Nature+Publishing+Group
-
 Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10208
Visualizing Chemical Reaction on Bimetal Surfaces
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park from the Department of Chemistry, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal–oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal–oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide–metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide–metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum–nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum–nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum–nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum–nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal–metal oxide interface, which demonstrates a straightforward process for the strong metal–support interaction effect. The formation of these interfacial metal–metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide–metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal–oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.
Professors Park and Yousung Jung from the Department of Chemical and Biomolecular Engineering and the Graduate School of EEWS conducted this research in collaboration with Professor Bongjin Mun from the Department of Physics at GIST.
Figure 1. Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions.
Figure 2. Formation of Pt-CoO interface leads to the catalytic enhancement of PtCo bimetal catalysts.
2018.07.25 View 10208 -
 Fast-Charging Lithium-Oxygen Batteries
(Professor Hye Ryung Byon)
KAIST researchers have paved the way for fast-charging lithium-oxygen batteries.
Professor Hye Ryung Byon from the Department of Chemistry and Professor Yousung Jung from the Graduate School of EEWS led a joint research team to develop lithium-oxygen batteries exhibiting 80% round-trip efficiency even at high charging rates, solving the problem of existing lithium-oxygen batteries which generally showed drastically lower efficiencies when the charge current rate was increased.
This study exploits the size and shape lithium peroxide, a discharge product, which is known to cause the very problems mentioned above. In doing so, the researchers have lowered the overpotential, which is the difference between the thermodynamic reversible potential and the measured potential, and simultaneously improved battery efficiency. Of particular interest is the fact that these high-performance lithium-oxygen batteries can be realized without costly catalysts.
One remarkable property of lithium-oxygen batteries is that they can accommodate three to five times the energy density of lithium-ion batteries commonly used today. Therefore, lithium-oxygen batteries would render longer driving distance to electric vehicles or drones, which operate on the continued use of electrical power.
However, their weakness lies in that, during charge, the lithium peroxide remains undecomposed at low overpotential, resulting in eventually compromising the battery’s overall performance. This is due to the poor ionic and electrical conductivity of lithium peroxide.
To tackle this issue, the researchers could form one-dimensional amorphous lithium peroxide nanostructures through the use of a mesoporous carbon electrode, CMK-3. When compared against non-mesoporous electrodes, CMK-3 showed exceptionally lower overpotential, thereby enhancing the round-trip efficiency of lithium-oxygen batteries.
The amorphous lithium peroxide produced along the electrode has a small volume and a large surface area contacting electrolyte solution, which is presumably endowed with high conductivity to speed up the charging of the lithium-oxygen batteries.
This research underpins the feasibility of overcoming the fundamental limitations of lithium-oxygen batteries even without the addition of expensive catalytic materials, but rather by the re-configuration of the size and shape of the lithium peroxide.
The findings of this research were published in Nature Communications on February 14.
Figure 1. Transmission electron microscopy (TEM) images
Figure 2. Galvanostatic rate capability
Figure 3. Density functional calculation and Bader charge analysis
2018.05.30 View 10121
Fast-Charging Lithium-Oxygen Batteries
(Professor Hye Ryung Byon)
KAIST researchers have paved the way for fast-charging lithium-oxygen batteries.
Professor Hye Ryung Byon from the Department of Chemistry and Professor Yousung Jung from the Graduate School of EEWS led a joint research team to develop lithium-oxygen batteries exhibiting 80% round-trip efficiency even at high charging rates, solving the problem of existing lithium-oxygen batteries which generally showed drastically lower efficiencies when the charge current rate was increased.
This study exploits the size and shape lithium peroxide, a discharge product, which is known to cause the very problems mentioned above. In doing so, the researchers have lowered the overpotential, which is the difference between the thermodynamic reversible potential and the measured potential, and simultaneously improved battery efficiency. Of particular interest is the fact that these high-performance lithium-oxygen batteries can be realized without costly catalysts.
One remarkable property of lithium-oxygen batteries is that they can accommodate three to five times the energy density of lithium-ion batteries commonly used today. Therefore, lithium-oxygen batteries would render longer driving distance to electric vehicles or drones, which operate on the continued use of electrical power.
However, their weakness lies in that, during charge, the lithium peroxide remains undecomposed at low overpotential, resulting in eventually compromising the battery’s overall performance. This is due to the poor ionic and electrical conductivity of lithium peroxide.
To tackle this issue, the researchers could form one-dimensional amorphous lithium peroxide nanostructures through the use of a mesoporous carbon electrode, CMK-3. When compared against non-mesoporous electrodes, CMK-3 showed exceptionally lower overpotential, thereby enhancing the round-trip efficiency of lithium-oxygen batteries.
The amorphous lithium peroxide produced along the electrode has a small volume and a large surface area contacting electrolyte solution, which is presumably endowed with high conductivity to speed up the charging of the lithium-oxygen batteries.
This research underpins the feasibility of overcoming the fundamental limitations of lithium-oxygen batteries even without the addition of expensive catalytic materials, but rather by the re-configuration of the size and shape of the lithium peroxide.
The findings of this research were published in Nature Communications on February 14.
Figure 1. Transmission electron microscopy (TEM) images
Figure 2. Galvanostatic rate capability
Figure 3. Density functional calculation and Bader charge analysis
2018.05.30 View 10121 -
 New Material for Generating Energy-Efficient Spin Currents
(Professor Byong-Guk Park (left) and Professor Kab-Jin Kim)
Magnetic random access memory (MRAM) is emerging as next-generation memory. It allows information to be kept even without an external power supply and its unique blend of high density and high speed operation is driving global semiconductor manufacturers to develop new versions continuously.
A KAIST team, led by Professor Byong-Guk Park in the Department of Materials Science and Engineering and Professor Kab-Jin Kim in the Department of Physics, recently has developed a new material which enables the efficient generation of a spin current, the core part of operating MRAM. This new material consisting of ferromagnet-transition metal bilayers can randomly control the direction of the generated spin current unlike the existing ones.
They also described a mechanism for spin-current generation at the interface between the bottom ferromagnetic layer and the non-magnetic spacer layer, which gives torques on the top magnetic layer that are consistent with the measured magnetization dependence.
