Chem
-
 KAIST Develops Technology for the Precise Diagnosis of Electric Vehicle Batteries Using Small Currents
Accurately diagnosing the state of electric vehicle (EV) batteries is essential for their efficient management and safe use. KAIST researchers have developed a new technology that can diagnose and monitor the state of batteries with high precision using only small amounts of current, which is expected to maximize the batteries’ long-term stability and efficiency.
KAIST (represented by President Kwang Hyung Lee) announced on the 17th of October that a research team led by Professors Kyeongha Kwon and Sang-Gug Lee from the School of Electrical Engineering had developed electrochemical impedance spectroscopy (EIS) technology that can be used to improve the stability and performance of high-capacity batteries in electric vehicles.
EIS is a powerful tool that measures the impedance* magnitude and changes in a battery, allowing the evaluation of battery efficiency and loss. It is considered an important tool for assessing the state of charge (SOC) and state of health (SOH) of batteries. Additionally, it can be used to identify thermal characteristics, chemical/physical changes, predict battery life, and determine the causes of failures. *Battery Impedance: A measure of the resistance to current flow within the battery that is used to assess battery performance and condition.
However, traditional EIS equipment is expensive and complex, making it difficult to install, operate, and maintain. Moreover, due to sensitivity and precision limitations, applying current disturbances of several amperes (A) to a battery can cause significant electrical stress, increasing the risk of battery failure or fire and making it difficult to use in practice.
< Figure 1. Flow chart for diagnosis and prevention of unexpected combustion via the use of the electrochemical impedance spectroscopy (EIS) for the batteries for electric vehicles. >
To address this, the KAIST research team developed and validated a low-current EIS system for diagnosing the condition and health of high-capacity EV batteries. This EIS system can precisely measure battery impedance with low current disturbances (10mA), minimizing thermal effects and safety issues during the measurement process.
In addition, the system minimizes bulky and costly components, making it easy to integrate into vehicles. The system was proven effective in identifying the electrochemical properties of batteries under various operating conditions, including different temperatures and SOC levels.
Professor Kyeongha Kwon (the corresponding author) explained, “This system can be easily integrated into the battery management system (BMS) of electric vehicles and has demonstrated high measurement accuracy while significantly reducing the cost and complexity compared to traditional high-current EIS methods. It can contribute to battery diagnosis and performance improvements not only for electric vehicles but also for energy storage systems (ESS).”
This research, in which Young-Nam Lee, a doctoral student in the School of Electrical Engineering at KAIST participated as the first author, was published in the prestigious international journal IEEE Transactions on Industrial Electronics (top 2% in the field; IF 7.5) on September 5th. (Paper Title: Small-Perturbation Electrochemical Impedance Spectroscopy System With High Accuracy for High-Capacity Batteries in Electric Vehicles, Link: https://ieeexplore.ieee.org/document/10666864)
< Figure 2. Impedance measurement results of large-capacity batteries for electric vehicles. ZEW (commercial EW; MP10, Wonatech) versus ZMEAS (proposed system) >
This research was supported by the Basic Research Program of the National Research Foundation of Korea, the Next-Generation Intelligent Semiconductor Technology Development Program of the Korea Evaluation Institute of Industrial Technology, and the AI Semiconductor Graduate Program of the Institute of Information & Communications Technology Planning & Evaluation.
2024.10.17 View 6291
KAIST Develops Technology for the Precise Diagnosis of Electric Vehicle Batteries Using Small Currents
Accurately diagnosing the state of electric vehicle (EV) batteries is essential for their efficient management and safe use. KAIST researchers have developed a new technology that can diagnose and monitor the state of batteries with high precision using only small amounts of current, which is expected to maximize the batteries’ long-term stability and efficiency.
KAIST (represented by President Kwang Hyung Lee) announced on the 17th of October that a research team led by Professors Kyeongha Kwon and Sang-Gug Lee from the School of Electrical Engineering had developed electrochemical impedance spectroscopy (EIS) technology that can be used to improve the stability and performance of high-capacity batteries in electric vehicles.
EIS is a powerful tool that measures the impedance* magnitude and changes in a battery, allowing the evaluation of battery efficiency and loss. It is considered an important tool for assessing the state of charge (SOC) and state of health (SOH) of batteries. Additionally, it can be used to identify thermal characteristics, chemical/physical changes, predict battery life, and determine the causes of failures. *Battery Impedance: A measure of the resistance to current flow within the battery that is used to assess battery performance and condition.
However, traditional EIS equipment is expensive and complex, making it difficult to install, operate, and maintain. Moreover, due to sensitivity and precision limitations, applying current disturbances of several amperes (A) to a battery can cause significant electrical stress, increasing the risk of battery failure or fire and making it difficult to use in practice.
