteins of SFTSV also can contribute to protection against SFTSV infection.
Professor Park said, “This is the first study demonstrating complete protection against lethal SFTSV challenge using an immunocompetent, middle-sized animal model with clinical manifestations of SFTSV infection. We believe this study provides valuable insights into designing preventive vaccines for SFTSV.”
2020.01.31 View 5490
KAIST Vaccine for Tick-Borne Disease ‘SFTS’ Protects Against Lethal Infection
A KAIST research team reported the development of a DNA vaccine for Severe Fever with Thrombocytopenia Syndrome Virus (SFTSV) which completely protects against lethal infection in ferrets. The team confirmed that ferrets immunized with DNA vaccines encoding all SFTSV proteins showed 100% survival rate without detectable viremia and did not develop any clinical symptoms. This study was published in Nature Communications on August 23.
Severe Fever with Thrombocytopenia Syndrome (SFTS) is a newly emerging tick-borne infectious disease. The disease causes fever, severe thrombocytopenia, leukocytopenia as well as vomiting and diarrhea. Severe cases end up with organ system failure often accompanied by hemorrhages, and its mortality rate stands at 10–20%.
The viral disease has been endemic to East Asia but the spread of the tick vector to North America increases the likelihood of potential outbreak beyond the Far East Asia. The World Health Organization (WHO) has also put SFTSV into the priority pathogen requiring urgent attention category. Currently, no vaccine has been available to prevent SFTS.
The research team led by Professor Su-Hyung Park noted that DNA vaccines induce broader immunity to multiple antigens than traditional ones. Moreover, DNA vaccines stimulate both T cell and antibody immunity, which make them suitable for vaccine development.
They constructed DNA vaccines that encode full-length Gn, Gc, N, NS, and RNA polymerase genes based on common sequences of 31 SFTSV strains isolated from patients. Their vaccine candidates induced both neutralizing antibody response and multifunctional SFTSV-specific T cell response in mice and ferrets.
To investigate the vaccine’s efficacy in vivo, the research team applied a recently developed ferret model that recapitulates fatal clinical symptoms in SFTSV infection in humans. Vaccinated ferrets were completely protected from lethal SFTSV challenge without SFTSV detection in their blood, whereas all control ferrets died within 10 days’ post-infection.
The KAIST team found that anti-envelope antibodies play an important role in protective immunity, suggesting that envelope glycoproteins of SFTSV may be the most effective antigens for inducing protective immunity. Moreover, the study revealed that T cell responses specific to non-envelope proteins of SFTSV also can contribute to protection against SFTSV infection.
Professor Park said, “This is the first study demonstrating complete protection against lethal SFTSV challenge using an immunocompetent, middle-sized animal model with clinical manifestations of SFTSV infection. We believe this study provides valuable insights into designing preventive vaccines for SFTSV.”
2020.01.31 View 5490 -
 Transformative Electronics Systems to Broaden Wearable Applications
Imagine a handheld electronic gadget that can soften and deform when attached to our skin. This will be the future of electronics we all dreamed of. A research team at KAIST says their new platform called 'Transformative Electronics Systems' will open a new class of electronics, allowing reconfigurable electronic interfaces to be optimized for a variety of applications.
A team working under Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST has invented a multifunctional electronic platform that can mechanically transform its shape, flexibility, and stretchability. This platform, which was reported in Science Advances, allows users to seamlessly and precisely tune its stiffness and shape.
"This new class of electronics will not only offer robust, convenient interfaces for use in both tabletop or handheld setups, but also allow seamless integration with the skin when applied onto our bodies," said Professor Jeong.
The transformative electronics consist of a special gallium metal structure, hermetically encapsulated and sealed within a soft silicone material, combined with electronics that are designed to be flexible and stretchable. The mechanical transformation of the electronic systems is specifically triggered by temperature change events controlled by the user.
"Gallium is an interesting key material. It is biocompatible, has high rigidity in solid form, and melts at a temperature comparable to the skin's temperature," said lead author Sang-Hyuk Byun, a researcher at KAIST.
Once the transformative electronic platform comes in contact with a human body, the gallium metal encapsulated inside the silicone changes to a liquid state and softens the whole electronic structure, making it stretchable, flexible, and wearable. The gallium metal then solidifies again once the structure is peeled off the skin, making the electronic circuits stiff and stable. When flexible electronic circuits were integrated onto these transformative platforms, it empowered them with the ability to become either flexible and stretchable or rigid.
"This technology could not have been achieved without interdisciplinary efforts," said co-lead author Joo Yong Sim, who is a researcher with ETRI. "We worked together with electrical, mechanical, and biomedical engineers, as well as material scientists and neuroscientists to make this breakthrough."
This universal electronics platform allowed researchers to demonstrate applications that were highly adaptable and customizable, such as a multi-purpose personal electronics with variable stiffness and stretchability, a pressure sensor with tuneable bandwidth and sensitivity, and a neural probe that softens upon implantation into brain tissue.
Applicable for both traditional and emerging electronics technologies, this breakthrough can potentially reshape the consumer electronics industry, especially in the biomedical and robotic domains. The researchers believe that with further development, this novel electronics technology can significantly impact the way we use electronics in our daily life.