When applying this to spin-orbit torque magnetic memory, it shows the increased efficiency of spin torque and generation of the spin current without an external magnetic field. High-speed operation, the distinct feature of spin-orbit torque-based MRAM that carries its non-volatility, can significantly reduce the standby power better than SRAM.
This new material will expect to speed up the commercialization of MRAM. The research team said that this magnetic memory will further be applied to mobile, wearable, and IoT devices.
This study, conducted in collaboration with Professor Kyung-Jin Lee from Korea University and Dr. Mark Stiles from the National Institute of Standards and Technology in the US, was featured in Nature Materials in March. The research was funded by the Creative Materials Discovery Program of the Ministry of Science and ICT.
(Figure: Ferromagnet-transition metal bilayers which can randomly control the direction of the generated spin current)
2018.05.11 View 10449
New Material for Generating Energy-Efficient Spin Currents
(Professor Byong-Guk Park (left) and Professor Kab-Jin Kim)
Magnetic random access memory (MRAM) is emerging as next-generation memory. It allows information to be kept even without an external power supply and its unique blend of high density and high speed operation is driving global semiconductor manufacturers to develop new versions continuously.
A KAIST team, led by Professor Byong-Guk Park in the Department of Materials Science and Engineering and Professor Kab-Jin Kim in the Department of Physics, recently has developed a new material which enables the efficient generation of a spin current, the core part of operating MRAM. This new material consisting of ferromagnet-transition metal bilayers can randomly control the direction of the generated spin current unlike the existing ones.
They also described a mechanism for spin-current generation at the interface between the bottom ferromagnetic layer and the non-magnetic spacer layer, which gives torques on the top magnetic layer that are consistent with the measured magnetization dependence.
When applying this to spin-orbit torque magnetic memory, it shows the increased efficiency of spin torque and generation of the spin current without an external magnetic field. High-speed operation, the distinct feature of spin-orbit torque-based MRAM that carries its non-volatility, can significantly reduce the standby power better than SRAM.
This new material will expect to speed up the commercialization of MRAM. The research team said that this magnetic memory will further be applied to mobile, wearable, and IoT devices.
This study, conducted in collaboration with Professor Kyung-Jin Lee from Korea University and Dr. Mark Stiles from the National Institute of Standards and Technology in the US, was featured in Nature Materials in March. The research was funded by the Creative Materials Discovery Program of the Ministry of Science and ICT.
(Figure: Ferromagnet-transition metal bilayers which can randomly control the direction of the generated spin current)
2018.05.11 View 10449 -
 Animal Cyborg: Behavioral Control by 'Toy' Craving Circuit
Children love to get toys from parents for their birthday present. This craving toward items also involves object hoarding disorders and shopping addiction. However, the biological meaning of why the brain pursues objects or items has remained unknown. Part of the answer may lie with a neural circuit in the hypothalamus associated with “object craving,” says neuroscientist Daesoo Kim from the Department of Biological Sciences at KAIST.
His research team found that some neurons in the hypothalamus are activated during playing with toys in mice. Thanks to optogenetics, they proved that these neurons in the hypothalamus actually governs obsessive behavior toward non-food objects in mice.
“When we stimulate a neuron in the hypothalamus of mice, they anxiously chased target objects. We found evidence that the neural circuits in the medial preoptic area (MPA) modulate “object craving,” the appetite for possessing objects” said Professor Kim.
Researchers also proved that the MPA circuit facilitate hunting behavior in response to crickets, a natural prey to mice, showing the role of this circuit for catching prey. Further, the MPA nerves send excitatory signals to the periaqueductal gray (PAG), located around the cerebral aqueduct, to create such behavior. The team named this circuit the ‘MPA-PAG’ circuit.
The team showed that they could control mammalian behavior for the first time with this scheme of MPA-Induced Drive Assisted Steering (MIDAS), in which a mouse chase the target objects in the front of head during stimulation of the MPA-PAG circuit. MIDAS allows mice to overcome obstacles to move in a desired path using optogenetics.
(Professor Daesoo Kim)
Professor Kim, who teamed up with Professor Phill Seung Lee in the Department of Mechanical Engineering, explained the significance of the research, “This study provides evidence to treat brain disorders such as compulsive hoarding and kleptomania. It also contributes to the development of technology to control the behavior of animals and humans using strong innate motivation, and thus could impact neuro-economics, defense, and disaster relief.”
He said the team would like to complete the neural circuit map governing behaviors of possession and hunting in the near future by exploring correlations with other neural behaviors controlling possessing and hunting activities.
This research was funded by the Samsung Science and Technology Foundation and published in Nature Neuroscience in March 2018.
(Figure 1: Schematics showing possessive behavior induced by the MPA neural circuit)
(Figure 2: Schematics of the MIDAS system that controls mammals behavior using the desire to possess. A MIDAS mouse is following the bait object controlled wirelessly.)
2018.04.23 View 9881
Animal Cyborg: Behavioral Control by 'Toy' Craving Circuit
Children love to get toys from parents for their birthday present. This craving toward items also involves object hoarding disorders and shopping addiction. However, the biological meaning of why the brain pursues objects or items has remained unknown. Part of the answer may lie with a neural circuit in the hypothalamus associated with “object craving,” says neuroscientist Daesoo Kim from the Department of Biological Sciences at KAIST.
His research team found that some neurons in the hypothalamus are activated during playing with toys in mice. Thanks to optogenetics, they proved that these neurons in the hypothalamus actually governs obsessive behavior toward non-food objects in mice.
“When we stimulate a neuron in the hypothalamus of mice, they anxiously chased target objects. We found evidence that the neural circuits in the medial preoptic area (MPA) modulate “object craving,” the appetite for possessing objects” said Professor Kim.
Researchers also proved that the MPA circuit facilitate hunting behavior in response to crickets, a natural prey to mice, showing the role of this circuit for catching prey. Further, the MPA nerves send excitatory signals to the periaqueductal gray (PAG), located around the cerebral aqueduct, to create such behavior. The team named this circuit the ‘MPA-PAG’ circuit.
The team showed that they could control mammalian behavior for the first time with this scheme of MPA-Induced Drive Assisted Steering (MIDAS), in which a mouse chase the target objects in the front of head during stimulation of the MPA-PAG circuit. MIDAS allows mice to overcome obstacles to move in a desired path using optogenetics.