< Figure 1. Flow chart for diagnosis and prevention of unexpected combustion via the use of the electrochemical impedance spectroscopy (EIS) for the batteries for electric vehicles. >
To address this, the KAIST research team developed and validated a low-current EIS system for diagnosing the condition and health of high-capacity EV batteries. This EIS system can precisely measure battery impedance with low current disturbances (10mA), minimizing thermal effects and safety issues during the measurement process.
In addition, the system minimizes bulky and costly components, making it easy to integrate into vehicles. The system was proven effective in identifying the electrochemical properties of batteries under various operating conditions, including different temperatures and SOC levels.
Professor Kyeongha Kwon (the corresponding author) explained, “This system can be easily integrated into the battery management system (BMS) of electric vehicles and has demonstrated high measurement accuracy while significantly reducing the cost and complexity compared to traditional high-current EIS methods. It can contribute to battery diagnosis and performance improvements not only for electric vehicles but also for energy storage systems (ESS).”
This research, in which Young-Nam Lee, a doctoral student in the School of Electrical Engineering at KAIST participated as the first author, was published in the prestigious international journal IEEE Transactions on Industrial Electronics (top 2% in the field; IF 7.5) on September 5th. (Paper Title: Small-Perturbation Electrochemical Impedance Spectroscopy System With High Accuracy for High-Capacity Batteries in Electric Vehicles, Link: https://ieeexplore.ieee.org/document/10666864)
< Figure 2. Impedance measurement results of large-capacity batteries for electric vehicles. ZEW (commercial EW; MP10, Wonatech) versus ZMEAS (proposed system) >
This research was supported by the Basic Research Program of the National Research Foundation of Korea, the Next-Generation Intelligent Semiconductor Technology Development Program of the Korea Evaluation Institute of Industrial Technology, and the AI Semiconductor Graduate Program of the Institute of Information & Communications Technology Planning & Evaluation.
2024.10.17 View 6291 -
 Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8188
Novel High-performance and Sustainable Paper Coating Material created by KAIST-Yonsei University Research Team to reduce microplastic pollution
What if there is a biodegradable packaging material with high performance without leaving microplastics?
Plastic pollution presents a global challenge that must be solved. In particular, packaging accounts for 30-50% of the total plastic consumption. While paper packaging is eco-friendly, it lacks crucial functionalities like moisture resistance and strength. Traditional coating materials exacerbate plastic pollution, prompting the need for sustainable alternatives.
Polyethylene (PE) and ethylene vinyl alcohol (EVOH) are typically used as coating materials to improve the low barrier properties of paper packaging, but these substances do not decompose and worsen microplastic pollution when disposed of in the natural environment. In response to this problem, packaging materials made from bio-based substances and biodegradable plastics have been developed, but in most cases, as the packaging performance improves, the biodegradability diminishes rapidly.
KAIST announced that a joint research team led by Professor Jaewook Myung of the Department of Civil and Environmental Engineering, Professor Hanseul Yang of the Department of Life Sciences, and Professor Jongcheol Seo of the Department of Packaging and Logistics <Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
at Yonsei University tackled the challenge of balancing packaging performance and sustainability. They successfully developed a sustainable, marine biodegradable, high-performance paper coating material.
* Biodegradable plastic: A plastic that can be decomposed by microorganisms in natural environments such as soil and ocean or artificial conditions such as industrial composting and anaerobic digestion by microorganisms.
*Microplastics: Tiny pieces of plastic less than 5 mm, produced during the decomposition of bulk plastic materials. Microplastics can persist in the sea for more than decades, causing severe marine pollution.
The team utilized boric acid-crosslinked poly(vinyl alcohol) (PVA), a biodegradable plastic, to coat the paper, thereby enhancing its biodegradability, barrier properties, and strength. The resulting coated paper exhibited superior performance compared to conventional plastics, with excellent barrier properties and physical strength, even in humid conditions.
<Figure 1. (a) Chemical structure of boric acid-crosslinked poly(vinyl alcohol) coating on paper, (b-c) Oxygen and water vapor barrier properties, (d-f) Tensile strength in dry and moist conditions. OTR: Oxygen transmission rate, WVTR: Water vapor transmission rate.>
The team also conducted an in-depth examination of biodegradation and biocompatibility to systematically evaluate the sustainability of the newly developed coated paper. Biodegradation was assessed by simulating the marine environment, known for its challenging biodegradability conditions. The team employed a respiratory system-based bioreactor to measure the degree of carbon mineralization into carbon dioxide. After 111 days of biodegradation, it was found that the coated papers achieved 59-82% biodegradation depending on the coating component. The phenomenon in which marine bacteria are decomposing the coating material was captured through a scanning electron microscope. In addition, in vitro biocompatibility was confirmed through human embryonic kidney and mouse embryonic fibroblast cells, as well as high in-vivo biocompatibility of the coated paper was verified through mouse experiments.