< Transformative electronics in soft mode,which becomes wearable for outdoor applications.>
Video Material:
https://youtu.be/im0J18TfShk
Publication: Sang-Hyuk Byun, Joo Yong Sim, Zhanan Zhou, Juhyun Lee, Raza Qazi, Marie C. Walicki, Kyle E. Parker, Matthew P. Haney, Su Hwan Choi, Ahnsei Shon, Graydon B. Gereau, John Bilbily, Shuo Li, Yuhao Liu, Woon-Hong Yeo, Jordan G. McCall, Jianliang Xiao, and Jae-Woong Jeong. 2019. Mechanically transformative electronics, sensors, and implantable devices. Science Advances. Volume 5. No. 11. 12 pages. https://doi.org/10.1126/sciadv.aay0418
Link to download the full-text paper: https://advances.sciencemag.org/content/advances/5/11/eaay0418.full.pdf
Profile: Prof. Jae-Woong Jeong, PhD jjeong1@kaist.ac.kr
https://www.jeongresearch.org/
Professor
Bio-Integrated Electronics and Systems Laboratory
School of Electrical Engineering
Korea Advanced Institute of Science and Technology (KAIST) https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Sang-Hyuk Byun, PhD Candidate
shbun95@kaist.ac.kr
(END)
2020.01.31 View 7567
Transformative Electronics Systems to Broaden Wearable Applications
Imagine a handheld electronic gadget that can soften and deform when attached to our skin. This will be the future of electronics we all dreamed of. A research team at KAIST says their new platform called 'Transformative Electronics Systems' will open a new class of electronics, allowing reconfigurable electronic interfaces to be optimized for a variety of applications.
A team working under Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST has invented a multifunctional electronic platform that can mechanically transform its shape, flexibility, and stretchability. This platform, which was reported in Science Advances, allows users to seamlessly and precisely tune its stiffness and shape.
"This new class of electronics will not only offer robust, convenient interfaces for use in both tabletop or handheld setups, but also allow seamless integration with the skin when applied onto our bodies," said Professor Jeong.
The transformative electronics consist of a special gallium metal structure, hermetically encapsulated and sealed within a soft silicone material, combined with electronics that are designed to be flexible and stretchable. The mechanical transformation of the electronic systems is specifically triggered by temperature change events controlled by the user.
"Gallium is an interesting key material. It is biocompatible, has high rigidity in solid form, and melts at a temperature comparable to the skin's temperature," said lead author Sang-Hyuk Byun, a researcher at KAIST.
Once the transformative electronic platform comes in contact with a human body, the gallium metal encapsulated inside the silicone changes to a liquid state and softens the whole electronic structure, making it stretchable, flexible, and wearable. The gallium metal then solidifies again once the structure is peeled off the skin, making the electronic circuits stiff and stable. When flexible electronic circuits were integrated onto these transformative platforms, it empowered them with the ability to become either flexible and stretchable or rigid.
"This technology could not have been achieved without interdisciplinary efforts," said co-lead author Joo Yong Sim, who is a researcher with ETRI. "We worked together with electrical, mechanical, and biomedical engineers, as well as material scientists and neuroscientists to make this breakthrough."
This universal electronics platform allowed researchers to demonstrate applications that were highly adaptable and customizable, such as a multi-purpose personal electronics with variable stiffness and stretchability, a pressure sensor with tuneable bandwidth and sensitivity, and a neural probe that softens upon implantation into brain tissue.
Applicable for both traditional and emerging electronics technologies, this breakthrough can potentially reshape the consumer electronics industry, especially in the biomedical and robotic domains. The researchers believe that with further development, this novel electronics technology can significantly impact the way we use electronics in our daily life.
< Transformative electronics in soft mode,which becomes wearable for outdoor applications.>
Video Material:
https://youtu.be/im0J18TfShk
Publication: Sang-Hyuk Byun, Joo Yong Sim, Zhanan Zhou, Juhyun Lee, Raza Qazi, Marie C. Walicki, Kyle E. Parker, Matthew P. Haney, Su Hwan Choi, Ahnsei Shon, Graydon B. Gereau, John Bilbily, Shuo Li, Yuhao Liu, Woon-Hong Yeo, Jordan G. McCall, Jianliang Xiao, and Jae-Woong Jeong. 2019. Mechanically transformative electronics, sensors, and implantable devices. Science Advances. Volume 5. No. 11. 12 pages. https://doi.org/10.1126/sciadv.aay0418
Link to download the full-text paper: https://advances.sciencemag.org/content/advances/5/11/eaay0418.full.pdf
Profile: Prof. Jae-Woong Jeong, PhD jjeong1@kaist.ac.kr
https://www.jeongresearch.org/
Professor
Bio-Integrated Electronics and Systems Laboratory
School of Electrical Engineering
Korea Advanced Institute of Science and Technology (KAIST) https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Sang-Hyuk Byun, PhD Candidate
shbun95@kaist.ac.kr
(END)
2020.01.31 View 7567 -
 Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 10726
Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 10726 -
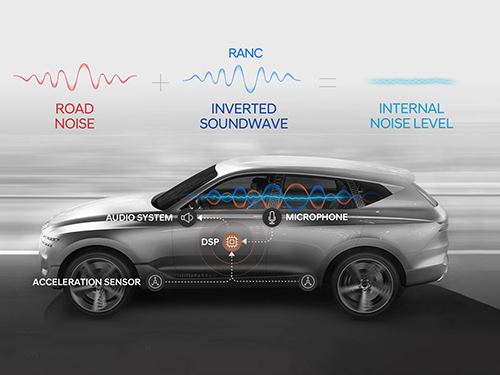 A System Controlling Road Active Noise to Hit the Road
The research team led by Professor Youngjin Park of the Department of Mechanical Engineering has developed a road noise active noise control (RANC) system to be commercialized in partnership with Hyundai Motor Group.
On December 11, Hyundai Motor Group announced the successful development of the RANC system, which significantly reduces the road noise flowing into cars. The carmaker has completed the domestic and American patent applications for the location of sensors and the signal selection method, the core technology of RANC.
RANC is a technology for reducing road noise during driving. This system consists of an acceleration sensor, digital signal processor (the control computer to analyze sound signals), microphone, amplifier, and audio system. To make the system as simple as possible, the audio system utilizes the original audio system embedded in the car instead of a separate system.
The acceleration sensor first calculates the vibration from the road into the car. The location of the sensor is important for accurately identifying the vibration path. The research team was able to find the optimal sensor location through a number of tests.