(Professor Daesoo Kim)
Professor Kim, who teamed up with Professor Phill Seung Lee in the Department of Mechanical Engineering, explained the significance of the research, “This study provides evidence to treat brain disorders such as compulsive hoarding and kleptomania. It also contributes to the development of technology to control the behavior of animals and humans using strong innate motivation, and thus could impact neuro-economics, defense, and disaster relief.”
He said the team would like to complete the neural circuit map governing behaviors of possession and hunting in the near future by exploring correlations with other neural behaviors controlling possessing and hunting activities.
This research was funded by the Samsung Science and Technology Foundation and published in Nature Neuroscience in March 2018.
(Figure 1: Schematics showing possessive behavior induced by the MPA neural circuit)
(Figure 2: Schematics of the MIDAS system that controls mammals behavior using the desire to possess. A MIDAS mouse is following the bait object controlled wirelessly.)
2018.04.23 View 9881 -
 KAIST Develops Sodium Ion Batteries using Copper Sulfide
A KAIST research team recently developed sodium ion batteries using copper sulfide anode. This finding will contribute to advancing the commercialization of sodium ion batteries (SIBs) and reducing the production cost of any electronic products with batteries.
Professor Jong Min Yuk and Emeritus Professor Jeong Yong Lee from Department of Materials Science and Engineering succeeded in developing a new anode material suitable for use in a SIB. Compared to the existing anode materials, the copper sulfide anode was measured to exhibit 1.5 times better cyclability with projected 40% reduction in cost.
Batteries used in various applications including mobile phones are lithium ion batteries, mostly referred as Li-ion batteries or LIBs. Though they are popularly used until now, large-scale energy storage systems require much inexpensive and abundant materials. Hence, a SIB has attracted enormous attention for their advantage over a lithium counterpart.
However, one main obstacle to commercialization of SIB is the lack of suitable anodes that exhibit high capacity and the cycling stability of the battery. Hence, the research team recognized this need for a good anode material that could offer high electrical conductivity and theoretical capacity. The material was found to be copper sulfide, preferably in nanoplates, which “prefers to make an alloy with sodium and is thus promising for high capacity and long-term cyclability.”
Further analysis presented in the study reveals that copper sulfide undergoes crystallographic tuning to make a room for sodium insertion. Results indicate that the sodium ion-insertion capacity of copper sulfide is as much as 1.5 times that of lithium ions for graphite. Furthermore, a battery with this new anode material retains 90% of its original capacity for 250 charge-discharge cycles.
With the natural abundance of sodium in seawater, this development may contribute to reduction in battery costs, which can be translated into up to 30% cut in the price of various consumer electronics.
Professor Lee expressed his hope for “the production of next-generation, high-performance sodium ion batteries”.
Professor Yuk said, “These days, people are showing a great deal of interest in products related to renewable energy due to recent micro-dust issues ongoing in Korea. This study may help Korea get a head-start on renewable energy products”.
This research, led by PhD candidate Jae Yeol Park and Dr. Sung Joo Kim, was published online in Nature Communications on March 2.
Figure 1. The sodiation process of copper sulfide
2018.04.17 View 6872
KAIST Develops Sodium Ion Batteries using Copper Sulfide
A KAIST research team recently developed sodium ion batteries using copper sulfide anode. This finding will contribute to advancing the commercialization of sodium ion batteries (SIBs) and reducing the production cost of any electronic products with batteries.
Professor Jong Min Yuk and Emeritus Professor Jeong Yong Lee from Department of Materials Science and Engineering succeeded in developing a new anode material suitable for use in a SIB. Compared to the existing anode materials, the copper sulfide anode was measured to exhibit 1.5 times better cyclability with projected 40% reduction in cost.
Batteries used in various applications including mobile phones are lithium ion batteries, mostly referred as Li-ion batteries or LIBs. Though they are popularly used until now, large-scale energy storage systems require much inexpensive and abundant materials. Hence, a SIB has attracted enormous attention for their advantage over a lithium counterpart.
However, one main obstacle to commercialization of SIB is the lack of suitable anodes that exhibit high capacity and the cycling stability of the battery. Hence, the research team recognized this need for a good anode material that could offer high electrical conductivity and theoretical capacity. The material was found to be copper sulfide, preferably in nanoplates, which “prefers to make an alloy with sodium and is thus promising for high capacity and long-term cyclability.”
Further analysis presented in the study reveals that copper sulfide undergoes crystallographic tuning to make a room for sodium insertion. Results indicate that the sodium ion-insertion capacity of copper sulfide is as much as 1.5 times that of lithium ions for graphite. Furthermore, a battery with this new anode material retains 90% of its original capacity for 250 charge-discharge cycles.
With the natural abundance of sodium in seawater, this development may contribute to reduction in battery costs, which can be translated into up to 30% cut in the price of various consumer electronics.
Professor Lee expressed his hope for “the production of next-generation, high-performance sodium ion batteries”.
Professor Yuk said, “These days, people are showing a great deal of interest in products related to renewable energy due to recent micro-dust issues ongoing in Korea. This study may help Korea get a head-start on renewable energy products”.
This research, led by PhD candidate Jae Yeol Park and Dr. Sung Joo Kim, was published online in Nature Communications on March 2.
Figure 1. The sodiation process of copper sulfide
2018.04.17 View 6872 -
 Producing 50x More Stable Adsorbent
A KAIST research team developed a technology to increase the stability of amine-containing adsorbents by fifty times, moving one step further toward commercializing stable adsorbents that last longer.
Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering and his team succeeded in developing amine-containing adsorbents that show high oxidative stability.
The capture of the greenhouse gas carbon dioxide is an active ongoing research field, and some of the latest advancements point to amine-containing adsorbents as an efficient and environment-friendly way to capture carbon dioxide. However, existing amine-containing adsorbents are known to be unstable under oxidation, which chemically breaks down the adsorbent, thereby making it difficult to rely on amine-containing adsorbents for repeated and continued use.
The researchers have discovered that the miniscule amount of iron and copper present in the amine accelerate the oxidative breakdown of the amine-containing adsorbent. Upon this discovery, they proposed the use of a chelator substance, which essentially suppresses the activation of the impurities.
