Medicine
-
 Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
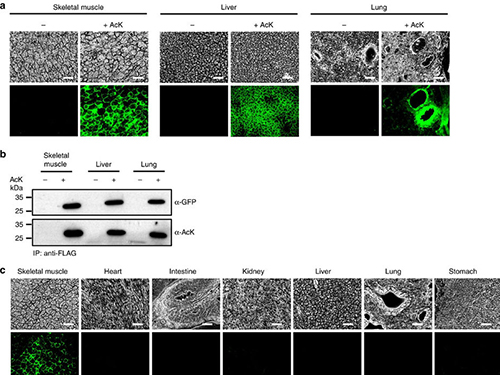
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 9905
Expanding the Genetic Code of Mus Musculus
Professor Hee-Sung Park of the Department of Chemistry, who garnered attention for his novel strategy of installing authentic post-translational modifications into recombinant proteins, expanded his research portfolio to another level. Professor Park’s team was the first to report the generation of a mouse strain with an expanded genetic code, allowing site-specific incorporation of unnatural amino acids.
Professor Park published the research on the new chemical biology method for achieving selective chemical modifications in proteins in Science last September. The research team, this time in collaboration with Professor Chan Bae Park of the Department of Physiology at the Ajou University School of Medicine, demonstrated temporal and spatial control of protein acetylation in various organs of the transgenic mouse using a recombinant green fluorescent protein as a model protein. This research was published in the online edition of Nature Communications on February 21.
This approach enables the rapid onset of position-specific acetylation of a target protein at any developmental stage, facilitating temporal and spatial control of protein acetylation in various organs of the transgenic mouse. Such temporal and spatial control of protein acetylation will be of prime importance for investigating many essential biological processes and human diseases at the tissue and organism level.
Almost all human proteins, the products of about 25,000 genes, are known to undergo various post-translational modifications during and after synthesis. Post-translation modifications regulate the function of cellular proteins, playing a key role in many essential processes such as delivering signals and body growth. However, the unusual protein modifications, aroused from genetic and/or environmental factors, trigger severe diseases including cancer, dementia, and diabetes.
The team inserted transgenes into the mouse genome to allocate the site-specific addition of unnatural amino acids. The researchers inserted a modified version of lysine into the house mice, which allowed for the control of the acetylation. They used recombinant green fluorescent proteins from transgenic house mice as models for control of the acetylation.
The team was also able to regulate the acetylation of specific temporal and spatial frames in the mice, restraining the abnormality in proteins to certain organs such as the liver and kidneys. The research team said the strategy will provide a powerful tool for systematic in vivo study of cellular proteins in the most commonly used mammalian model organisms for human physiology and disease. Professor Park said, “This method can be easily extended to generate a wide range of custom-made transgenic mouse strains for further investigating diverse proteins of interest.” He added, “This method can be further extended to generate a wide range of custom-made transgenic mouse strains, opening a new paradigm for investigating anti-cancer and cerebral disease treatments.
This work was supported by grants from KAIST Systems Healthcare and the Medicinal Bioconvergence Research Center and the Intelligent Synthetic Biology Center of the Global Frontier Project funded by the Ministry of Science, ICT & Future Planning and the Ministry of Food and Drug Safety.
(Figure:Temporal and spatial control of in vivo protein acetylation)
(a) Temporal expression of acetylated GFPuv in the AcK-GFPamber mouse. The expression of GFPuv in skeletal muscle, liver, and lung tissues was detected only in the AcK-injected mouse. Scale bar, 200 µm. (b) Western blotting of anti-FLAG-immunoprecipitated proteins from tissues of the AcK-GFPamber mouse. Acetylated GFPuv was produced after AcK injection. (c) Spatial expression of acetylated GFPuv in the AcK-GFPamber mouse. Acetylated GFPuv was observed only in skeletal muscle when AcK was directly delivered to the tissues. Sacle bar, 200 µm.
2017.03.27 View 9905 -
 More Donations Arrive to Establish the New Medicine Research and Development Center on Campus
A raft of businesses continues to make donations to establish a new medicine research and development center on campus. The Department of Biological Sciences at KAIST is leading the fundraising campaign.
On November 9, 2015, Nikon Instruments Korea Co., Ltd. contributed USD 8,500 to the fundraising, followed by Carl Zeiss AG and Three-Shine Inc., which donated USD 12,800 and 8,500, respectively.
Bruno Lin, an Executive Director at Carl Zeiss AG in Korea, said, “I’m very glad to participate in this fundraising initiative for the Biological Sciences Department at KAIST, one rapidly reaching out to the world.”
From the left in the picture are Vice President Tae-Hoon Kim, Director Gyu-Hyeok Lee, and Executive Director Bruno Lin of Carl Zeiss AG, Byung-Ha Oh, Dean of the Biological Sciences Department, and Professor Eunjoon Kim.
From the left in the picture are Byung-Ha Oh, Dean of the Biological Sciences Department, President Chun-Gui Park of Three-Shine Inc., and Professor Daesoo Kim.
President Chun of Three-Shine Inc., said, “We hope that the Department of Biological Sciences at KAIST, aided by the construction of new research center, will produce practical research achievements and stand on the frontier of new medicine development research in Korea.”
The New Medicine Research and Development Center will be equipped with state-of-the-art, purpose-built research facilities to support convergent, interdisciplinary research in biomedicine.
2015.11.27 View 8043
More Donations Arrive to Establish the New Medicine Research and Development Center on Campus
A raft of businesses continues to make donations to establish a new medicine research and development center on campus. The Department of Biological Sciences at KAIST is leading the fundraising campaign.
On November 9, 2015, Nikon Instruments Korea Co., Ltd. contributed USD 8,500 to the fundraising, followed by Carl Zeiss AG and Three-Shine Inc., which donated USD 12,800 and 8,500, respectively.
Bruno Lin, an Executive Director at Carl Zeiss AG in Korea, said, “I’m very glad to participate in this fundraising initiative for the Biological Sciences Department at KAIST, one rapidly reaching out to the world.”
From the left in the picture are Vice President Tae-Hoon Kim, Director Gyu-Hyeok Lee, and Executive Director Bruno Lin of Carl Zeiss AG, Byung-Ha Oh, Dean of the Biological Sciences Department, and Professor Eunjoon Kim.