The System Dynamics and Applied Control Laboratory of Professor Park researched ways to significantly reduce road noise with Hyundai Motor Group for four years from 1993 as a G7 national project and published the results in international journals. In 2002, the researchers published an article titled “Noise Quietens Driving” in Nature, where they announced the first success in reducing road noise in actual cars. The achievement did not lead to commercialization, however, due to the lack of auxiliary technologies at the time, digital amplifiers and DSP for cars for example, and pricing issues.
Since 2013, Professor Park’s research team has participated in one technology transfer and eight university-industry projects. Based on these efforts, the team was able to successfully develop the RANC system with domestic technology in partnership with Hyundai’s NVH Research Lab (Research Fellow, Dr. Gangdeok Lee; Ph.D. in aviation engineering, 1996), Optomech (Founder, Professor Gyeongsu Kim; Ph.D. in mechanical engineering, 1999), ARE (CEO Hyeonseok Kim; Ph.D. in mechanical engineering, 1998), WeAcom, and BurnYoung.
Professor Park’s team led the project by performing theory-based research during the commercialization stage in collaboration with Hyundai Motor Group.
For the commercialization of the RANC system, Hyundai Motor Group is planning to collaborate with the global car audio company Harman to increase the degree of completion and apply the RANC system to the GV 80, the first SUV model of the Genesis brand.
“I am very delighted as an engineer to see the research I worked on from my early days at KAIST be commercialized after 20 years,” noted Professor Park. “I am thrilled to make a contribution to such commercialization with my students in my lab.”
2019.12.27 View 11987
A System Controlling Road Active Noise to Hit the Road
The research team led by Professor Youngjin Park of the Department of Mechanical Engineering has developed a road noise active noise control (RANC) system to be commercialized in partnership with Hyundai Motor Group.
On December 11, Hyundai Motor Group announced the successful development of the RANC system, which significantly reduces the road noise flowing into cars. The carmaker has completed the domestic and American patent applications for the location of sensors and the signal selection method, the core technology of RANC.
RANC is a technology for reducing road noise during driving. This system consists of an acceleration sensor, digital signal processor (the control computer to analyze sound signals), microphone, amplifier, and audio system. To make the system as simple as possible, the audio system utilizes the original audio system embedded in the car instead of a separate system.
The acceleration sensor first calculates the vibration from the road into the car. The location of the sensor is important for accurately identifying the vibration path. The research team was able to find the optimal sensor location through a number of tests.
The System Dynamics and Applied Control Laboratory of Professor Park researched ways to significantly reduce road noise with Hyundai Motor Group for four years from 1993 as a G7 national project and published the results in international journals. In 2002, the researchers published an article titled “Noise Quietens Driving” in Nature, where they announced the first success in reducing road noise in actual cars. The achievement did not lead to commercialization, however, due to the lack of auxiliary technologies at the time, digital amplifiers and DSP for cars for example, and pricing issues.
Since 2013, Professor Park’s research team has participated in one technology transfer and eight university-industry projects. Based on these efforts, the team was able to successfully develop the RANC system with domestic technology in partnership with Hyundai’s NVH Research Lab (Research Fellow, Dr. Gangdeok Lee; Ph.D. in aviation engineering, 1996), Optomech (Founder, Professor Gyeongsu Kim; Ph.D. in mechanical engineering, 1999), ARE (CEO Hyeonseok Kim; Ph.D. in mechanical engineering, 1998), WeAcom, and BurnYoung.
Professor Park’s team led the project by performing theory-based research during the commercialization stage in collaboration with Hyundai Motor Group.
For the commercialization of the RANC system, Hyundai Motor Group is planning to collaborate with the global car audio company Harman to increase the degree of completion and apply the RANC system to the GV 80, the first SUV model of the Genesis brand.
“I am very delighted as an engineer to see the research I worked on from my early days at KAIST be commercialized after 20 years,” noted Professor Park. “I am thrilled to make a contribution to such commercialization with my students in my lab.”
2019.12.27 View 11987 -
 New Liquid Metal Wearable Pressure Sensor Created for Health Monitoring Applications
Soft pressure sensors have received significant research attention in a variety of fields, including soft robotics, electronic skin, and wearable electronics. Wearable soft pressure sensors have great potential for the real-time health monitoring and for the early diagnosis of diseases.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering developed a highly sensitive wearable pressure sensor for health monitoring applications. This work was reported in Advanced Healthcare Materials on November 21 as a front cover article.
This technology is capable of sensitive, precise, and continuous measurement of physiological and physical signals and shows great potential for health monitoring applications and the early diagnosis of diseases.
A soft pressure sensor is required to have high compliance, high sensitivity, low cost, long-term performance stability, and environmental stability in order to be employed for continuous health monitoring. Conventional solid-state soft pressure sensors using functional materials including carbon nanotubes and graphene have showed great sensing performance. However, these sensors suffer from limited stretchability, signal drifting, and long-term instability due to the distance between the stretchable substrate and the functional materials.
To overcome these issues, liquid-state electronics using liquid metal have been introduced for various wearable applications. Of these materials, Galinstan, a eutectic metal alloy of gallium, indium, and tin, has great mechanical and electrical properties that can be employed in wearable applications. But today’s liquid metal-based pressure sensors have low-pressure sensitivity, limiting their applicability for health monitoring devices.
The research team developed a 3D-printed rigid microbump array-integrated, liquid metal-based soft pressure sensor. With the help of 3D printing, the integration of a rigid microbump array and the master mold for a liquid metal microchannel could be achieved simultaneously, reducing the complexity of the manufacturing process. Through the integration of the rigid microbump and the microchannel, the new pressure sensor has an extremely low detection limit and enhanced pressure sensitivity compared to previously reported liquid metal-based pressure sensors. The proposed sensor also has a negligible signal drift over 10,000 cycles of pressure, bending, and stretching and exhibited excellent stability when subjected to various environmental conditions.