The team demonstrates that the proposed method renders the adsorbent up to 50 times slower in its deactivation rate due to oxidation, compared to conventional polyethyleneimine (PEI) / silica adsorbents. Figure 1 illustrates the superior performance of this oxidation-stable amine-containing adsorbent (shown in black squares), whose carbon dioxide-capturing capacity deteriorates by only a small amount (~8%). Meanwhile, the carbon dioxide-capturing capacity of the PEI/silica adsorbent (shown in red diamonds) degrades dramatically after being exposed to oxidative aging for 30 days.
This stability under oxidation is expected to have brought amine-containing adsorbents one step closer to commercialization. As such, first author Woosung Choi describes the significance of this study as “having brought solid carbon dioxide adsorbents to commercializable standards”. In fact, Professor Choi explains that commercialization steps for his team’s carbon dioxide adsorbents are already underway. He further set forth his aim to “develop the world’s best carbon dioxide capture adsorbent”.
This research, led by the PhD candidate Woosung Choi, was published online in Nature Communications on February 20.
Figure 1. Carbon dioxide working capacity against oxidative aging time. Performance of the proposed method (black) degrades much more slowly (~50x) than that of existing methods. The novel adsorbent is thus shown to be more robust to oxidation.
2018.04.16 View 6159
Producing 50x More Stable Adsorbent
A KAIST research team developed a technology to increase the stability of amine-containing adsorbents by fifty times, moving one step further toward commercializing stable adsorbents that last longer.
Professor Minkee Choi from the Department of Chemical and Biomolecular Engineering and his team succeeded in developing amine-containing adsorbents that show high oxidative stability.
The capture of the greenhouse gas carbon dioxide is an active ongoing research field, and some of the latest advancements point to amine-containing adsorbents as an efficient and environment-friendly way to capture carbon dioxide. However, existing amine-containing adsorbents are known to be unstable under oxidation, which chemically breaks down the adsorbent, thereby making it difficult to rely on amine-containing adsorbents for repeated and continued use.
The researchers have discovered that the miniscule amount of iron and copper present in the amine accelerate the oxidative breakdown of the amine-containing adsorbent. Upon this discovery, they proposed the use of a chelator substance, which essentially suppresses the activation of the impurities.
The team demonstrates that the proposed method renders the adsorbent up to 50 times slower in its deactivation rate due to oxidation, compared to conventional polyethyleneimine (PEI) / silica adsorbents. Figure 1 illustrates the superior performance of this oxidation-stable amine-containing adsorbent (shown in black squares), whose carbon dioxide-capturing capacity deteriorates by only a small amount (~8%). Meanwhile, the carbon dioxide-capturing capacity of the PEI/silica adsorbent (shown in red diamonds) degrades dramatically after being exposed to oxidative aging for 30 days.
This stability under oxidation is expected to have brought amine-containing adsorbents one step closer to commercialization. As such, first author Woosung Choi describes the significance of this study as “having brought solid carbon dioxide adsorbents to commercializable standards”. In fact, Professor Choi explains that commercialization steps for his team’s carbon dioxide adsorbents are already underway. He further set forth his aim to “develop the world’s best carbon dioxide capture adsorbent”.
This research, led by the PhD candidate Woosung Choi, was published online in Nature Communications on February 20.
Figure 1. Carbon dioxide working capacity against oxidative aging time. Performance of the proposed method (black) degrades much more slowly (~50x) than that of existing methods. The novel adsorbent is thus shown to be more robust to oxidation.
2018.04.16 View 6159 -
 KAIST Finds the Principle of Electric Wind in Plasma
(From left: Professor Wonho Choe and PhD Sanghoo Park)
A KAIST team identified the basic principle of electric wind in plasma. This finding will contribute to developing technology in various applications of plasma, including fluid control technology.
Professor Wonho Choe from the Department of Physics and his team identified the main principle of neutral gas flow in plasma, known as ‘electric wind’, in collaboration with Professor Se Youn Moon’s team at Chonbuk National University.
Electric wind in plasma is a well-known consequence of interactions arising from collisions between charged particles (electrons or ions) and neutral particles. It refers to the flow of neutral gas that occurs when charged particles accelerate and collide with a neutral gas.
This is a way to create air movement without mechanical movement, such as fan wings, and it is gaining interest as a next-generation technology to replace existing fans. However, there was no experimental evidence of the cause.
To identify the cause, the team used atmospheric pressure plasma. As a result, the team succeeded in identifying streamer propagation and space charge drift from electrohydrodynamic (EHD) force in a qualitative manner.
According to the team, streamer propagation has very little effect on electric wind, but space charge drift that follows streamer propagation and collapse was the main cause of electric wind.
The team also identified that electrons, instead of negatively charged ions, were key components of electric wind generation in certain plasmas.
Furthermore, electric wind with the highest speed of 4 m/s was created in a helium jet plasma, which is one fourth the speed of a typhoon. These results indicate that the study could provide basic principles to effectively control the speed of electric wind.
Professor Choe said, “These findings set a significant foundation to understand the interactions between electrons or ions and neutral particles that occur in weakly ionized plasmas, such as atmospheric pressure plasmas. This can play an important role in expanding the field of fluid-control applications using plasmas which becomes economically and commercially interest.”
This research, led by PhD Sanghoo Park, was published online in Nature Communications on January 25.
Figure 1. Plasma jet image
Figure 2. The differences in electric wind speeds and voltage pulse
2018.03.02 View 7915
KAIST Finds the Principle of Electric Wind in Plasma
(From left: Professor Wonho Choe and PhD Sanghoo Park)
A KAIST team identified the basic principle of electric wind in plasma. This finding will contribute to developing technology in various applications of plasma, including fluid control technology.
Professor Wonho Choe from the Department of Physics and his team identified the main principle of neutral gas flow in plasma, known as ‘electric wind’, in collaboration with Professor Se Youn Moon’s team at Chonbuk National University.
Electric wind in plasma is a well-known consequence of interactions arising from collisions between charged particles (electrons or ions) and neutral particles. It refers to the flow of neutral gas that occurs when charged particles accelerate and collide with a neutral gas.
This is a way to create air movement without mechanical movement, such as fan wings, and it is gaining interest as a next-generation technology to replace existing fans. However, there was no experimental evidence of the cause.