Through this study, the joint research team proposed a coating strategy that can improve packaging performance while upholding sustainability to address the drawbacks of paper packaging. The boric acid-crosslinked PVA-coated paper eliminates the need for artificial composting conditions or sewage treatment facilities. Being biodegradable in natural environments and characterized by low toxicity, this newly coated paper does not exacerbate environmental pollution when accidentally discarded. Thus, it presents a sustainable substitute for plastic packaging materials.
<Figure 2. (a) Normal paper and boric acid-crosslinked poly(vinyl alcohol) coated paper, (b) Biodegradation of the coated paper by marine bacteria, (c) Result of cytotoxicity test using human embryonic kidney and mouse embryonic fibroblast cells. (d) Vital organs after one-month exposure of the coated papers to mice.>
Professor Jaewook Myung at KAIST, who led the sustainability study of coated paper, said, "The development of a marine biodegradable high-performance paper coating is the result of combining the innovative technologies of three leading research teams in each field." He said, “We will continue to develop sustainable materials with excellent performance.” Meanwhile, Professor Jongchul Seo of Yonsei University, who led the research on the development of high-performance paper coating, mentioned, “Through this research, we have developed a sustainable paper packaging technology that can replace non-degradable plastic packaging, and we expect the research outcome will be applied in industry,”.
<Figure 3. End-of-life scenario of papers coated by BA-crosslinked PVA in the marine environment. The coated papers potentially be disintegrated by marine microorganisms and ocean waves and tides. The depolymerization of PVA coating and paper is then mediated by extracellular depolymerases such as oxidases and cellulases, after which the small subunits (oligomers and monomers) are assimilated by microbial cells. The carbon components in the coated papers are ultimately mineralized into CO2, posing no harm in the ocean.>
The work was published in Green Chemistry and Food Chemistry journals. This study was conducted with the support of the Korea Research Foundation and the Korea Institute for Agriculture, Food and Rural Affairs Technology Planning and Evaluation, etc.
*Title of paper published in Green Chemistry: Boric acid-crosslinked poly(vinyl alcohol): biodegradable, biocompatible, robust, and high-barrier paper coating
※ Selected as the article for the back cover of the journal .
- Authors: Shinhyeong Choe, Seulki You, Kitae Park, Youngju Kim, Jehee Park, Yongjun Cho, Jongchul Seo, Hanseul Yang, and Jaewook Myung)
- Date: April 17, 2024
- DOI: 10.1039/D4GC00618F
*Title of paper published in Food Chemistry: Effect of epichlorohydrin treatment on the coating process and performance of high-barrier paper packaging
- Authors: Kitae Park, Shinhyeong Choe, Kambiz Sadeghi, Pradeep Kumar Panda, Jaewook Myung, Dowan Kim, and Jongchul Seo
- Date: February 19, 2024
- DOI: 10.1016/j.foodchem.2024.138772
<Figure 4. Back cover art of Green Chemistry journal of the latest volume, describing the boric acid cross-linked poly(vinyl alcohol) coated paper featuring marine biodegradability, biocompatibility, high barrier properties, and robustness developed through this study.>
2024.05.22 View 8188 -
 KAIST Research Team Creates the Scent of Jasmine from Microorganisms
The fragrance of jasmine and ylang-ylang, used widely in the manufacturing of cosmetics, foods, and beverages, can be produced by direct extraction from their respective flowers. In reality, this makes it difficult for production to meet demand, so companies use benzyl acetate, a major aromatic component of the two fragrances that is chemically synthesized from raw materials derived from petroleum.
On February 26, a KAIST research team led by Research Professor Kyeong Rok Choi from the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering revealed the development of the first microbial process to effectively produce benzyl acetate, an industrially useful compound, from renewable carbon sources such as glucose. The results were published in their paper titled “A microbial process for the production of benzyl acetate”.
< Figure 1. Production of benzyl acetate through co-culture of upstream and downstream strains harboring the benzoic acid-dependent pathway. >
The team, led by Distinguished Professor Lee, aimed to produce benzyl acetate through an environmentally friendly and sustainable method, and developed an Escherichia coli strand to convert glucose into benzyl acetate through system metabolic engineering*.
*System metabolic engineering: a field of research founded by Distinguished Professor Lee to effectively develop microbial cell plants, a core component of the bio-industry that will replace the existing chemical industry, which is highly dependent on petroleum.
The research team developed a metabolic pathway that biosynthesizes benzyl acetate from benzoic acid derived from glucose, and successfully produced benzyl acetate by co-culturing** the strain.
**co-culture: simultaneously synthesizing two or more types of microorganisms in a mixture
However, it has been confirmed that the enzyme used to convert benzoic acid into benzyl acetate in this co-culturing technique acts non-specifically on an intermediate product during benzoic acid biosynthesis, producing a by-product called cinnamyl acetate. This process consumes the intermediate product needed for benzoic acid biosynthesis, thereby reducing the production efficiency of the target compound, benzyl acetate.