These performance outcomes make it an excellent sensor for various health monitoring devices. First, the research team demonstrated a wearable wristband device that can continuously monitor one’s pulse during exercise and be employed in a noninvasive cuffless BP monitoring system based on PTT calculations. Then, they introduced a wireless wearable heel pressure monitoring system that integrates three 3D-BLiPS with a wireless communication module.
Professor Park said, “It was possible to measure health indicators including pulse and blood pressure continuously as well as pressure of body parts using our proposed soft pressure sensor. We expect it to be used in health care applications, such as the prevention and the monitoring of the pressure-driven diseases such as pressure ulcers in the near future. There will be more opportunities for future research including a whole-body pressure monitoring system related to other physical parameters.”
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT.
< Figure 1. The front cover image of Advanced Healthcare Materials, Volume 8, Issue 22. >
< Figure 2. Highly sensitive liquid metal-based soft pressure sensor integrated with 3D-printed microbump array. >
< Figure 3. High pressure sensitivity and reliable sensing performances of the proposed sensor and wireless heel pressure monitoring application. >
-ProfileProfessor Inkyu ParkMicro/Nano Transducers Laboratoryhttp://mintlab1.kaist.ac.kr/
Department of Mechanical EngineeringKAIST
2019.12.20 View 14618
New Liquid Metal Wearable Pressure Sensor Created for Health Monitoring Applications
Soft pressure sensors have received significant research attention in a variety of fields, including soft robotics, electronic skin, and wearable electronics. Wearable soft pressure sensors have great potential for the real-time health monitoring and for the early diagnosis of diseases.
A KAIST research team led by Professor Inkyu Park from the Department of Mechanical Engineering developed a highly sensitive wearable pressure sensor for health monitoring applications. This work was reported in Advanced Healthcare Materials on November 21 as a front cover article.
This technology is capable of sensitive, precise, and continuous measurement of physiological and physical signals and shows great potential for health monitoring applications and the early diagnosis of diseases.
A soft pressure sensor is required to have high compliance, high sensitivity, low cost, long-term performance stability, and environmental stability in order to be employed for continuous health monitoring. Conventional solid-state soft pressure sensors using functional materials including carbon nanotubes and graphene have showed great sensing performance. However, these sensors suffer from limited stretchability, signal drifting, and long-term instability due to the distance between the stretchable substrate and the functional materials.
To overcome these issues, liquid-state electronics using liquid metal have been introduced for various wearable applications. Of these materials, Galinstan, a eutectic metal alloy of gallium, indium, and tin, has great mechanical and electrical properties that can be employed in wearable applications. But today’s liquid metal-based pressure sensors have low-pressure sensitivity, limiting their applicability for health monitoring devices.
The research team developed a 3D-printed rigid microbump array-integrated, liquid metal-based soft pressure sensor. With the help of 3D printing, the integration of a rigid microbump array and the master mold for a liquid metal microchannel could be achieved simultaneously, reducing the complexity of the manufacturing process. Through the integration of the rigid microbump and the microchannel, the new pressure sensor has an extremely low detection limit and enhanced pressure sensitivity compared to previously reported liquid metal-based pressure sensors. The proposed sensor also has a negligible signal drift over 10,000 cycles of pressure, bending, and stretching and exhibited excellent stability when subjected to various environmental conditions.
These performance outcomes make it an excellent sensor for various health monitoring devices. First, the research team demonstrated a wearable wristband device that can continuously monitor one’s pulse during exercise and be employed in a noninvasive cuffless BP monitoring system based on PTT calculations. Then, they introduced a wireless wearable heel pressure monitoring system that integrates three 3D-BLiPS with a wireless communication module.
Professor Park said, “It was possible to measure health indicators including pulse and blood pressure continuously as well as pressure of body parts using our proposed soft pressure sensor. We expect it to be used in health care applications, such as the prevention and the monitoring of the pressure-driven diseases such as pressure ulcers in the near future. There will be more opportunities for future research including a whole-body pressure monitoring system related to other physical parameters.”
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT.
< Figure 1. The front cover image of Advanced Healthcare Materials, Volume 8, Issue 22. >
< Figure 2. Highly sensitive liquid metal-based soft pressure sensor integrated with 3D-printed microbump array. >
< Figure 3. High pressure sensitivity and reliable sensing performances of the proposed sensor and wireless heel pressure monitoring application. >
-ProfileProfessor Inkyu ParkMicro/Nano Transducers Laboratoryhttp://mintlab1.kaist.ac.kr/
Department of Mechanical EngineeringKAIST
2019.12.20 View 14618 -
 ‘Carrier-Resolved Photo-Hall’ to Push Semiconductor Advances
(Professor Shin and Dr. Gunawan (left))
An IBM-KAIST research team described a breakthrough in a 140-year-old mystery in physics. The research reported in Nature last month unlocks the physical characteristics of semiconductors in much greater detail and aids in the development of new and improved semiconductor materials.
Research team under Professor Byungha Shin at the Department of Material Sciences and Engineering and Dr. Oki Gunawan at IBM discovered a new formula and technique that enables the simultaneous extraction of both majority and minority carrier information such as their density and mobility, as well as gain additional insights about carrier lifetimes, diffusion lengths, and the recombination process. This new discovery and technology will help push semiconductor advances in both existing and emerging technologies.
Semiconductors are the basic building blocks of today’s digital electronics age, providing us with a multitude of devices that benefit our modern life. To truly appreciate the physics of semiconductors, it is very important to understand the fundamental properties of the charge carriers inside the materials, whether those particles are positive or negative, their speed under an applied electric field, and how densely they are packed into the material.