To identify the cause, the team used atmospheric pressure plasma. As a result, the team succeeded in identifying streamer propagation and space charge drift from electrohydrodynamic (EHD) force in a qualitative manner.
According to the team, streamer propagation has very little effect on electric wind, but space charge drift that follows streamer propagation and collapse was the main cause of electric wind.
The team also identified that electrons, instead of negatively charged ions, were key components of electric wind generation in certain plasmas.
Furthermore, electric wind with the highest speed of 4 m/s was created in a helium jet plasma, which is one fourth the speed of a typhoon. These results indicate that the study could provide basic principles to effectively control the speed of electric wind.
Professor Choe said, “These findings set a significant foundation to understand the interactions between electrons or ions and neutral particles that occur in weakly ionized plasmas, such as atmospheric pressure plasmas. This can play an important role in expanding the field of fluid-control applications using plasmas which becomes economically and commercially interest.”
This research, led by PhD Sanghoo Park, was published online in Nature Communications on January 25.
Figure 1. Plasma jet image
Figure 2. The differences in electric wind speeds and voltage pulse
2018.03.02 View 7915 -
 KAIST to Develop Technology to Control Topological Defects
(Professor Chan-Ho Yang and PhD candidate Kwang-Eun Kim)
Professor Chan-Ho Yang and his team from the Department of Physics developed technology to create and remove topological defects in ferroelectric nanostructures.
This technology will contribute to developing topological defect-based storage that will allow the saving of massive amounts of information in a stable manner.
Topology refers to the property of matter upon deformation, in which a circle and a triangle are considered to be the same topologically.
During the announcement of the 2016 Nobel Prize in Physics, the concept of topology was explained with a bagel with a hole, cinnamon bread without a hole, and a glass cup. Although the cinnamon bread and the glass cup have different appearances, they are topologically the same since neither has a hole. In the same sense, the bagel and the cinnamon bread are topologically different.
In other words, topology of matter is conserved and its properties cannot be altered by continuous deformation.
Using this topological texture can produce information storage devices that can protect the stored information from external stimuli, but the data can still be written and erased, resulting in ideal non-volatile memory.
Unlike ferroelectrics, magnetic topological defect structures such as the ferromagnetic vortex and skyrmion have already been implemented.
Ferroelectrics, which have aligned electric dipoles without external electric fields, can stabilize topological defect structures to a smaller size using less energy; however, further research on ferroelectrics has not been carried out sufficiently. This is due to a lack of research on stabilizing topological defect structures and how to control them in an experimental setting.
To overcome this problem, the team applied inhomogeneous deformations to ferroelectric nanostructures to successfully stabilize the topological defect structures. The team manufactured a ferroelectric nanoplate structure on a special board, which can exert strong compression from the bottom surface while the sides and the upper surfaces of the structure is free from deformation.
This structure led to radial compressive strain relaxation, in which deformations of the lattice stabilize the vortex structure of ferroelectrics.
This could lead to the establishment of the core principle of topological ferroelectric memory of high density, high efficiency, and high stability.
Professor Yang said, “Ferroelectrics are nonconductor but topological ferroelectric quasiparticles could carry electrical conductivity locally. This finding could be expanded to new quantum device research.”
This research, led by the PhD candidate Kwang-Eun Kim, was published in Nature Communications on January 26.
The study was co-conducted by Professor Si-Young Choi and Dr. Tae Yeong Koo from POSTECH, Professor Long-Qing Chen from The Pennsylvania State University, and Professor Ramamoorthy Ramesh from the University of California at Berkeley.
Figure 1. Five different topological structures produced by controlling the number of topological defects
2018.02.19 View 7861
KAIST to Develop Technology to Control Topological Defects
(Professor Chan-Ho Yang and PhD candidate Kwang-Eun Kim)
Professor Chan-Ho Yang and his team from the Department of Physics developed technology to create and remove topological defects in ferroelectric nanostructures.
This technology will contribute to developing topological defect-based storage that will allow the saving of massive amounts of information in a stable manner.
Topology refers to the property of matter upon deformation, in which a circle and a triangle are considered to be the same topologically.
During the announcement of the 2016 Nobel Prize in Physics, the concept of topology was explained with a bagel with a hole, cinnamon bread without a hole, and a glass cup. Although the cinnamon bread and the glass cup have different appearances, they are topologically the same since neither has a hole. In the same sense, the bagel and the cinnamon bread are topologically different.
In other words, topology of matter is conserved and its properties cannot be altered by continuous deformation.
Using this topological texture can produce information storage devices that can protect the stored information from external stimuli, but the data can still be written and erased, resulting in ideal non-volatile memory.
Unlike ferroelectrics, magnetic topological defect structures such as the ferromagnetic vortex and skyrmion have already been implemented.
Ferroelectrics, which have aligned electric dipoles without external electric fields, can stabilize topological defect structures to a smaller size using less energy; however, further research on ferroelectrics has not been carried out sufficiently. This is due to a lack of research on stabilizing topological defect structures and how to control them in an experimental setting.
To overcome this problem, the team applied inhomogeneous deformations to ferroelectric nanostructures to successfully stabilize the topological defect structures. The team manufactured a ferroelectric nanoplate structure on a special board, which can exert strong compression from the bottom surface while the sides and the upper surfaces of the structure is free from deformation.
This structure led to radial compressive strain relaxation, in which deformations of the lattice stabilize the vortex structure of ferroelectrics.
This could lead to the establishment of the core principle of topological ferroelectric memory of high density, high efficiency, and high stability.
Professor Yang said, “Ferroelectrics are nonconductor but topological ferroelectric quasiparticles could carry electrical conductivity locally. This finding could be expanded to new quantum device research.”
This research, led by the PhD candidate Kwang-Eun Kim, was published in Nature Communications on January 26.
The study was co-conducted by Professor Si-Young Choi and Dr. Tae Yeong Koo from POSTECH, Professor Long-Qing Chen from The Pennsylvania State University, and Professor Ramamoorthy Ramesh from the University of California at Berkeley.
Figure 1. Five different topological structures produced by controlling the number of topological defects
2018.02.19 View 7861 -
 New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6096
New Arylation Inducing Reaction Developed
(Professor Chang(left) and Professor Baik)
KAIST researchers have identified a reaction mechanism that selectively introduces aryl groups at the desired position of a molecule at room temperature. A team, co-led by Professor Sukbok Chang and Mu-Hyun Baik of the Department of Chemistry, used an iridium catalyst for the reaction. The team also proved that the reaction proceeds by an unusual mechanism by employing computer simulations that were substantiated with targeted experimental probes.