To overcome this problem, Distinguished Professor Lee and his team devised a delayed co-culture method, where they first produced benzoic acid in the earlier stages of fermentation by only culturing the top strain that produces benzoic acid from glucose, and later inoculated the bottom strain to convert the accumulated benzoic acid in the culture medium into benzyl acetate.
By applying this co-culture technique, the team suppressed the formation of by-products without further strain improvement or applying additional enzymes, and multiplied the concentration of the target compound by 10 times, producing 2.2 g/L of benzyl acetate. In addition, the team confirmed its potential for the commercial production of benzyl acetate through a technical economic analysis on this microbial process.
< Figure 2. Delayed co-culture of the Bn1 and Bn-BnAc3 strains for improved production of benzyl acetate through the benzoic acid-independent pathway.>
Research Professor Keyong Rok Choi, who was the first author of this paper, said, “This work is significant in that we have developed an effective microbial process to produce the industrially useful compound benzyl acetate, and also in that we have suggested a new approach to overcome the target chemical efficiency diminution and by-product formation issues caused commonly through non-specific enzyme activities during metabolic engineering.”
Distinguished Professor Lee said, “If we can increase the variety and number of microbial processes that produce useful chemicals through sustainable methods and at the same time develop effective strategies to solve chronic and inevitable problems that arise during microbial strain development, we will be able to accelerate the transition from the petrochemical industry into the eco-friendly and sustainable bio-industry.
This work was published online in Nature Chemical Engineering, issued by Nature.
This research was supported by the ‘Implementation of Intelligent Cell Factory Technology (PI: Distinguished Professor Sang Yup Lee) Project by the Ministry of Science and ICT, and the ‘Development of Protein Production Technology from Inorganic Substances through Microbiological Metabolic System Control’ (PI: Research Professor Kyeong Rok Choi) by the Agricultural Microbiological Project Group at the Rural Development Administration.
2024.03.05 View 7222
KAIST Research Team Creates the Scent of Jasmine from Microorganisms
The fragrance of jasmine and ylang-ylang, used widely in the manufacturing of cosmetics, foods, and beverages, can be produced by direct extraction from their respective flowers. In reality, this makes it difficult for production to meet demand, so companies use benzyl acetate, a major aromatic component of the two fragrances that is chemically synthesized from raw materials derived from petroleum.
On February 26, a KAIST research team led by Research Professor Kyeong Rok Choi from the BioProcess Engineering Research Center and Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering revealed the development of the first microbial process to effectively produce benzyl acetate, an industrially useful compound, from renewable carbon sources such as glucose. The results were published in their paper titled “A microbial process for the production of benzyl acetate”.
< Figure 1. Production of benzyl acetate through co-culture of upstream and downstream strains harboring the benzoic acid-dependent pathway. >
The team, led by Distinguished Professor Lee, aimed to produce benzyl acetate through an environmentally friendly and sustainable method, and developed an Escherichia coli strand to convert glucose into benzyl acetate through system metabolic engineering*.
*System metabolic engineering: a field of research founded by Distinguished Professor Lee to effectively develop microbial cell plants, a core component of the bio-industry that will replace the existing chemical industry, which is highly dependent on petroleum.
The research team developed a metabolic pathway that biosynthesizes benzyl acetate from benzoic acid derived from glucose, and successfully produced benzyl acetate by co-culturing** the strain.
**co-culture: simultaneously synthesizing two or more types of microorganisms in a mixture
However, it has been confirmed that the enzyme used to convert benzoic acid into benzyl acetate in this co-culturing technique acts non-specifically on an intermediate product during benzoic acid biosynthesis, producing a by-product called cinnamyl acetate. This process consumes the intermediate product needed for benzoic acid biosynthesis, thereby reducing the production efficiency of the target compound, benzyl acetate.
To overcome this problem, Distinguished Professor Lee and his team devised a delayed co-culture method, where they first produced benzoic acid in the earlier stages of fermentation by only culturing the top strain that produces benzoic acid from glucose, and later inoculated the bottom strain to convert the accumulated benzoic acid in the culture medium into benzyl acetate.
By applying this co-culture technique, the team suppressed the formation of by-products without further strain improvement or applying additional enzymes, and multiplied the concentration of the target compound by 10 times, producing 2.2 g/L of benzyl acetate. In addition, the team confirmed its potential for the commercial production of benzyl acetate through a technical economic analysis on this microbial process.
< Figure 2. Delayed co-culture of the Bn1 and Bn-BnAc3 strains for improved production of benzyl acetate through the benzoic acid-independent pathway.>
Research Professor Keyong Rok Choi, who was the first author of this paper, said, “This work is significant in that we have developed an effective microbial process to produce the industrially useful compound benzyl acetate, and also in that we have suggested a new approach to overcome the target chemical efficiency diminution and by-product formation issues caused commonly through non-specific enzyme activities during metabolic engineering.”