From the left in the picture are Byung-Ha Oh, Dean of the Biological Sciences Department, President Chun-Gui Park of Three-Shine Inc., and Professor Daesoo Kim.
President Chun of Three-Shine Inc., said, “We hope that the Department of Biological Sciences at KAIST, aided by the construction of new research center, will produce practical research achievements and stand on the frontier of new medicine development research in Korea.”
The New Medicine Research and Development Center will be equipped with state-of-the-art, purpose-built research facilities to support convergent, interdisciplinary research in biomedicine.
2015.11.27 View 8043 -
 Nikon Instruments Korea Donates a Fund to KAIST's Department of Biological Sciences
Representatives from Nikon Instruments Korea Co., Ltd., a producer of microscopes and measuring instruments, visited the KAIST campus on September 25, 2015, and donated USD 9,000 to the Department of Biological Sciences at KAIST.
A small ceremony to mark the donation took place at the department’s conference room.
In the picture from left to right were Professor Won-Do Heo, Department Head Byung-Ha Oh, Professor Sangyong Jon, President Sam-Sup Jang of Korea Instech, and Director Ik-Soo Yoo of Nikon Instruments Korea.
The department announced that the fund would be used to build its new research center to house the state-of-the-art research equipment and tools for the development of new medicine.
2015.10.03 View 5493
Nikon Instruments Korea Donates a Fund to KAIST's Department of Biological Sciences
Representatives from Nikon Instruments Korea Co., Ltd., a producer of microscopes and measuring instruments, visited the KAIST campus on September 25, 2015, and donated USD 9,000 to the Department of Biological Sciences at KAIST.
A small ceremony to mark the donation took place at the department’s conference room.
In the picture from left to right were Professor Won-Do Heo, Department Head Byung-Ha Oh, Professor Sangyong Jon, President Sam-Sup Jang of Korea Instech, and Director Ik-Soo Yoo of Nikon Instruments Korea.
The department announced that the fund would be used to build its new research center to house the state-of-the-art research equipment and tools for the development of new medicine.
2015.10.03 View 5493 -
 KAIST & the Classic 500 Co Sign for Mobile Healthcare Research
KAIST and The Classic 500 Co., Ltd., an elder care provider based in Seoul, signed a memorandum of understanding to expand medical services by cooperating on the research of medical services and IT on March 24, 2015.
Twenty people from the two institutions, including President Steve Kang, Dong-Hyun Bak, CEO of The Classic 500 and Mun-Sul Jeong, a former KAIST Chairman of the Board, attended the signing ceremony.
Under the agreement, the two institutions will cooperate on mobile healthcare research and the development of a telemedicine system. They will also research and develop a system to better serve society with medical services.
The Classic 500, established by Konkuk University in Korea, provides nursing care services and assisted living facilities for senior citizens.
2015.03.26 View 9022
KAIST & the Classic 500 Co Sign for Mobile Healthcare Research
KAIST and The Classic 500 Co., Ltd., an elder care provider based in Seoul, signed a memorandum of understanding to expand medical services by cooperating on the research of medical services and IT on March 24, 2015.
Twenty people from the two institutions, including President Steve Kang, Dong-Hyun Bak, CEO of The Classic 500 and Mun-Sul Jeong, a former KAIST Chairman of the Board, attended the signing ceremony.
Under the agreement, the two institutions will cooperate on mobile healthcare research and the development of a telemedicine system. They will also research and develop a system to better serve society with medical services.
The Classic 500, established by Konkuk University in Korea, provides nursing care services and assisted living facilities for senior citizens.
2015.03.26 View 9022 -
 Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
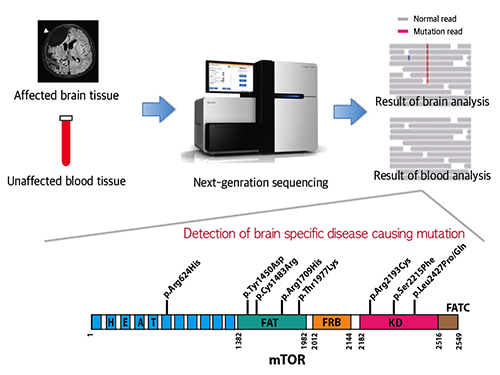
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 14530
Mutations Occurring Only in Brain Responsible for Intractable Epilepsy Identified
KAIST researchers have discovered that brain somatic mutations in MTOR gene induce intractable epilepsy and suggest a precision medicine to treat epileptic seizures.
Epilepsy is a brain disorder which afflicts more than 50 million people worldwide. Many epilepsy patients can control their symptoms through medication, but about 30% suffer from intractable epilepsy and are unable to manage the disease with drugs. Intractable epilepsy causes multiple seizures, permanent mental, physical, and developmental disabilities, and even death. Therefore, surgical removal of the affected area from the brain has been practiced as a treatment for patients with medically refractory seizures, but this too fails to provide a complete solution because only 60% of the patients who undergo surgery are rendered free of seizures.
A Korean research team led by Professor Jeong Ho Lee of the Graduate School of Medical Science and Engineering at the Korea Advanced Institute of Science and Technology (KAIST) and Professor Dong-Seok Kim of Epilepsy Research Center at Yonsei University College of Medicine has recently identified brain somatic mutations in the gene of mechanistic target of rapamycin (MTOR) as the cause of focal cortical dysplasia type II (FCDII), one of the most important and common inducers to intractable epilepsy, particularly in children. They propose a targeted therapy to lessen epileptic seizures by suppressing the activation of mTOR kinase, a signaling protein in the brain. Their research results were published online in Nature Medicine on March 23, 2015.
FCDII contributes to the abnormal developments of the cerebral cortex, ranging from cortical disruption to severe forms of cortical dyslamination, balloon cells, and dysplastic neurons. The research team studied 77 FCDII patients with intractable epilepsy who had received a surgery to remove the affected regions from the brain. The researchers used various deep sequencing technologies to conduct comparative DNA analysis of the samples obtained from the patients’ brain and blood, or saliva. They reported that about 16% of the studied patients had somatic mutations in their brain. Such mutations, however, did not take place in their blood or saliva DNA.