Hydrocarbon is an omnipresent material in nature. But its low reactivity makes it difficult to process to value-added products at the room temperature. Thus, designing catalysts that can accelerate the reaction remains an important challenge in chemistry.
In particular, since most chemicals used in medicine, pharmacy, or material chemistry contain aryl groups, an effective reaction to selectively introduce the aryl group has been an area of intensive research in organic chemistry.
In order to introduce an aryl group into stable carbon-hydrogen (C-H) bond, activation of the C-H bond with a halogen atom or organic metal is required prior to the introduction of the aryl group, or C-H functionalization directly on C-H bond is needed. Direct functionalization is more effective and economical, but most reactions require harsh reaction conditions such as high temperature or excess additives. And adding the aryl fragment selectively to only one among the many possible sites in the molecule is difficult. The new catalyst developed by these KAIST researchers is highly selective.
This work is the latest example of a successful teamwork between experimental and theoretical research groups: Computer simulations revealed that traditional approaches to arylation required high energies because the intermediates produced during the reaction are too low in energy. Based on this insight, the researchers thought of changing the character of the intermediate by oxidizing it, which was predicted to be a great way of increasing the reactivity of the catalyst. Subsequent experimental work showed that this design strategy is highly effective resulting in unprecedented chemical transformations.
Professor Chang said, “We have been able to carry out location-selective arylation at room temperature, as well as identifying a new reaction pathway, different from the conventionally suggested mechanism.” He continued, “This research is significant for identifying the reaction pathway and developing a novel selective reaction method that does not require high temperature or additives based on the mechanistic understanding. This work is a triumph of rational design, rather than fortuitous discovery.” The research findings were published online in Nature Chemistry on December 11, 2017.
(Figure 1: X-ray crystal structure transmetallation intermediate)
(Figure 2: Correlation between oxidation state of intermediate and energy barrier required for reductive elimination of intermediate as calculated using density function from computational chemistry )
(Figure 3: Arylation mechanism using iridium catalyst as suggested by the research team)
2018.01.11 View 6096 -
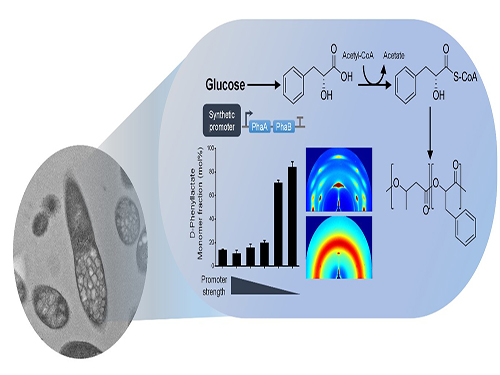 One-Step Production of Aromatic Polyesters by E. coli Strains
KAIST systems metabolic engineers defined a novel strategy for microbial aromatic polyesters production fused with synthetic biology from renewable biomass. The team of Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering produced aromatic polyesters from Escherichia coli (E. coli) strains by applying microbial fermentation, employing direct microbial fermentation from renewable feedstock carbohydrates.
This is the first report to determine a platform strain of engineered E. coli capable of producing environmentally friendly aromatic polyesters. This engineered E. coli strain, if desired, has the potential to be used as a platform strain capable of producing various high-valued aromatic polyesters from renewable biomass. This research was published in Nature Communications on January 8.
Conventionally, aromatic polyesters boast solid strength and heat stability so that there has been a great deal of interest in fermentative production of aromatic polyesters from renewable non-food biomass, but without success.
However, aromatic polyesters are only made by feeding the cells with corresponding aromatic monomers as substrates, and have not been produced by direct fermentation from renewable feedstock carbohydrates such as glucose.
To address this issue, the team prescribed the detailed procedure for aromatic polyester production through identifying CoA-transferase that activates phenylalkanoates into their corresponding CoA derivatives. In this process, researchers employed metabolic engineering of E. coli to produce phenylalkanoates from glucose based on genome-scale metabolic flux analysis. In particular, the KAIST team made a modulation of gene expression to produce various aromatic polyesters having different monomer fractions.
The research team successfully produced aromatic polyesters, a non-natural polymer using the strategy that combines systems metabolic engineering and synthetic biology. They succeeded in biosynthesis of various kinds of aromatic polyesters through the system, thus proving the technical excellence of the environmentally friendly biosynthetic system of this research. Furthermore, his team also proved the potential of expanding the range of aromatic polyesters from renewable resources, which is expected to play an important role in the bio-plastic industry.
Professor Lee said, “An eco-friendly and sustainable chemical industry is the key global agenda every nation faces. We are making a research focus to a biochemical industry free from petroleum dependence, and conducting diverse research activities to address the issue. This novel technology we are presenting will serve as an opportunity to advance the biochemical industry moving forward.”
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) and also by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation of Korea.
Figure: Biosynthesis of aromatic polyesters by metabolically engineered E. coli.This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced aromatic polyesters from glucose.
2018.01.09 View 7642
One-Step Production of Aromatic Polyesters by E. coli Strains
KAIST systems metabolic engineers defined a novel strategy for microbial aromatic polyesters production fused with synthetic biology from renewable biomass. The team of Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering produced aromatic polyesters from Escherichia coli (E. coli) strains by applying microbial fermentation, employing direct microbial fermentation from renewable feedstock carbohydrates.
This is the first report to determine a platform strain of engineered E. coli capable of producing environmentally friendly aromatic polyesters. This engineered E. coli strain, if desired, has the potential to be used as a platform strain capable of producing various high-valued aromatic polyesters from renewable biomass. This research was published in Nature Communications on January 8.
Conventionally, aromatic polyesters boast solid strength and heat stability so that there has been a great deal of interest in fermentative production of aromatic polyesters from renewable non-food biomass, but without success.
However, aromatic polyesters are only made by feeding the cells with corresponding aromatic monomers as substrates, and have not been produced by direct fermentation from renewable feedstock carbohydrates such as glucose.