Distinguished Professor Lee said, “If we can increase the variety and number of microbial processes that produce useful chemicals through sustainable methods and at the same time develop effective strategies to solve chronic and inevitable problems that arise during microbial strain development, we will be able to accelerate the transition from the petrochemical industry into the eco-friendly and sustainable bio-industry.
This work was published online in Nature Chemical Engineering, issued by Nature.
This research was supported by the ‘Implementation of Intelligent Cell Factory Technology (PI: Distinguished Professor Sang Yup Lee) Project by the Ministry of Science and ICT, and the ‘Development of Protein Production Technology from Inorganic Substances through Microbiological Metabolic System Control’ (PI: Research Professor Kyeong Rok Choi) by the Agricultural Microbiological Project Group at the Rural Development Administration.
2024.03.05 View 7222 -
 KAIST Research team develops anti-icing film that only requires sunlight
A KAIST research team has developed an anti-icing and de-icing film coating technology that can apply the photothermal effect of gold nanoparticles to industrial sites without the need for heating wires, periodic spray or oil coating of anti-freeze substances, and substrate design alterations.
The group led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering (Fluid & Interface Laboratory) and Professor Dong Ki Yoon from the Department of Chemistry (Soft Material Assembly Group) revealed on January 3 to have together developed an original technique that can uniformly pattern gold nanorod (GNR) particles in quadrants through simple evaporation, and have used this to develop an anti-icing and de-icing surface.
Many scientists in recent years have tried to control substrate surfaces through various coating techniques, and those involving the patterning of functional nanomaterials have gained special attention. In particular, GNR is considered a promising candidate nanomaterial for its biocompatibility, chemical stability, relatively simple synthesis, and its stable and unique property of surface plasmon resonance. To maximize the performance of GNR, it is important to achieve a high uniformity during film deposition, and a high level of rod alignment. However, achieving both criteria has thus far been a difficult challenge.
< Figure 1. Conceptual image to display Hydrodynamic mechanisms for the formation of a homogeneous quadrant cellulose nanocrystal(CNC) matrix. >
To solve this, the joint research team utilized cellulose nanocrystal (CNC), a next-generation functional nanomaterial that can easily be extracted from nature. By co-assembling GNR on CNC quadrant templates, the team could uniformly dry the film and successfully obtain a GNR film with a uniform alignment in a ring-shape. Compared to existing coffee-ring films, the highly uniform and aligned GNR film developed through this research showed enhanced plasmonic photothermal properties, and the team showed that it could carry out anti-icing and de-icing functions by simply irradiating light in the visible wavelength range.
< Figure 2. Optical and thermal performance evaluation results of gold nanorod film and demonstration of plasmonic heater for anti-icing and de-icing. >
Professor Hyoungsoo Kim said, “This technique can be applied to plastic, as well as flexible surfaces. By using it on exterior materials and films, it can generate its own heat energy, which would greatly save energy through voluntary thermal energy harvesting across various applications including cars, aircrafts, and windows in residential or commercial spaces, where frosting becomes a serious issue in the winter.” Professor Dong Ki Yoon added, “This research is significant in that we can now freely pattern the CNC-GNR composite, which was previously difficult to create into films, over a large area. We can utilize this as an anti-icing material, and if we were to take advantage of the plasmonic properties of gold, we can also use it like stained-glass to decorate glass surfaces.”
This research was conducted by Ph.D. candidate Jeongsu Pyeon from the Department of Mechanical Engineering, and his co-first author Dr. Soon Mo Park (a KAIST graduate, currently a post-doctoral associate at Cornell University), and was pushed in the online volume of Nature Communication on December 8, 2023 under the title “Plasmonic Metasurfaces of Cellulose Nanocrystal Matrices with Quadrants of Aligned Gold Nanorods for Photothermal Anti-Icing." Recognized for its achievement, the research was also selected as an editor’s highlight for the journals Materials Science and Chemistry, and Inorganic and Physical Chemistry.
This research was supported by the Individual Basic Mid-Sized Research Fund from the National Research Foundation of Korea and the Center for Multiscale Chiral Architectures.
2024.01.16 View 11010
KAIST Research team develops anti-icing film that only requires sunlight
A KAIST research team has developed an anti-icing and de-icing film coating technology that can apply the photothermal effect of gold nanoparticles to industrial sites without the need for heating wires, periodic spray or oil coating of anti-freeze substances, and substrate design alterations.
The group led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering (Fluid & Interface Laboratory) and Professor Dong Ki Yoon from the Department of Chemistry (Soft Material Assembly Group) revealed on January 3 to have together developed an original technique that can uniformly pattern gold nanorod (GNR) particles in quadrants through simple evaporation, and have used this to develop an anti-icing and de-icing surface.