Professor Jeong Ho Lee of KAIST said, “This is an important finding. Unlike our previous belief that genetic mutations causing intractable epilepsy exist anywhere in the human body including blood, specific gene mutations incurred only in the brain can lead to intractable epilepsy. From our animal models, we could see how a small fraction of mutations carrying neurons in the brain could affect its entire function.”
The research team recapitulated the pathogenesis of intractable epilepsy by inducing the focal cortical expression of mutated mTOR in the mouse brain via electroporation method and observed as the mouse develop epileptic symptoms. They then treated these mice with the drug called “rapamycin” to inhibit the activity of mTOR protein and observed that it suppressed the development of epileptic seizures with cytomegalic neurons.
“Our study offers the first evidence that brain-somatic activating mutations in MTOR cause FCDII and identifies mTOR as a treatment target for intractable epilepsy,” said co-author Dr. Dong-Seok Kim, a neurosurgeon at Yonsei Medical Center with the country’s largest surgical experiences in treating patients with this condition.
The research paper is titled “Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy.” (Digital Object Identifier #: 10.1038/nm.3824)
Picture 1: A schematic image to show how to detect brain specific mutation using next-generation sequencing technology with blood-brain paired sample. Simple comparison of non-overlapping mutations between affected and unaffected tissues is able to detect brain specific mutations.
Picture 2: A schematic image to show how to generate focal cortical dysplasia mouse model. This mouse model open the new window of drug screening for seizure patients.
Picture 3: Targeted medicine can rescue the focal cortical dysplasia symptoms including cytomegalic neuron & intractable epilepsy.
2015.03.25 View 14530 -
 Professor Sangyong Jon Appointed Fellow of AIMBE
Professor Sangyong Jon of the Department of Biological Sciences at KAIST has been appointed a member of the American Institute for Medical and Biological Engineering (AIMBE) fellowship.
Established in 1991, AIMBE is a non-profit organization based in Washington, D.C., representing 50,000 individuals and the top 2% of medical and biological engineers. AIMBE provides policy advice and advocacy for medical and biological engineering for the benefit of humanity. It has had about 1,500 fellows over the past 25 years. Among the members, only 110 are non-American nationalities.
Following the appointment of Dr. Hae-Bang Lee, the former senior researcher at the Korean Research Institute of Chemical Technology, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, Professor Jon is the third Korean to become an AIMBE fellow. He had an induction ceremony for the appointment of his fellowship at the AIMBE’s Annual Event held on March 15-17, 2015 in Washington, D.C.
An authority on nanomedicine, Professor Jon has developed many original technologies including multi-functional Theranostics nano particles for the diagnosis and treatment of diseases. He received the Most Cited Paper Award from Theranostics, an academic journal specialized in nanomedicine, last February.
Additionally, Professor Jon is a leading researcher in the field of translational medicine, using a multi-disciplinary, highly collaborative, “Bench to Bedside” approach for disease treatment and prevention. He created a biotechnology venture company and transferred research developments to the industry in Korea.
2015.03.12 View 13094
Professor Sangyong Jon Appointed Fellow of AIMBE
Professor Sangyong Jon of the Department of Biological Sciences at KAIST has been appointed a member of the American Institute for Medical and Biological Engineering (AIMBE) fellowship.
Established in 1991, AIMBE is a non-profit organization based in Washington, D.C., representing 50,000 individuals and the top 2% of medical and biological engineers. AIMBE provides policy advice and advocacy for medical and biological engineering for the benefit of humanity. It has had about 1,500 fellows over the past 25 years. Among the members, only 110 are non-American nationalities.
Following the appointment of Dr. Hae-Bang Lee, the former senior researcher at the Korean Research Institute of Chemical Technology, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST, Professor Jon is the third Korean to become an AIMBE fellow. He had an induction ceremony for the appointment of his fellowship at the AIMBE’s Annual Event held on March 15-17, 2015 in Washington, D.C.
An authority on nanomedicine, Professor Jon has developed many original technologies including multi-functional Theranostics nano particles for the diagnosis and treatment of diseases. He received the Most Cited Paper Award from Theranostics, an academic journal specialized in nanomedicine, last February.
Additionally, Professor Jon is a leading researcher in the field of translational medicine, using a multi-disciplinary, highly collaborative, “Bench to Bedside” approach for disease treatment and prevention. He created a biotechnology venture company and transferred research developments to the industry in Korea.
2015.03.12 View 13094 -
 System Approach Using Metabolite Structural Similarity Toward TOM Suggested
A Korean research team at KAIST suggests that a system approach using metabolite structural similarity helps to elucidate the mechanisms of action of traditional oriental medicine.
Traditional oriental medicine (TOM) has been practiced in Asian countries for centuries, and is gaining increasing popularity around the world. Despite its efficacy in various symptoms, TOM has been practiced without precise knowledge of its mechanisms of action. Use of TOM largely comes from empirical knowledge practiced over a long period of time. The fact that some of the compounds found in TOM have led to successful modern drugs such as artemisinin for malaria and taxol (Paclitaxel) for cancer has spurred modernization of TOM.
A research team led by Sang-Yup Lee at KAIST has focused on structural similarities between compounds in TOM and human metabolites to help explain TOM’s mechanisms of action. This systems approach using structural similarities assumes that compounds which are structurally similar to metabolites could affect relevant metabolic pathways and reactions by biosynthesizing structurally similar metabolites.
Structural similarity analysis has helped to identify mechanisms of action of TOM. This is described in a recent study entitled “A systems approach to traditional oriental medicine,” published online in Nature Biotechnology on March 6, 2015. In this study, the research team conducted structural comparisons of all the structurally known compounds in TOM and human metabolites on a large-scale. As a control, structures of all available approved drugs were also compared against human metabolites. This structural analysis provides two important results. First, the identification of metabolites structurally similar to TOM compounds helped to narrow down the candidate target pathways and reactions for the effects from TOM compounds. Second, it suggested that a greater fraction of all the structurally known TOM compounds appeared to be more similar to human metabolites than the approved drugs. This second finding indicates that TOM has a great potential to interact with diverse metabolic pathways with strong efficacy. This finding, in fact, shows that TOM compounds might be advantageous for the multitargeting required to cure complex diseases. “Once we have narrowed down candidate target pathways and reactions using this structural similarity approach, additional in silico tools will be necessary to characterize the mechanisms of action of many TOM compounds at a molecular level,” said Hyun Uk Kim, a research professor at KAIST.