Physicist Edwin Hall found a way to determine those properties in 1879, when he discovered that a magnetic field will deflect the movement of electronic charges inside a conductor and that the amount of deflection can be measured as a voltage perpendicular to the flow of the charge. Decades after Hall’s discovery, researchers also recognized that they can measure the Hall effect with light via “photo-Hall experiments”. During such experiments, the light generates multiple carriers or electron–hole pairs in the semiconductors.
Unfortunately, the basic Hall effect only provided insights into the dominant charge carrier (or majority carrier). Researchers were unable to extract the properties of both carriers (the majority and minority carriers) simultaneously. The property information of both carriers is crucial for many applications that involve light such as solar cells and other optoelectronic devices.
In the photo-Hall experiment by the KAIST-IBM team, both carriers contribute to changes in conductivity and the Hall coefficient. The key insight comes from measuring the conductivity and Hall coefficient as a function of light intensity. Hidden in the trajectory of the conductivity, the Hall coefficient curve reveals crucial new information: the difference in the mobility of both carriers. As discussed in the paper, this relationship can be expressed elegantly as: Δµ = d (σ²H)/dσ
The research team solved for both majority and minority carrier mobility and density as a function of light intensity, naming the new technique Carrier-Resolved Photo Hall (CRPH) measurement. With known light illumination intensity, the carrier lifetime can be established in a similar way.
Beyond advances in theoretical understanding, advances in experimental techniques were also critical for enabling this breakthrough. The technique requires a clean Hall signal measurement, which can be challenging for materials where the Hall signal is weak due to low mobility or when extra unwanted signals are present, such as under strong light illumination.
The newly developed photo-Hall technique allows the extraction of an astonishing amount of information from semiconductors. In contrast to only three parameters obtained in the classic Hall measurements, this new technique yields up to seven parameters at every tested level of light intensity. These include the mobility of both the electron and hole; their carrier density under light; the recombination lifetime; and the diffusion lengths for electrons, holes, and ambipolar types. All of these can be repeated N times (i.e. the number of light intensity settings used in the experiment).
Professor Shin said, “This novel technology sheds new light on understanding the physical characteristics of semiconductor materials in great detail.” Dr. Gunawan added, “This will will help accelerate the development of next-generation semiconductor technology such as better solar cells, better optoelectronics devices, and new materials and devices for artificial intelligence technology.”
Profile:
Professor Byungha Shin
Department of Materials Science and Engineering
KAIST
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
2019.11.18 View 14547
‘Carrier-Resolved Photo-Hall’ to Push Semiconductor Advances
(Professor Shin and Dr. Gunawan (left))
An IBM-KAIST research team described a breakthrough in a 140-year-old mystery in physics. The research reported in Nature last month unlocks the physical characteristics of semiconductors in much greater detail and aids in the development of new and improved semiconductor materials.
Research team under Professor Byungha Shin at the Department of Material Sciences and Engineering and Dr. Oki Gunawan at IBM discovered a new formula and technique that enables the simultaneous extraction of both majority and minority carrier information such as their density and mobility, as well as gain additional insights about carrier lifetimes, diffusion lengths, and the recombination process. This new discovery and technology will help push semiconductor advances in both existing and emerging technologies.
Semiconductors are the basic building blocks of today’s digital electronics age, providing us with a multitude of devices that benefit our modern life. To truly appreciate the physics of semiconductors, it is very important to understand the fundamental properties of the charge carriers inside the materials, whether those particles are positive or negative, their speed under an applied electric field, and how densely they are packed into the material.
Physicist Edwin Hall found a way to determine those properties in 1879, when he discovered that a magnetic field will deflect the movement of electronic charges inside a conductor and that the amount of deflection can be measured as a voltage perpendicular to the flow of the charge. Decades after Hall’s discovery, researchers also recognized that they can measure the Hall effect with light via “photo-Hall experiments”. During such experiments, the light generates multiple carriers or electron–hole pairs in the semiconductors.
Unfortunately, the basic Hall effect only provided insights into the dominant charge carrier (or majority carrier). Researchers were unable to extract the properties of both carriers (the majority and minority carriers) simultaneously. The property information of both carriers is crucial for many applications that involve light such as solar cells and other optoelectronic devices.
In the photo-Hall experiment by the KAIST-IBM team, both carriers contribute to changes in conductivity and the Hall coefficient. The key insight comes from measuring the conductivity and Hall coefficient as a function of light intensity. Hidden in the trajectory of the conductivity, the Hall coefficient curve reveals crucial new information: the difference in the mobility of both carriers. As discussed in the paper, this relationship can be expressed elegantly as: Δµ = d (σ²H)/dσ
The research team solved for both majority and minority carrier mobility and density as a function of light intensity, naming the new technique Carrier-Resolved Photo Hall (CRPH) measurement. With known light illumination intensity, the carrier lifetime can be established in a similar way.
Beyond advances in theoretical understanding, advances in experimental techniques were also critical for enabling this breakthrough. The technique requires a clean Hall signal measurement, which can be challenging for materials where the Hall signal is weak due to low mobility or when extra unwanted signals are present, such as under strong light illumination.
The newly developed photo-Hall technique allows the extraction of an astonishing amount of information from semiconductors. In contrast to only three parameters obtained in the classic Hall measurements, this new technique yields up to seven parameters at every tested level of light intensity. These include the mobility of both the electron and hole; their carrier density under light; the recombination lifetime; and the diffusion lengths for electrons, holes, and ambipolar types. All of these can be repeated N times (i.e. the number of light intensity settings used in the experiment).
Professor Shin said, “This novel technology sheds new light on understanding the physical characteristics of semiconductor materials in great detail.” Dr. Gunawan added, “This will will help accelerate the development of next-generation semiconductor technology such as better solar cells, better optoelectronics devices, and new materials and devices for artificial intelligence technology.”