To address this issue, the team prescribed the detailed procedure for aromatic polyester production through identifying CoA-transferase that activates phenylalkanoates into their corresponding CoA derivatives. In this process, researchers employed metabolic engineering of E. coli to produce phenylalkanoates from glucose based on genome-scale metabolic flux analysis. In particular, the KAIST team made a modulation of gene expression to produce various aromatic polyesters having different monomer fractions.
The research team successfully produced aromatic polyesters, a non-natural polymer using the strategy that combines systems metabolic engineering and synthetic biology. They succeeded in biosynthesis of various kinds of aromatic polyesters through the system, thus proving the technical excellence of the environmentally friendly biosynthetic system of this research. Furthermore, his team also proved the potential of expanding the range of aromatic polyesters from renewable resources, which is expected to play an important role in the bio-plastic industry.
Professor Lee said, “An eco-friendly and sustainable chemical industry is the key global agenda every nation faces. We are making a research focus to a biochemical industry free from petroleum dependence, and conducting diverse research activities to address the issue. This novel technology we are presenting will serve as an opportunity to advance the biochemical industry moving forward.”
This work was supported by the Intelligent Synthetic Biology Center through the Global Frontier Project (2011-0031963) and also by the Technology Development Program to Solve Climate Changes on Systems Metabolic Engineering for Biorefineries (NRF-2012M1A2A2026556 and NRF-2012M1A2A2026557) from the Ministry of Science and ICT through the National Research Foundation of Korea.
Figure: Biosynthesis of aromatic polyesters by metabolically engineered E. coli.This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced aromatic polyesters from glucose.
2018.01.09 View 7642 -
 Technology to Find Optimum Drug Target for Cancer Developed
(Professor Kwang-Hyun Cho (right) and lead author Dr. Minsoo Choi)
A KAIST research team led by Professor Kwang-Hyun Cho of the Department of Bio and Brain Engineering developed technology to find the optimum drug target according to the type of cancer cell. The team used systems biology to analyze molecular network dynamics that reflect genetic mutations in cancer cells and to predict drug response. The technology could contribute greatly to future anti-cancer drug development.
There are many types of genetic variations found in cancer cells, including gene mutations and copy number variations. These variations differ in cancer cells even within the same type of cancer, and thus the drug response varies cell by cell. Cancer researchers worked towards identifying frequently occurring genetic variations in cancer patients and, in particular, the mutations that can be used as an index for specific drugs. Previous studies focused on identifying a single genetic mutation or creating an analysis of the structural characteristics of a gene network. However, this approach was limited in its inability to explain the biological properties of cancer which are induced by various gene and protein interactions in cancer cells, which result in differences in drug response.
Gene mutations in cancer cells not only affect the function of the affected gene, but also other genes that interact with the mutated gene and proteins. As a consequence, one mutation could lead to changes in the dynamical properties of the molecular network. Therefore, the responses to anti-cancer drugs by cancer cells differ. The current treatment approach that ignores molecular network dynamics and targets a few cancer-related genes is only effective on a fraction of patients, while many other patients exhibit resistance to the drug.
Professor Cho’s team integrated a large-scale computer simulation using super-computing and cellular experiments to analyze changes in molecular network dynamics in cancer cells.
This led to development of technology to find the optimum drug target according to the type of cancer cells by predicting drug response. This technology was applied to the molecular network of known tumor suppressor p53. The team used large-scale cancer cell genomic data available from The Cancer Cell Line Encyclopedia (CCLE) to construct different molecular networks specific to the characteristics of genetic variations.
Perturbation analysis on drug response in each molecular network was used to quantify changes in cancer cells from drug response and similar networks were clustered. Then, computer simulations were used to analyze the synergetic effects in terms of efficacy and combination to predict the level of drug response. Based on the simulation results from various cancer cell lines including lung, breast, bone, skin, kidney, and ovary cancers were used in drug response experiments for compare analysis.
This technique can be applied in any molecular network to identify the optimum drug target for personalized medicine.
The research team suggests that the technology can analyze varying drug response due to the heterogeneity of cancer cells by considering the overall modulatory interactions rather than focusing only on a specific gene or protein. Further, the technology aids the prediction of causes of drug resistance and thus the identification of the optimum drug target to inhibit the resistance. This could be core source technology that can be used in drug repositioning, a process of applying existing drugs to new disease targets.
Professor Cho said, “Genetic variations in cancer cells are the cause of diverse drug response, but a complete analysis had not yet been made.” He continued, “Systems biology allowed the simulation of drug responses by cancer cell molecular networks to identify fundamental principles of drug response and optimum drug targets using a new conceptual approach.”
This research was published in Nature Communications on December 5 and was funded by Ministry of Science and ICT and National Research Foundation of Korea.
(Figure 1. Drug response prediction for each cancer cell type from computer simulation and cellular experiment verification for comparison)
(Figure 2. Drug response prediction based on cancer cell molecular network dynamics and clustering of cancer cells by their molecular networks)
(Figure 3. Identification of drug target for each cancer cell type by cellular molecular network analysis and establishment for personalized medicine strategy for each cancer patient)
2017.12.15 View 7236
Technology to Find Optimum Drug Target for Cancer Developed
(Professor Kwang-Hyun Cho (right) and lead author Dr. Minsoo Choi)
A KAIST research team led by Professor Kwang-Hyun Cho of the Department of Bio and Brain Engineering developed technology to find the optimum drug target according to the type of cancer cell. The team used systems biology to analyze molecular network dynamics that reflect genetic mutations in cancer cells and to predict drug response. The technology could contribute greatly to future anti-cancer drug development.
There are many types of genetic variations found in cancer cells, including gene mutations and copy number variations. These variations differ in cancer cells even within the same type of cancer, and thus the drug response varies cell by cell. Cancer researchers worked towards identifying frequently occurring genetic variations in cancer patients and, in particular, the mutations that can be used as an index for specific drugs. Previous studies focused on identifying a single genetic mutation or creating an analysis of the structural characteristics of a gene network. However, this approach was limited in its inability to explain the biological properties of cancer which are induced by various gene and protein interactions in cancer cells, which result in differences in drug response.