TOM’s multicomponent, multitarget approach wherein multiple components show synergistic effects to treat symptoms is highly distinctive. The researchers investigated previously observed effects recorded since 2000 of a set of TOM compounds with known mechanisms of action. TOM compounds’ synergistic combinations largely consist of a major compound providing the intended efficacy to the target site and supporting compounds which maximize the efficacy of the major compound. In fact, such combination designs appear to mirror the Kun-Shin-Choa-Sa design principle of TOM.
That principle, Kun-Shin-Choa-Sa (君臣佐使 or Jun-Chen-Zuo-Shi in Chinese) literally means “king-minister-assistant-ambassador.” In ancient East Asian medicine, treating human diseases and taking good care of the human body are analogous to the politics of governing a nation. Just as good governance requires that a king be supported by ministers, assistants and/or ambassadors, treating diseases or good care of the body required the combined use of herbal medicines designed based on the concept of Kun-Shin-Choa-Sa. Here, the Kun (king or the major component) indicates the major medicine (or herb) conveying the major drug efficacy, and is supported by three different types of medicines: the Shin (minister or the complementary component) for enhancing and/or complementing the efficacy of the Kun, Choa (assistant or the neutralizing component) for reducing any side effects caused by the Kun and reducing the minor symptoms accompanying major symptom, and Sa (ambassador or the delivery/retaining component) which facilitated the delivery of the Kun to the target site, and retaining the Kun for prolonged availability in the cells.
The synergistic combinations of TOM compounds reported in the literature showed four different types of synergisms: complementary action (similar to Kun-Shin), neutralizing action (similar to Kun-Choa), facilitating action or pharmacokinetic potentiation (both similar to Kun-Choa or Kun-Sa). Additional structural analyses for these compounds with synergism show that they appeared to affect metabolism of amino acids, co-factors and vitamins as major targets.
Professor Sang Yup Lee remarks, “This study lays a foundation for the integration of traditional oriental medicine with modern drug discovery and development. The systems approach taken in this analysis will be used to elucidate mechanisms of action of unknown TOM compounds which will then be subjected to rigorous validation through clinical and in silico experiments.”
Sources: Kim, H.U. et al. “A systems approach to traditional oriental medicine.” Nature Biotechnology. 33: 264-268 (2015).
This work was supported by the Bio-Synergy Research Project (2012M3A9C4048759) of the Ministry of Science, ICT and Future Planning through the National Research Foundation. This work was also supported by the Novo Nordisk Foundation.
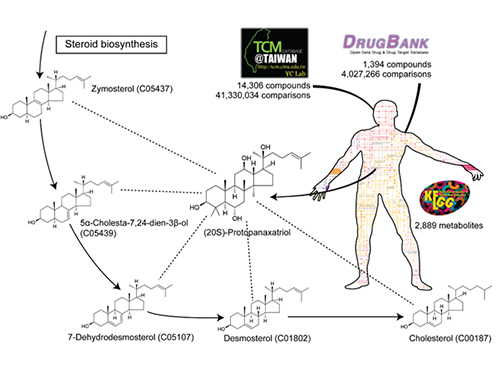
The picture below presents the structural similarity analysis of comparing compounds in traditional oriental medicine and those in all available approved drugs against the structures of human metabolites.
2015.03.09 View 10889
System Approach Using Metabolite Structural Similarity Toward TOM Suggested
A Korean research team at KAIST suggests that a system approach using metabolite structural similarity helps to elucidate the mechanisms of action of traditional oriental medicine.
Traditional oriental medicine (TOM) has been practiced in Asian countries for centuries, and is gaining increasing popularity around the world. Despite its efficacy in various symptoms, TOM has been practiced without precise knowledge of its mechanisms of action. Use of TOM largely comes from empirical knowledge practiced over a long period of time. The fact that some of the compounds found in TOM have led to successful modern drugs such as artemisinin for malaria and taxol (Paclitaxel) for cancer has spurred modernization of TOM.
A research team led by Sang-Yup Lee at KAIST has focused on structural similarities between compounds in TOM and human metabolites to help explain TOM’s mechanisms of action. This systems approach using structural similarities assumes that compounds which are structurally similar to metabolites could affect relevant metabolic pathways and reactions by biosynthesizing structurally similar metabolites.
Structural similarity analysis has helped to identify mechanisms of action of TOM. This is described in a recent study entitled “A systems approach to traditional oriental medicine,” published online in Nature Biotechnology on March 6, 2015. In this study, the research team conducted structural comparisons of all the structurally known compounds in TOM and human metabolites on a large-scale. As a control, structures of all available approved drugs were also compared against human metabolites. This structural analysis provides two important results. First, the identification of metabolites structurally similar to TOM compounds helped to narrow down the candidate target pathways and reactions for the effects from TOM compounds. Second, it suggested that a greater fraction of all the structurally known TOM compounds appeared to be more similar to human metabolites than the approved drugs. This second finding indicates that TOM has a great potential to interact with diverse metabolic pathways with strong efficacy. This finding, in fact, shows that TOM compounds might be advantageous for the multitargeting required to cure complex diseases. “Once we have narrowed down candidate target pathways and reactions using this structural similarity approach, additional in silico tools will be necessary to characterize the mechanisms of action of many TOM compounds at a molecular level,” said Hyun Uk Kim, a research professor at KAIST.
TOM’s multicomponent, multitarget approach wherein multiple components show synergistic effects to treat symptoms is highly distinctive. The researchers investigated previously observed effects recorded since 2000 of a set of TOM compounds with known mechanisms of action. TOM compounds’ synergistic combinations largely consist of a major compound providing the intended efficacy to the target site and supporting compounds which maximize the efficacy of the major compound. In fact, such combination designs appear to mirror the Kun-Shin-Choa-Sa design principle of TOM.