Profile:
Professor Byungha Shin
Department of Materials Science and Engineering
KAIST
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
2019.11.18 View 14547 -
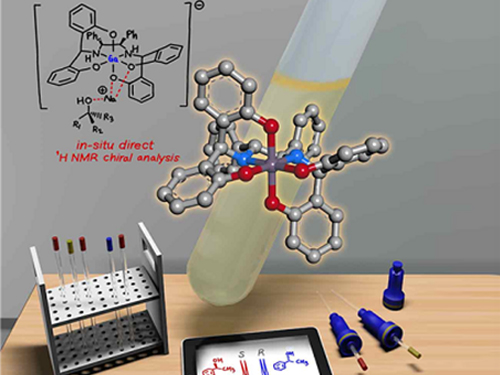 Gallium-Based Solvating Agent Efficiently Analyzes Optically Active Alcohols
A KAIST research team has developed a gallium-based metal complex enabling the rapid chiral analysis of alcohols. A team working under Professor Hyunwoo Kim reported the efficient new alcohol analysis method using nuclear magnetic resonance (NMR) spectroscopy in iScience.
Enantiopure chiral alcohols are ubiquitous in nature and widely utilized as pharmaceuticals. This importance of chirality in synthetic and medicinal chemistry has advanced the search for rapid and facile methods to determine the enantiomeric purities of compounds. To date, chiral analysis has been performed using high-performance liquid chromatography (HPLC) with chiral columns.
Along with the HPLC technique, chiral analysis using NMR spectroscopy has gained tremendous attention as an alternative to traditionally employed chromatographic methods due to its simplicity and rapid detection for real-time measurement. However, this method carries drawbacks such as line-broadening, narrow substrate scope, and poor resolution. Thus, compared with popular methods of chromatographic analysis, NMR spectroscopy is infrequently used for chiral analysis.
In principle, a chiral solvating agent is additionally required for the NMR measurement of chiral alcohols to obtain two distinct signals. However, NMR analysis of chiral alcohols has been challenging due to weak binding interactions with chiral solvating agents. To overcome the intrinsic difficulty of relatively weak molecular interactions that are common for alcohols, many researchers have used multifunctional alcohols to enhance interactions with solvating agents.
Instead, the KAIST team successfully varied the physical properties of metal complexes to induce stronger interactions with alcohols rather than the strategy of using multifunctional analytes, in the hopes of developing a universal chiral solvating agent for alcohols. Compared to the current method of chiral analysis used in the pharmaceutical industry, alcohols that do not possess chromophores can also be directly analyzed with the gallium complexes.
Professor Kim said that this method could be a complementary chiral analysis technique at the industry level in the near future. He added that since the developed gallium complex can determine enantiomeric excess within minutes, it can be further utilized to monitor asymmetric synthesis. This feature will benefit a large number of researchers in the organic chemistry community, as well as the pharmaceutical industry.
(Figure: Schematic view of the in-situ direct 1H NMR chiral analysis.)
-Profile:
Professor Hyunwoo Kim
Department of Chemistry
KAIST
http://mdos.kaist.ac.kr
hwk34@kaist.ac.kr
For more on this article,
please go to https://doi.org/10.1016/j.isci2019.07051
2019.11.14 View 11481
Gallium-Based Solvating Agent Efficiently Analyzes Optically Active Alcohols
A KAIST research team has developed a gallium-based metal complex enabling the rapid chiral analysis of alcohols. A team working under Professor Hyunwoo Kim reported the efficient new alcohol analysis method using nuclear magnetic resonance (NMR) spectroscopy in iScience.
Enantiopure chiral alcohols are ubiquitous in nature and widely utilized as pharmaceuticals. This importance of chirality in synthetic and medicinal chemistry has advanced the search for rapid and facile methods to determine the enantiomeric purities of compounds. To date, chiral analysis has been performed using high-performance liquid chromatography (HPLC) with chiral columns.
Along with the HPLC technique, chiral analysis using NMR spectroscopy has gained tremendous attention as an alternative to traditionally employed chromatographic methods due to its simplicity and rapid detection for real-time measurement. However, this method carries drawbacks such as line-broadening, narrow substrate scope, and poor resolution. Thus, compared with popular methods of chromatographic analysis, NMR spectroscopy is infrequently used for chiral analysis.
In principle, a chiral solvating agent is additionally required for the NMR measurement of chiral alcohols to obtain two distinct signals. However, NMR analysis of chiral alcohols has been challenging due to weak binding interactions with chiral solvating agents. To overcome the intrinsic difficulty of relatively weak molecular interactions that are common for alcohols, many researchers have used multifunctional alcohols to enhance interactions with solvating agents.
Instead, the KAIST team successfully varied the physical properties of metal complexes to induce stronger interactions with alcohols rather than the strategy of using multifunctional analytes, in the hopes of developing a universal chiral solvating agent for alcohols. Compared to the current method of chiral analysis used in the pharmaceutical industry, alcohols that do not possess chromophores can also be directly analyzed with the gallium complexes.
Professor Kim said that this method could be a complementary chiral analysis technique at the industry level in the near future. He added that since the developed gallium complex can determine enantiomeric excess within minutes, it can be further utilized to monitor asymmetric synthesis. This feature will benefit a large number of researchers in the organic chemistry community, as well as the pharmaceutical industry.
(Figure: Schematic view of the in-situ direct 1H NMR chiral analysis.)
-Profile:
Professor Hyunwoo Kim
Department of Chemistry
KAIST
http://mdos.kaist.ac.kr
hwk34@kaist.ac.kr
For more on this article,
please go to https://doi.org/10.1016/j.isci2019.07051
2019.11.14 View 11481 -
 AI to Determine When to Intervene with Your Driving
(Professor Uichin Lee (left) and PhD candidate Auk Kim)
Can your AI agent judge when to talk to you while you are driving? According to a KAIST research team, their in-vehicle conservation service technology will judge when it is appropriate to contact you to ensure your safety.