Many scientists in recent years have tried to control substrate surfaces through various coating techniques, and those involving the patterning of functional nanomaterials have gained special attention. In particular, GNR is considered a promising candidate nanomaterial for its biocompatibility, chemical stability, relatively simple synthesis, and its stable and unique property of surface plasmon resonance. To maximize the performance of GNR, it is important to achieve a high uniformity during film deposition, and a high level of rod alignment. However, achieving both criteria has thus far been a difficult challenge.
< Figure 1. Conceptual image to display Hydrodynamic mechanisms for the formation of a homogeneous quadrant cellulose nanocrystal(CNC) matrix. >
To solve this, the joint research team utilized cellulose nanocrystal (CNC), a next-generation functional nanomaterial that can easily be extracted from nature. By co-assembling GNR on CNC quadrant templates, the team could uniformly dry the film and successfully obtain a GNR film with a uniform alignment in a ring-shape. Compared to existing coffee-ring films, the highly uniform and aligned GNR film developed through this research showed enhanced plasmonic photothermal properties, and the team showed that it could carry out anti-icing and de-icing functions by simply irradiating light in the visible wavelength range.
< Figure 2. Optical and thermal performance evaluation results of gold nanorod film and demonstration of plasmonic heater for anti-icing and de-icing. >
Professor Hyoungsoo Kim said, “This technique can be applied to plastic, as well as flexible surfaces. By using it on exterior materials and films, it can generate its own heat energy, which would greatly save energy through voluntary thermal energy harvesting across various applications including cars, aircrafts, and windows in residential or commercial spaces, where frosting becomes a serious issue in the winter.” Professor Dong Ki Yoon added, “This research is significant in that we can now freely pattern the CNC-GNR composite, which was previously difficult to create into films, over a large area. We can utilize this as an anti-icing material, and if we were to take advantage of the plasmonic properties of gold, we can also use it like stained-glass to decorate glass surfaces.”
This research was conducted by Ph.D. candidate Jeongsu Pyeon from the Department of Mechanical Engineering, and his co-first author Dr. Soon Mo Park (a KAIST graduate, currently a post-doctoral associate at Cornell University), and was pushed in the online volume of Nature Communication on December 8, 2023 under the title “Plasmonic Metasurfaces of Cellulose Nanocrystal Matrices with Quadrants of Aligned Gold Nanorods for Photothermal Anti-Icing." Recognized for its achievement, the research was also selected as an editor’s highlight for the journals Materials Science and Chemistry, and Inorganic and Physical Chemistry.
This research was supported by the Individual Basic Mid-Sized Research Fund from the National Research Foundation of Korea and the Center for Multiscale Chiral Architectures.
2024.01.16 View 11010 -
 KAIST presents strategies for environmentally friendly and sustainable polyamides production
- Provides current research trends in bio-based polyamide production
- Research on bio-based polyamides production gains importance for achieving a carbon-neutral society
Global industries focused on carbon neutrality, under the slogan "Net-Zero," are gaining increasing attention. In particular, research on microbial production of polymers, replacing traditional chemical methods with biological approaches, is actively progressing.
Polyamides, represented by nylon, are linear polymers widely used in various industries such as automotive, electronics, textiles, and medical fields. They possess beneficial properties such as high tensile strength, electrical insulation, heat resistance, wear resistance, and biocompatibility. Since the commercialization of nylon in 1938, approximately 7 million tons of polyamides are produced worldwide annually. Considering their broad applications and significance, producing polyamides through bio-based methods holds considerable environmental and industrial importance.
KAIST (President Kwang-Hyung Lee) announced that a research team led by Distinguished Professor Sang Yup Lee, including Dr. Jong An Lee and doctoral candidate Ji Yeon Kim from the Department of Chemical and Biomolecular Engineering, published a paper titled "Current Advancements in Bio-Based Production of Polyamides”. The paper was featured on the cover of the monthly issue of "Trends in Chemistry” by Cell Press.
As part of climate change response technologies, bio-refineries involve using biotechnological and chemical methods to produce industrially important chemicals and biofuels from renewable biomass without relying on fossil resources. Notably, systems metabolic engineering, pioneered by KAIST's Distinguished Professor Sang Yup Lee, is a research field that effectively manipulates microbial metabolic pathways to produce valuable chemicals, forming the core technology for bio-refineries. The research team has successfully developed high-performance strains producing a variety of compounds, including succinic acid, biodegradable plastics, biofuels, and natural products, using systems metabolic engineering tools and strategies.
The research team predicted that if bio-based polyamide production technology, which is widely used in the production of clothing and textiles, becomes widespread, it will attract attention as a future technology that can respond to the climate crisis due to its environment-friendly production technology. In this study, the research team comprehensively reviewed the bio-based polyamide production strategies. They provided insights into the advancements in polyamide monomer production using metabolically engineered microorganisms and highlighted the recent trends in bio-based polyamide advancements utilizing these monomers. Additionally, they reviewed the strategies for synthesizing bio-based polyamides through chemical conversion of natural oils and discussed the biodegradability and recycling of the polyamides. Furthermore, the paper presented the future direction in which metabolic engineering can be applied for the bio-based polyamide production, contributing to environmentally friendly and sustainable society.