That principle, Kun-Shin-Choa-Sa (君臣佐使 or Jun-Chen-Zuo-Shi in Chinese) literally means “king-minister-assistant-ambassador.” In ancient East Asian medicine, treating human diseases and taking good care of the human body are analogous to the politics of governing a nation. Just as good governance requires that a king be supported by ministers, assistants and/or ambassadors, treating diseases or good care of the body required the combined use of herbal medicines designed based on the concept of Kun-Shin-Choa-Sa. Here, the Kun (king or the major component) indicates the major medicine (or herb) conveying the major drug efficacy, and is supported by three different types of medicines: the Shin (minister or the complementary component) for enhancing and/or complementing the efficacy of the Kun, Choa (assistant or the neutralizing component) for reducing any side effects caused by the Kun and reducing the minor symptoms accompanying major symptom, and Sa (ambassador or the delivery/retaining component) which facilitated the delivery of the Kun to the target site, and retaining the Kun for prolonged availability in the cells.
The synergistic combinations of TOM compounds reported in the literature showed four different types of synergisms: complementary action (similar to Kun-Shin), neutralizing action (similar to Kun-Choa), facilitating action or pharmacokinetic potentiation (both similar to Kun-Choa or Kun-Sa). Additional structural analyses for these compounds with synergism show that they appeared to affect metabolism of amino acids, co-factors and vitamins as major targets.
Professor Sang Yup Lee remarks, “This study lays a foundation for the integration of traditional oriental medicine with modern drug discovery and development. The systems approach taken in this analysis will be used to elucidate mechanisms of action of unknown TOM compounds which will then be subjected to rigorous validation through clinical and in silico experiments.”
Sources: Kim, H.U. et al. “A systems approach to traditional oriental medicine.” Nature Biotechnology. 33: 264-268 (2015).
This work was supported by the Bio-Synergy Research Project (2012M3A9C4048759) of the Ministry of Science, ICT and Future Planning through the National Research Foundation. This work was also supported by the Novo Nordisk Foundation.
The picture below presents the structural similarity analysis of comparing compounds in traditional oriental medicine and those in all available approved drugs against the structures of human metabolites.
2015.03.09 View 10889 -
 The Bio-Synergy Research Center, KAIST, Hosts an Annual Meeting
The Ministry of Science, ICT and Future Planning of the Republic of Korea founded the Bio-Synergy Research Center (BSRC) at KAIST in 2013 to develop source technology and generate new knowledge by conducting convergence research projects in natural resources with information technology (IT) and biotechnology.
The BSRC hosted an annual meeting on November 21, 2014, at the KAIST campus and reviewed the progress it made this year with the participation of President Steve Kang of KAIST, Commissioner Young-min Kim of KIPO, and Director Doheon Lee of BSRC.
The Korean Intellectual Property Office (KIPO) provided BSRC with its database in Korean traditional medicine that includes a vast amount of information about disease symptoms, native medicinal herbs and plant extracts, prescriptions, and chemical compounds used for medication. The database, “Compound Combination-Oriented Natural Product Database with Unified Terminology (COCUNUT),” holds approximately one million data sets in four major categories: prescriptions, medicinal resources, medicine components and functions, and diseases.
Based on COCUNUT, BSRC has been working on the standardization of Korean traditional medicine such as the development of data mapping and text mining technology and the analysis of big data in accordance with the said categories. Using IT and biotechnology, the center has also created a virtual human body to explain how traditional medicine works in human body, thereby contributing to the development of new natural materials for medicine.
2014.12.03 View 8294
The Bio-Synergy Research Center, KAIST, Hosts an Annual Meeting
The Ministry of Science, ICT and Future Planning of the Republic of Korea founded the Bio-Synergy Research Center (BSRC) at KAIST in 2013 to develop source technology and generate new knowledge by conducting convergence research projects in natural resources with information technology (IT) and biotechnology.
The BSRC hosted an annual meeting on November 21, 2014, at the KAIST campus and reviewed the progress it made this year with the participation of President Steve Kang of KAIST, Commissioner Young-min Kim of KIPO, and Director Doheon Lee of BSRC.
The Korean Intellectual Property Office (KIPO) provided BSRC with its database in Korean traditional medicine that includes a vast amount of information about disease symptoms, native medicinal herbs and plant extracts, prescriptions, and chemical compounds used for medication. The database, “Compound Combination-Oriented Natural Product Database with Unified Terminology (COCUNUT),” holds approximately one million data sets in four major categories: prescriptions, medicinal resources, medicine components and functions, and diseases.
Based on COCUNUT, BSRC has been working on the standardization of Korean traditional medicine such as the development of data mapping and text mining technology and the analysis of big data in accordance with the said categories. Using IT and biotechnology, the center has also created a virtual human body to explain how traditional medicine works in human body, thereby contributing to the development of new natural materials for medicine.
2014.12.03 View 8294 -
 Visit by Sir Paul Maxime Nurse, President of the Royal Society
Sir Paul Maxime
Nurse, who is an English geneticist and cell biologist, visited KAIST and gave
a lecture entitled The Great Ideas of
Biology on March 11, 2014.
Sir Paul was awarded the 2001 Nobel Prize in
Physiology or Medicine with Leland H. Hartwell and R. Timothy Hunt for their discoveries
of protein molecules that control the division of cells in the cell cycle.
He was Professor of Microbiology at the University
of Oxford, CEO of the Imperial Cancer Research Fund and Cancer Research UK, and
President of Rockefeller University in New York. Sir Paul is currently the President of
the Royal Society as well as Director and Chief Executive of the Francis Crick
Institute.
Founded in London in 1660, the Royal Society is composed of the world’s most distinguished scientists drawn from all areas of
science, engineering, and medicine.
Below is a summary of his lecture, The Great Ideas of Biology:
Four major ideas of biology
are the theory of genes, evolution by natural selection, the proposal that the
cell is the fundamental unit of all life, and the chemical composition of a cell.
When considering the
question “what is life?” these ideas come together. The special way cells reproduce
provides the conditions by which natural selection takes place, allowing living
organisms to evolve. The organization of chemistry within the cell provides
explanations for life’s phenomena.
In addition, an emerging idea
is the nature of biological self-organization with which living cells and organisms
process information and acquire specific forms. These great ideas have
influenced one another and changed the way we perceive biology and science
today.
2014.03.11 View 11046
Visit by Sir Paul Maxime Nurse, President of the Royal Society
Sir Paul Maxime
Nurse, who is an English geneticist and cell biologist, visited KAIST and gave
a lecture entitled The Great Ideas of
Biology on March 11, 2014.
Sir Paul was awarded the 2001 Nobel Prize in
Physiology or Medicine with Leland H. Hartwell and R. Timothy Hunt for their discoveries
of protein molecules that control the division of cells in the cell cycle.