Gene mutations in cancer cells not only affect the function of the affected gene, but also other genes that interact with the mutated gene and proteins. As a consequence, one mutation could lead to changes in the dynamical properties of the molecular network. Therefore, the responses to anti-cancer drugs by cancer cells differ. The current treatment approach that ignores molecular network dynamics and targets a few cancer-related genes is only effective on a fraction of patients, while many other patients exhibit resistance to the drug.
Professor Cho’s team integrated a large-scale computer simulation using super-computing and cellular experiments to analyze changes in molecular network dynamics in cancer cells.
This led to development of technology to find the optimum drug target according to the type of cancer cells by predicting drug response. This technology was applied to the molecular network of known tumor suppressor p53. The team used large-scale cancer cell genomic data available from The Cancer Cell Line Encyclopedia (CCLE) to construct different molecular networks specific to the characteristics of genetic variations.
Perturbation analysis on drug response in each molecular network was used to quantify changes in cancer cells from drug response and similar networks were clustered. Then, computer simulations were used to analyze the synergetic effects in terms of efficacy and combination to predict the level of drug response. Based on the simulation results from various cancer cell lines including lung, breast, bone, skin, kidney, and ovary cancers were used in drug response experiments for compare analysis.
This technique can be applied in any molecular network to identify the optimum drug target for personalized medicine.
The research team suggests that the technology can analyze varying drug response due to the heterogeneity of cancer cells by considering the overall modulatory interactions rather than focusing only on a specific gene or protein. Further, the technology aids the prediction of causes of drug resistance and thus the identification of the optimum drug target to inhibit the resistance. This could be core source technology that can be used in drug repositioning, a process of applying existing drugs to new disease targets.
Professor Cho said, “Genetic variations in cancer cells are the cause of diverse drug response, but a complete analysis had not yet been made.” He continued, “Systems biology allowed the simulation of drug responses by cancer cell molecular networks to identify fundamental principles of drug response and optimum drug targets using a new conceptual approach.”
This research was published in Nature Communications on December 5 and was funded by Ministry of Science and ICT and National Research Foundation of Korea.
(Figure 1. Drug response prediction for each cancer cell type from computer simulation and cellular experiment verification for comparison)
(Figure 2. Drug response prediction based on cancer cell molecular network dynamics and clustering of cancer cells by their molecular networks)
(Figure 3. Identification of drug target for each cancer cell type by cellular molecular network analysis and establishment for personalized medicine strategy for each cancer patient)
2017.12.15 View 7236 -
 A New Spin Current Generating Material Developed
(Professor Park(left) and Ph.D. candidate Kim)
Magnetic random-access memory (MRAM) is a non-volatile device made of thin magnetic film that can maintain information without an external power supply, in contrast to conventional silicon-based semiconductor memory. It also has the potential for high-density integration and high-speed operation.
The operation of MRAM involves the control of the magnetization direction by exerting spin current-induced torque on a magnetic material. Spin current is generated using electricity in conventional MRAM, but this study developed materials technology that generates spin current using heat.
A KAIST research team led by Professor Byong-Guk Park of the Department of Materials Science and Engineering developed a material that generates spin current from heat, which can be utilized for a new operation principle for MRAM.
There have been theoretical reports on the spin Nernst effect, the phenomenon of the thermal generation of spin current, but is yet to have been experimentally proven due to technological limitations. However, the research team introduced a spin Nernst magnetoresistance measurement method using tungsten (W) and platinum (Pt) with high spin orbit coupling which allows for the experimental identification of the spin Nernst effect. They also demonstrated that the efficiency of spin current generation from heat is similar to that of spin current generated from electricity.
Professor Park said, “This research has great significance in experimentally proving spin current generation from heat, a new physical phenomenon. We aim to develop the technology as a new operational method for MRAM through further research. This can lower power consumption, and is expected to contribute to the advancement of electronics requiring low power requirement such as wearable, mobile, and IOT devices”.
This research was conducted as a joint research project with Professor Kyung-Jin Lee at Korea University and Professor Jong-Ryul Jeong at Chungnam National University. It was published in Nature Communications online on November 9 titled “Observation of transverse spin Nernst magnetoresistance induced by thermal spin current in ferromagnet/non-magnet bilayers.” Ph.D. candidate Dong-Jun Kim at KAIST is the first author. This research was funded by the Ministry of Science and ICT.
(Schematic diagram of spin Nernst magnetoresistance)
(Research result of new spin current generating materials)
2017.12.08 View 8563
A New Spin Current Generating Material Developed
(Professor Park(left) and Ph.D. candidate Kim)
Magnetic random-access memory (MRAM) is a non-volatile device made of thin magnetic film that can maintain information without an external power supply, in contrast to conventional silicon-based semiconductor memory. It also has the potential for high-density integration and high-speed operation.
The operation of MRAM involves the control of the magnetization direction by exerting spin current-induced torque on a magnetic material. Spin current is generated using electricity in conventional MRAM, but this study developed materials technology that generates spin current using heat.
A KAIST research team led by Professor Byong-Guk Park of the Department of Materials Science and Engineering developed a material that generates spin current from heat, which can be utilized for a new operation principle for MRAM.
There have been theoretical reports on the spin Nernst effect, the phenomenon of the thermal generation of spin current, but is yet to have been experimentally proven due to technological limitations. However, the research team introduced a spin Nernst magnetoresistance measurement method using tungsten (W) and platinum (Pt) with high spin orbit coupling which allows for the experimental identification of the spin Nernst effect. They also demonstrated that the efficiency of spin current generation from heat is similar to that of spin current generated from electricity.
Professor Park said, “This research has great significance in experimentally proving spin current generation from heat, a new physical phenomenon. We aim to develop the technology as a new operational method for MRAM through further research. This can lower power consumption, and is expected to contribute to the advancement of electronics requiring low power requirement such as wearable, mobile, and IOT devices”.
This research was conducted as a joint research project with Professor Kyung-Jin Lee at Korea University and Professor Jong-Ryul Jeong at Chungnam National University. It was published in Nature Communications online on November 9 titled “Observation of transverse spin Nernst magnetoresistance induced by thermal spin current in ferromagnet/non-magnet bilayers.” Ph.D. candidate Dong-Jun Kim at KAIST is the first author. This research was funded by the Ministry of Science and ICT.
(Schematic diagram of spin Nernst magnetoresistance)
(Research result of new spin current generating materials)
2017.12.08 View 8563