Ji Yeon Kim, the co-first author of this paper from KAIST, stated "The importance of utilizing systems metabolic engineering tools and strategies for bio-based polyamides production is becoming increasingly prominent in achieving carbon neutrality."
Professor Sang Yup Lee emphasized, "Amid growing concerns about climate change, the significance of environmentally friendly and sustainable industrial development is greater than ever. Systems metabolic engineering is expected to have a significant impact not only on the chemical industry but also in various fields."
< [Figure 1] A schematic overview of the overall process for polyamides production >
This paper by Dr. Jong An Lee, PhD student Ji Yeon Kim, Dr. Jung Ho Ahn, and Master Yeah-Ji Ahn from the Department of Chemical and Biomolecular Engineering at KAIST was published in the December issue of 'Trends in Chemistry', an authoritative review journal in the field of chemistry published by Cell. It was published on December 7 as the cover paper and featured review.
※ Paper title: Current advancements in the bio-based production of polyamides
※ Author information: Jong An Lee, Ji Yeon Kim, Jung Ho Ahn, Yeah-Ji Ahn, and Sang Yup Lee
This research was conducted with the support from the development of platform technologies of microbial cell factories for the next-generation biorefineries project and C1 gas refinery program by Korean Ministry of Science and ICT.
< [Figure 2] Cover paper of the December issue of Trends in Chemistry >
2023.12.21 View 7352
KAIST presents strategies for environmentally friendly and sustainable polyamides production
- Provides current research trends in bio-based polyamide production
- Research on bio-based polyamides production gains importance for achieving a carbon-neutral society
Global industries focused on carbon neutrality, under the slogan "Net-Zero," are gaining increasing attention. In particular, research on microbial production of polymers, replacing traditional chemical methods with biological approaches, is actively progressing.
Polyamides, represented by nylon, are linear polymers widely used in various industries such as automotive, electronics, textiles, and medical fields. They possess beneficial properties such as high tensile strength, electrical insulation, heat resistance, wear resistance, and biocompatibility. Since the commercialization of nylon in 1938, approximately 7 million tons of polyamides are produced worldwide annually. Considering their broad applications and significance, producing polyamides through bio-based methods holds considerable environmental and industrial importance.
KAIST (President Kwang-Hyung Lee) announced that a research team led by Distinguished Professor Sang Yup Lee, including Dr. Jong An Lee and doctoral candidate Ji Yeon Kim from the Department of Chemical and Biomolecular Engineering, published a paper titled "Current Advancements in Bio-Based Production of Polyamides”. The paper was featured on the cover of the monthly issue of "Trends in Chemistry” by Cell Press.
As part of climate change response technologies, bio-refineries involve using biotechnological and chemical methods to produce industrially important chemicals and biofuels from renewable biomass without relying on fossil resources. Notably, systems metabolic engineering, pioneered by KAIST's Distinguished Professor Sang Yup Lee, is a research field that effectively manipulates microbial metabolic pathways to produce valuable chemicals, forming the core technology for bio-refineries. The research team has successfully developed high-performance strains producing a variety of compounds, including succinic acid, biodegradable plastics, biofuels, and natural products, using systems metabolic engineering tools and strategies.
The research team predicted that if bio-based polyamide production technology, which is widely used in the production of clothing and textiles, becomes widespread, it will attract attention as a future technology that can respond to the climate crisis due to its environment-friendly production technology. In this study, the research team comprehensively reviewed the bio-based polyamide production strategies. They provided insights into the advancements in polyamide monomer production using metabolically engineered microorganisms and highlighted the recent trends in bio-based polyamide advancements utilizing these monomers. Additionally, they reviewed the strategies for synthesizing bio-based polyamides through chemical conversion of natural oils and discussed the biodegradability and recycling of the polyamides. Furthermore, the paper presented the future direction in which metabolic engineering can be applied for the bio-based polyamide production, contributing to environmentally friendly and sustainable society.
Ji Yeon Kim, the co-first author of this paper from KAIST, stated "The importance of utilizing systems metabolic engineering tools and strategies for bio-based polyamides production is becoming increasingly prominent in achieving carbon neutrality."
Professor Sang Yup Lee emphasized, "Amid growing concerns about climate change, the significance of environmentally friendly and sustainable industrial development is greater than ever. Systems metabolic engineering is expected to have a significant impact not only on the chemical industry but also in various fields."
< [Figure 1] A schematic overview of the overall process for polyamides production >
This paper by Dr. Jong An Lee, PhD student Ji Yeon Kim, Dr. Jung Ho Ahn, and Master Yeah-Ji Ahn from the Department of Chemical and Biomolecular Engineering at KAIST was published in the December issue of 'Trends in Chemistry', an authoritative review journal in the field of chemistry published by Cell. It was published on December 7 as the cover paper and featured review.