He was Professor of Microbiology at the University
of Oxford, CEO of the Imperial Cancer Research Fund and Cancer Research UK, and
President of Rockefeller University in New York. Sir Paul is currently the President of
the Royal Society as well as Director and Chief Executive of the Francis Crick
Institute.
Founded in London in 1660, the Royal Society is composed of the world’s most distinguished scientists drawn from all areas of
science, engineering, and medicine.
Below is a summary of his lecture, The Great Ideas of Biology:
Four major ideas of biology
are the theory of genes, evolution by natural selection, the proposal that the
cell is the fundamental unit of all life, and the chemical composition of a cell.
When considering the
question “what is life?” these ideas come together. The special way cells reproduce
provides the conditions by which natural selection takes place, allowing living
organisms to evolve. The organization of chemistry within the cell provides
explanations for life’s phenomena.
In addition, an emerging idea
is the nature of biological self-organization with which living cells and organisms
process information and acquire specific forms. These great ideas have
influenced one another and changed the way we perceive biology and science
today.
2014.03.11 View 11046 -
 Opening Ceremony of Genetic Donguibogam held
- Medicine using traditional natural substances • Food product source technology development begins
- Over 150,000,000,000 Won for 10 years of work invested to develop source technology
- Opening ceremony held on November 26th at 3 p.m. in Bio & Brain Engineering Division Building
The research to develop medicine and food source technology using traditional natural substances hasbegun.The opening ceremony of the “Genetic Donguibogam” business group, with KAIST Department of Bio & Brain Engineering Professor Do Heon Lee as the leader, was held on November 26th at 3 p.m. in Dream Hall, Bio & Brain Engineering Division Building, KAIST, Daejeon. The attendees of the opening ceremony included Yo Eop Im, Head of the Future Technology Department of the Ministry of Science, ICT and Future Planning and around 200 experts in science and technology industry, including the National Research Foundation of Korea, KAIST, the Korea Institute of Science and Technology, Seoul National University and Yonsei University.
The business group was established to re-interpret traditional natural substances proved to be effective from experience and improve quality of life by researching its applications; and to develop integrated source technology using traditional natural substances. The group is to invest over 150,000,000,000 Won for 10 years of research to secure natural substance source technology in five stages: interpretation technology, analysis technology, verification technology, bio marker technology and human body effectiveness verification technology. Especially, the focus would be on the use of virtual body computer models and Omics* to analyse the effects of traditional natural substances mixture on human body, and to find new materials for healthcare.
This research model, it is hoped, will have a new item to pioneer in the world natural substance market as well as securing a technologically competitive edge in bio industry by developing source technology that investigates the effects of traditional natural substances using cutting edge science.
KAIST Department of Bio & Brain Engineering Professor and Head Do Heon Lee of the “Genetic Donguibogam” Business Group said, “We will push forward to develop source energy by integrating IT-BT technology with a computer virtual body to build a cooperation system with medicine and functional food industries.” He continued: “This will enable not only the creation of a new industry, but also customised medicine.”
The 12 partners of the group include KAIST, Korea Institute of Science and Technology, Seoul National University and Yonsei University and 200 experts. The research participation area will be widened to foreign research institutes and associated companies.
* Terminology
Noun) Omics is an academic discipline analysing mass information on metabolism of physiological phenomena in specific cells (transcriptome, proteome and protoplast) with an integrated approach to determine vital phenomena.
2013.12.11 View 9861
Opening Ceremony of Genetic Donguibogam held
- Medicine using traditional natural substances • Food product source technology development begins
- Over 150,000,000,000 Won for 10 years of work invested to develop source technology
- Opening ceremony held on November 26th at 3 p.m. in Bio & Brain Engineering Division Building
The research to develop medicine and food source technology using traditional natural substances hasbegun.The opening ceremony of the “Genetic Donguibogam” business group, with KAIST Department of Bio & Brain Engineering Professor Do Heon Lee as the leader, was held on November 26th at 3 p.m. in Dream Hall, Bio & Brain Engineering Division Building, KAIST, Daejeon. The attendees of the opening ceremony included Yo Eop Im, Head of the Future Technology Department of the Ministry of Science, ICT and Future Planning and around 200 experts in science and technology industry, including the National Research Foundation of Korea, KAIST, the Korea Institute of Science and Technology, Seoul National University and Yonsei University.
The business group was established to re-interpret traditional natural substances proved to be effective from experience and improve quality of life by researching its applications; and to develop integrated source technology using traditional natural substances. The group is to invest over 150,000,000,000 Won for 10 years of research to secure natural substance source technology in five stages: interpretation technology, analysis technology, verification technology, bio marker technology and human body effectiveness verification technology. Especially, the focus would be on the use of virtual body computer models and Omics* to analyse the effects of traditional natural substances mixture on human body, and to find new materials for healthcare.
This research model, it is hoped, will have a new item to pioneer in the world natural substance market as well as securing a technologically competitive edge in bio industry by developing source technology that investigates the effects of traditional natural substances using cutting edge science.
KAIST Department of Bio & Brain Engineering Professor and Head Do Heon Lee of the “Genetic Donguibogam” Business Group said, “We will push forward to develop source energy by integrating IT-BT technology with a computer virtual body to build a cooperation system with medicine and functional food industries.” He continued: “This will enable not only the creation of a new industry, but also customised medicine.”
The 12 partners of the group include KAIST, Korea Institute of Science and Technology, Seoul National University and Yonsei University and 200 experts. The research participation area will be widened to foreign research institutes and associated companies.
* Terminology
Noun) Omics is an academic discipline analysing mass information on metabolism of physiological phenomena in specific cells (transcriptome, proteome and protoplast) with an integrated approach to determine vital phenomena.
2013.12.11 View 9861 -
 KAIST's Partnership Agreement with the Imperial College of Science, Technology and Medicine, UK
KAIST signed an agreement on academic and research cooperation with the Imperial College of Science, Technology (Imperial College London) and Medicine in the United Kingdom (UK) on November 6th, 2013 in London.
The two universities have been implementing collaboration programs at the department level in the areas of plastic electronics since September 2012 and systems engineering and molecular simulation since February 2013, but have never had a formal partnership agreement.