Professor Uichin Lee from the Department of Industrial and Systems Engineering at KAIST and his research team have developed AI technology that automatically detects safe moments for AI agents to provide conversation services to drivers.
Their research focuses on solving the potential problems of distraction created by in-vehicle conversation services. If an AI agent talks to a driver at an inopportune moment, such as while making a turn, a car accident will be more likely to occur.
In-vehicle conversation services need to be convenient as well as safe. However, the cognitive burden of multitasking negatively influences the quality of the service. Users tend to be more distracted during certain traffic conditions. To address this long-standing challenge of the in-vehicle conversation services, the team introduced a composite cognitive model that considers both safe driving and auditory-verbal service performance and used a machine-learning model for all collected data.
The combination of these individual measures is able to determine the appropriate moments for conversation and most appropriate types of conversational services. For instance, in the case of delivering simple-context information, such as a weather forecast, driver safety alone would be the most appropriate consideration. Meanwhile, when delivering information that requires a driver response, such as a “Yes” or “No,” the combination of driver safety and auditory-verbal performance should be considered.
The research team developed a prototype of an in-vehicle conversation service based on a navigation app that can be used in real driving environments. The app was also connected to the vehicle to collect in-vehicle OBD-II/CAN data, such as the steering wheel angle and brake pedal position, and mobility and environmental data such as the distance between successive cars and traffic flow.
Using pseudo-conversation services, the research team collected a real-world driving dataset consisting of 1,388 interactions and sensor data from 29 drivers who interacted with AI conversational agents. Machine learning analysis based on the dataset demonstrated that the opportune moments for driver interruption could be correctly inferred with 87% accuracy.
The safety enhancement technology developed by the team is expected to minimize driver distractions caused by in-vehicle conversation services. This technology can be directly applied to current in-vehicle systems that provide conversation services. It can also be extended and applied to the real-time detection of driver distraction problems caused by the use of a smartphone while driving.
Professor Lee said, “In the near future, cars will proactively deliver various in-vehicle conversation services. This technology will certainly help vehicles interact with their drivers safely as it can fairly accurately determine when to provide conversation services using only basic sensor data generated by cars.”
The researchers presented their findings at the ACM International Joint Conference on Pervasive and Ubiquitous Computing (Ubicomp’19) in London, UK. This research was supported in part by Hyundai NGV and by the Next-Generation Information Computing Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
(Figure: Visual description of safe enhancement technology for in-vehicle conversation services)
2019.11.13 View 17812
AI to Determine When to Intervene with Your Driving
(Professor Uichin Lee (left) and PhD candidate Auk Kim)
Can your AI agent judge when to talk to you while you are driving? According to a KAIST research team, their in-vehicle conservation service technology will judge when it is appropriate to contact you to ensure your safety.
Professor Uichin Lee from the Department of Industrial and Systems Engineering at KAIST and his research team have developed AI technology that automatically detects safe moments for AI agents to provide conversation services to drivers.
Their research focuses on solving the potential problems of distraction created by in-vehicle conversation services. If an AI agent talks to a driver at an inopportune moment, such as while making a turn, a car accident will be more likely to occur.
In-vehicle conversation services need to be convenient as well as safe. However, the cognitive burden of multitasking negatively influences the quality of the service. Users tend to be more distracted during certain traffic conditions. To address this long-standing challenge of the in-vehicle conversation services, the team introduced a composite cognitive model that considers both safe driving and auditory-verbal service performance and used a machine-learning model for all collected data.
The combination of these individual measures is able to determine the appropriate moments for conversation and most appropriate types of conversational services. For instance, in the case of delivering simple-context information, such as a weather forecast, driver safety alone would be the most appropriate consideration. Meanwhile, when delivering information that requires a driver response, such as a “Yes” or “No,” the combination of driver safety and auditory-verbal performance should be considered.
The research team developed a prototype of an in-vehicle conversation service based on a navigation app that can be used in real driving environments. The app was also connected to the vehicle to collect in-vehicle OBD-II/CAN data, such as the steering wheel angle and brake pedal position, and mobility and environmental data such as the distance between successive cars and traffic flow.
Using pseudo-conversation services, the research team collected a real-world driving dataset consisting of 1,388 interactions and sensor data from 29 drivers who interacted with AI conversational agents. Machine learning analysis based on the dataset demonstrated that the opportune moments for driver interruption could be correctly inferred with 87% accuracy.
The safety enhancement technology developed by the team is expected to minimize driver distractions caused by in-vehicle conversation services. This technology can be directly applied to current in-vehicle systems that provide conversation services. It can also be extended and applied to the real-time detection of driver distraction problems caused by the use of a smartphone while driving.
Professor Lee said, “In the near future, cars will proactively deliver various in-vehicle conversation services. This technology will certainly help vehicles interact with their drivers safely as it can fairly accurately determine when to provide conversation services using only basic sensor data generated by cars.”
The researchers presented their findings at the ACM International Joint Conference on Pervasive and Ubiquitous Computing (Ubicomp’19) in London, UK. This research was supported in part by Hyundai NGV and by the Next-Generation Information Computing Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT.
(Figure: Visual description of safe enhancement technology for in-vehicle conversation services)
2019.11.13 View 17812 -
 Ultrafast Quantum Motion in a Nanoscale Trap Detected
< Professor Heung-Sun Sim (left) and Co-author Dr. Sungguen Ryu (right) >
KAIST researchers have reported the detection of a picosecond electron motion in a silicon transistor. This study has presented a new protocol for measuring ultrafast electronic dynamics in an effective time-resolved fashion of picosecond resolution. The detection was made in collaboration with Nippon Telegraph and Telephone Corp. (NTT) in Japan and National Physical Laboratory (NPL) in the UK and is the first report to the best of our knowledge.