President Steve Kang from KAIST and Provost James Stirling from Imperial College London signed the comprehensive cooperation agreement which will not only strengthen the existing collaborations between the two institutions but also explore areas of mutual interest in the interdisciplinary study of big data, as well as in the fields of mechanical engineering, synthetic biology, and quantum physics.
Workshops, seminars, lectures, and conferences will be jointly organized and held to facilitate the exchange of research staff and faculty and to promote collaborations in research assignments. The universities will also look into the possibility of exchange programs for undergraduate and graduate students.
The partnership agreement will be effective for five years.
Minister Moon-Gi Choi from the Republic of Korea’s Ministry of Science, Information and Communications Technology (ICT) & Future Planning attended the signing ceremony as well and congratulated the establishment of the partnership, saying:
“We are living in the age of highly advanced science and technology that requires us to have a new economic development paradigm for sustainable growth. Through convergence research based on the application of ICT and technology innovation, we will have new opportunities for development. I hope KAIST and the Imperial College London will be at the forefront of such endeavors in coming years.”With its history spanning over 100 years, the Imperial College London is a public research university located in London, UK, specializing in science, engineering, medicine, and business. The university is regarded as being one of the most prestigious universities in the world, having eminent alumni such as Thomas Henry Huxley (biologist), H.G. Wells (author), and Sir Alexander Fleming (pharmacologist).
From left to right: Provost James Stirling, Minister Moon-Gi Choi, and President Steve Kang
2013.11.12 View 9766
KAIST's Partnership Agreement with the Imperial College of Science, Technology and Medicine, UK
KAIST signed an agreement on academic and research cooperation with the Imperial College of Science, Technology (Imperial College London) and Medicine in the United Kingdom (UK) on November 6th, 2013 in London.
The two universities have been implementing collaboration programs at the department level in the areas of plastic electronics since September 2012 and systems engineering and molecular simulation since February 2013, but have never had a formal partnership agreement.
President Steve Kang from KAIST and Provost James Stirling from Imperial College London signed the comprehensive cooperation agreement which will not only strengthen the existing collaborations between the two institutions but also explore areas of mutual interest in the interdisciplinary study of big data, as well as in the fields of mechanical engineering, synthetic biology, and quantum physics.
Workshops, seminars, lectures, and conferences will be jointly organized and held to facilitate the exchange of research staff and faculty and to promote collaborations in research assignments. The universities will also look into the possibility of exchange programs for undergraduate and graduate students.
The partnership agreement will be effective for five years.
Minister Moon-Gi Choi from the Republic of Korea’s Ministry of Science, Information and Communications Technology (ICT) & Future Planning attended the signing ceremony as well and congratulated the establishment of the partnership, saying:
“We are living in the age of highly advanced science and technology that requires us to have a new economic development paradigm for sustainable growth. Through convergence research based on the application of ICT and technology innovation, we will have new opportunities for development. I hope KAIST and the Imperial College London will be at the forefront of such endeavors in coming years.”With its history spanning over 100 years, the Imperial College London is a public research university located in London, UK, specializing in science, engineering, medicine, and business. The university is regarded as being one of the most prestigious universities in the world, having eminent alumni such as Thomas Henry Huxley (biologist), H.G. Wells (author), and Sir Alexander Fleming (pharmacologist).
From left to right: Provost James Stirling, Minister Moon-Gi Choi, and President Steve Kang
2013.11.12 View 9766 -
 Therapy developed to induce Angiogenesis of Retina
- Junyeop Lee, Graduate School of Medical Sciences and Engineering
- Research results expected to be applied for treatment of diabetic retinopathy
A major clue to treatment of retinovascular disease, which causes blindness, has been found. The key to protection of the retinal nerve is the angiogenic protein that promotes healthy retinal vessel growth around the retina, which usually does not receive blood supply readily. This research offers a beginning to the possible improvement of therapy for diabetic retinopathy1 and retinopathy of prematurity2. Also important to the research is the fact that the ophthalmology specialist researcher, currently undergoing professional training, provided the results.
KAIST Graduate School of Medical Sciences and Engineering’s Junyeop Lee is the opthalmology specialist, who carried out the research under supervision by academic advisers Gyuyeong Go and Wookjun Yoo. The Ministry of Science, ICT and Future Planning as well as the National Research Foundation of Korea have funded his research. The research results have been published as a cover paper on ‘Science Translational Medicine’ on 18th August. This journal is a sister publication of Science, which is prestigious in the field of translational medicine that ties the basic science with clinical medicine. (Thesis title: Angiopoietin-1 Guides Directional Angiogenesis Through Integrin αvβ5 Signaling for Recovery of Ischemic Retinopathy)
The traditional treatment of diabetic retinopathy includes laser photocoagulation to destroy the retinal tissues or antibody therapeutics, which prevents vessel proliferation and blood leaking. The advantage of antibody therapeutics3 is that it retains the retinal nerves, however, it is not the fundamental solution but merely a temporary one, which requires repeated treatments.
The research team identified that Angiopoietin-14 protein, known as essential for growth and stabilization of vessels, also plays an important role in retinal vessel growth. The protein protects the retinal nerves, as well as provides improvement for retinal ischemia5 that is the root cause of vision loss due to retinal hemorrhages. It is expected to become a key to finding fundamental treatment method – by providing sufficient blood supply to the retina, thereby preserving the retinal nerve functions.
The results show that administration of Angiopoietin-1 to retinopathy mouse model promotes growth of healthy vessel growth, further preventing abnormal vessel growth, retinal hemorrhage and vision loss due to retinal ischemia.
Junyeop Lee said, “This research has identified that Angiopoietin-1 is an important factor in retinal vessel generation and stabilization. The paradigm will shift from traditional treatment method, which prevents vessel growth, to a new method that generates healthy vessels and strengthens vessel functions.”
1 Diabetic retinopathy: This retinovascular disease is a diabetic complication caused by insufficient blood supply. It is the major causes of blindness in adults.
2 Retinopathy of prematurity: The retinal vascular disease that occurs in premature infants with incomplete retinal vascular development. It is also the most common cause of blindness in children.
3 Antibody Therapeutics: Antibody developed to selectively inhibit abnormal blood vessel growth and leakage. Typical antibody therapeutics is Avastin and Lucentis, which hinder vascular endothelial growth factor (VEGF).
4 Angiopoietin-1: A critical growth factor that induces the production of healthy blood vessels and maintains the stability of the created vessel.
5 Retinal ischemia: State of ailment where retinal tissue blood supply is not sufficient.
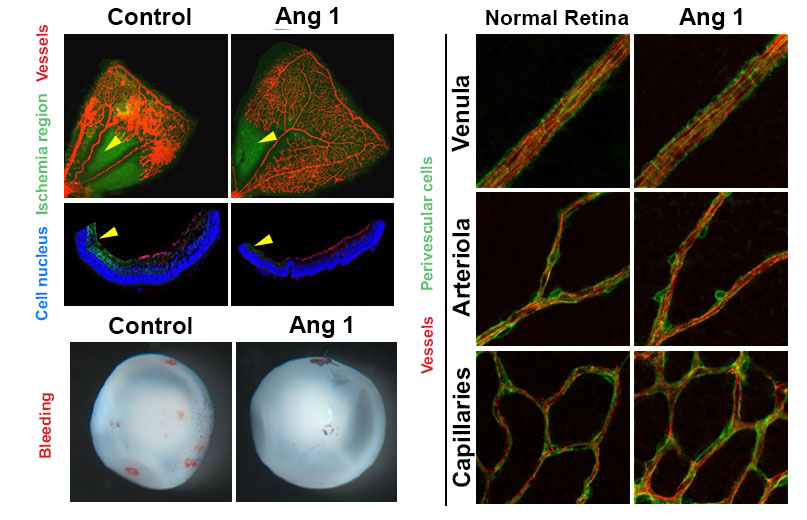
Figure 1. Retinopathy mouse models show that, in comparison to the control group, the VEGF-Trap treatment and Angiopoietin-1 (Ang1) treatment groups significantly suppresses the pathological vascular proliferation. In addition, the Ang 1 group show vessel growth toward the central avascular area (region of retinal ischemia), which is not observed in VEGF-Trap treatment.
Figure 2. Reduced retinal ischemia, retinal bleeding and blood vessel normalization by Angiopoietin-1. Retinal ischemic region (arrow) and retinal bleeding significantly reduced in the Angiopoietin-1 (Ang1) treatment model in comparison to control group (left). The newly generated vessels in Ang 1 model are structurally supported by perivascular cells as normal retinal vessels do (right).
2013.10.12 View 12077
Therapy developed to induce Angiogenesis of Retina
- Junyeop Lee, Graduate School of Medical Sciences and Engineering
- Research results expected to be applied for treatment of diabetic retinopathy
A major clue to treatment of retinovascular disease, which causes blindness, has been found. The key to protection of the retinal nerve is the angiogenic protein that promotes healthy retinal vessel growth around the retina, which usually does not receive blood supply readily. This research offers a beginning to the possible improvement of therapy for diabetic retinopathy1 and retinopathy of prematurity2. Also important to the research is the fact that the ophthalmology specialist researcher, currently undergoing professional training, provided the results.
KAIST Graduate School of Medical Sciences and Engineering’s Junyeop Lee is the opthalmology specialist, who carried out the research under supervision by academic advisers Gyuyeong Go and Wookjun Yoo. The Ministry of Science, ICT and Future Planning as well as the National Research Foundation of Korea have funded his research. The research results have been published as a cover paper on ‘Science Translational Medicine’ on 18th August. This journal is a sister publication of Science, which is prestigious in the field of translational medicine that ties the basic science with clinical medicine. (Thesis title: Angiopoietin-1 Guides Directional Angiogenesis Through Integrin αvβ5 Signaling for Recovery of Ischemic Retinopathy)
The traditional treatment of diabetic retinopathy includes laser photocoagulation to destroy the retinal tissues or antibody therapeutics, which prevents vessel proliferation and blood leaking. The advantage of antibody therapeutics3 is that it retains the retinal nerves, however, it is not the fundamental solution but merely a temporary one, which requires repeated treatments.
The research team identified that Angiopoietin-14 protein, known as essential for growth and stabilization of vessels, also plays an important role in retinal vessel growth. The protein protects the retinal nerves, as well as provides improvement for retinal ischemia5 that is the root cause of vision loss due to retinal hemorrhages. It is expected to become a key to finding fundamental treatment method – by providing sufficient blood supply to the retina, thereby preserving the retinal nerve functions.
The results show that administration of Angiopoietin-1 to retinopathy mouse model promotes growth of healthy vessel growth, further preventing abnormal vessel growth, retinal hemorrhage and vision loss due to retinal ischemia.
Junyeop Lee said, “This research has identified that Angiopoietin-1 is an important factor in retinal vessel generation and stabilization. The paradigm will shift from traditional treatment method, which prevents vessel growth, to a new method that generates healthy vessels and strengthens vessel functions.”
1 Diabetic retinopathy: This retinovascular disease is a diabetic complication caused by insufficient blood supply. It is the major causes of blindness in adults.
2 Retinopathy of prematurity: The retinal vascular disease that occurs in premature infants with incomplete retinal vascular development. It is also the most common cause of blindness in children.
3 Antibody Therapeutics: Antibody developed to selectively inhibit abnormal blood vessel growth and leakage. Typical antibody therapeutics is Avastin and Lucentis, which hinder vascular endothelial growth factor (VEGF).
4 Angiopoietin-1: A critical growth factor that induces the production of healthy blood vessels and maintains the stability of the created vessel.
5 Retinal ischemia: State of ailment where retinal tissue blood supply is not sufficient.
Figure 1. Retinopathy mouse models show that, in comparison to the control group, the VEGF-Trap treatment and Angiopoietin-1 (Ang1) treatment groups significantly suppresses the pathological vascular proliferation. In addition, the Ang 1 group show vessel growth toward the central avascular area (region of retinal ischemia), which is not observed in VEGF-Trap treatment.
Figure 2. Reduced retinal ischemia, retinal bleeding and blood vessel normalization by Angiopoietin-1. Retinal ischemic region (arrow) and retinal bleeding significantly reduced in the Angiopoietin-1 (Ang1) treatment model in comparison to control group (left). The newly generated vessels in Ang 1 model are structurally supported by perivascular cells as normal retinal vessels do (right).
2013.10.12 View 12077