When an electron is captured in a nanoscale trap in solids, its quantum mechanical wave function can exhibit spatial oscillation at sub-terahertz frequencies. Time-resolved detection of such picosecond dynamics of quantum waves is important, as the detection provides a way of understanding the quantum behavior of electrons in nano-electronics. It also applies to quantum information technologies such as the ultrafast quantum-bit operation of quantum computing and high-sensitivity electromagnetic-field sensing. However, detecting picosecond dynamics has been a challenge since the sub-terahertz scale is far beyond the latest bandwidth measurement tools.
A KAIST team led by Professor Heung-Sun Sim developed a theory of ultrafast electron dynamics in a nanoscale trap, and proposed a scheme for detecting the dynamics, which utilizes a quantum-mechanical resonant state formed beside the trap. The coupling between the electron dynamics and the resonant state is switched on and off at a picosecond so that information on the dynamics is read out on the electric current being generated when the coupling is switched on.
NTT realized, together with NPL, the detection scheme and applied it to electron motions in a nanoscale trap formed in a silicon transistor. A single electron was captured in the trap by controlling electrostatic gates, and a resonant state was formed in the potential barrier of the trap.
The switching on and off of the coupling between the electron and the resonant state was achieved by aligning the resonance energy with the energy of the electron within a picosecond. An electric current from the trap through the resonant state to an electrode was measured at only a few Kelvin degrees, unveiling the spatial quantum-coherent oscillation of the electron with 250 GHz frequency inside the trap.
Professor Sim said, “This work suggests a scheme of detecting picosecond electron motions in submicron scales by utilizing quantum resonance. It will be useful in dynamical control of quantum mechanical electron waves for various purposes in nano-electronics, quantum sensing, and quantum information”.
This work was published online at Nature Nanotechnology on November 4. It was partly supported by the Korea National Research Foundation through the SRC Center for Quantum Coherence in Condensed Matter. For more on the NTT news release this article, please visit https://www.ntt.co.jp/news2019/1911e/191105a.html
-ProfileProfessor Heung-Sun Sim
Department of PhysicsDirector, SRC Center for Quantum Coherence in Condensed Matterhttps://qet.kaist.ac.kr KAIST
-Publication:Gento Yamahata, Sungguen Ryu, Nathan Johnson, H.-S. Sim, Akira Fujiwara, and Masaya Kataoka. 2019. Picosecond coherent electron motion in a silicon single-electron source. Nature Nanotechnology (Online Publication). 6 pages. https://doi.org/10.1038/s41565-019-0563-2
2019.11.05 View 18233
Ultrafast Quantum Motion in a Nanoscale Trap Detected
< Professor Heung-Sun Sim (left) and Co-author Dr. Sungguen Ryu (right) >
KAIST researchers have reported the detection of a picosecond electron motion in a silicon transistor. This study has presented a new protocol for measuring ultrafast electronic dynamics in an effective time-resolved fashion of picosecond resolution. The detection was made in collaboration with Nippon Telegraph and Telephone Corp. (NTT) in Japan and National Physical Laboratory (NPL) in the UK and is the first report to the best of our knowledge.
When an electron is captured in a nanoscale trap in solids, its quantum mechanical wave function can exhibit spatial oscillation at sub-terahertz frequencies. Time-resolved detection of such picosecond dynamics of quantum waves is important, as the detection provides a way of understanding the quantum behavior of electrons in nano-electronics. It also applies to quantum information technologies such as the ultrafast quantum-bit operation of quantum computing and high-sensitivity electromagnetic-field sensing. However, detecting picosecond dynamics has been a challenge since the sub-terahertz scale is far beyond the latest bandwidth measurement tools.
A KAIST team led by Professor Heung-Sun Sim developed a theory of ultrafast electron dynamics in a nanoscale trap, and proposed a scheme for detecting the dynamics, which utilizes a quantum-mechanical resonant state formed beside the trap. The coupling between the electron dynamics and the resonant state is switched on and off at a picosecond so that information on the dynamics is read out on the electric current being generated when the coupling is switched on.
NTT realized, together with NPL, the detection scheme and applied it to electron motions in a nanoscale trap formed in a silicon transistor. A single electron was captured in the trap by controlling electrostatic gates, and a resonant state was formed in the potential barrier of the trap.
The switching on and off of the coupling between the electron and the resonant state was achieved by aligning the resonance energy with the energy of the electron within a picosecond. An electric current from the trap through the resonant state to an electrode was measured at only a few Kelvin degrees, unveiling the spatial quantum-coherent oscillation of the electron with 250 GHz frequency inside the trap.
Professor Sim said, “This work suggests a scheme of detecting picosecond electron motions in submicron scales by utilizing quantum resonance. It will be useful in dynamical control of quantum mechanical electron waves for various purposes in nano-electronics, quantum sensing, and quantum information”.
This work was published online at Nature Nanotechnology on November 4. It was partly supported by the Korea National Research Foundation through the SRC Center for Quantum Coherence in Condensed Matter. For more on the NTT news release this article, please visit https://www.ntt.co.jp/news2019/1911e/191105a.html
-ProfileProfessor Heung-Sun Sim
Department of PhysicsDirector, SRC Center for Quantum Coherence in Condensed Matterhttps://qet.kaist.ac.kr KAIST
-Publication:Gento Yamahata, Sungguen Ryu, Nathan Johnson, H.-S. Sim, Akira Fujiwara, and Masaya Kataoka. 2019. Picosecond coherent electron motion in a silicon single-electron source. Nature Nanotechnology (Online Publication). 6 pages. https://doi.org/10.1038/s41565-019-0563-2
2019.11.05 View 18233 -
 Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 21416
Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 21416 -
 A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 17857
A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 17857