※ Paper title: Current advancements in the bio-based production of polyamides
※ Author information: Jong An Lee, Ji Yeon Kim, Jung Ho Ahn, Yeah-Ji Ahn, and Sang Yup Lee
This research was conducted with the support from the development of platform technologies of microbial cell factories for the next-generation biorefineries project and C1 gas refinery program by Korean Ministry of Science and ICT.
< [Figure 2] Cover paper of the December issue of Trends in Chemistry >
2023.12.21 View 7352 -
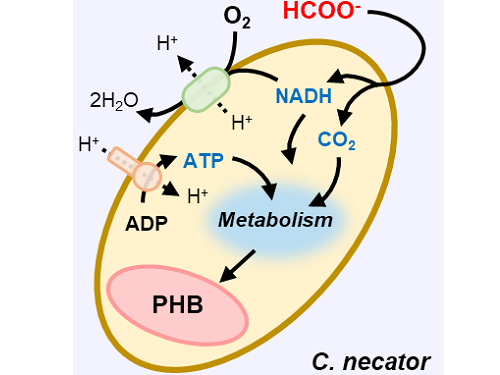 A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11858
A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11858 -
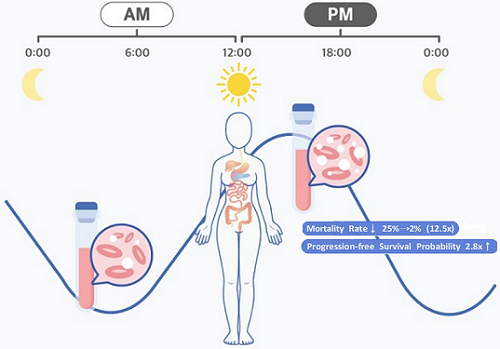 Afternoon chemotherapy proved to deliver more desirable results for female lymphoma patients
Chemotherapy is a commonly used regimen for cancer treatment, but it is also a double-edged sword. While the drugs are highly effective at killing cancer cells, they are also notorious for killing healthy cells in the body. As such, minimizing the drug’s damage to the patient’s body is necessary for improving the prognosis of chemotherapy.
Recently, “chrono-chemotherapy” have been gaining interest in the research community. As the name suggests, the aim is timing the delivery of the drugs when the body is least vulnerable to their harmful effects and while the cancer cells are at their most vulnerable.
< Figure 1. Chrono-chemotherapy considering circadian rhythm >
Chrono-chemotherapy exploits the fact that human physiological processes, including cell proliferation and differentiation, are regulated by an endogenous timer called the circadian clock. However, this has not been widely exploited in real-world clinical settings because, as of now, there is no systematic method for finding the optimal chemotherapy delivery time.
This problem was tackled by an interdisciplinary team of researchers from South Korea. They were led by principal investigators Jae Kyoung Kim (a mathematician from the Biomedical Mathematics Group, Institute for Basic Science) and Youngil Koh (an oncologist at Seoul National University Hospital). The researchers studied a group of patients suffering from diffuse large B-cell lymphoma (DLBCL).
Terminology
* Diffuse large B-cell lymphoma (DLBCL): Lymphoma is a type of blood cancer caused by the malignant transformation of lymphoid tissue cells. Lymphoma is divided into Hodgkin's lymphoma and non-Hodgkin's lymphoma (malignant lymphoma), and diffuse large B-cell lymphoma accounts for about 30 to 40% of non-Hodgkin's lymphoma.
The research team noticed that DLBCL patients at Seoul National University Hospital received chemotherapy on two different schedules, with some patients receiving morning treatment (8:30 a.m.) and others taking the drugs in the afternoon (2:30 p.m.). All patients received the same cancer treatment (R-CHOP), which is a combination of targeted therapy and chemotherapy, four to six times in the morning or afternoon at intervals of about three weeks.
They analyzed 210 patients to investigate whether there was any difference between morning and afternoon treatments. It was found that female patients who received the afternoon treatment had a 12.5 times reduced mortality rate (25% to 2%), while the cancer recurrence after 60 months decreased by 2.8 times (37% to 13%). In addition, chemotherapy side effects such as neutropenia were more common in female patients who received the morning treatment.
Surprisingly, there was no differences found in treatment efficiency depending on the treatment schedule in the cases of male patients.
To understand the cause of the gender differences, the research team analyzed upto 14,000 blood samples from the Seoul National University Hospital Health Examination Center. It was found that in females, white blood cell counts tended to decrease in the morning and increase in the afternoon. This indicates that the bone marrow proliferation rate was higher in the morning than in the afternoon because there is a upto 12 hour delay between bone marrow proliferation and blood cell production.
This means that if a female patient receives chemotherapy in the morning when bone marrow is actively producing blood cells, the possibility of adverse side effects becomes greater. These results are consistent with the findings from recent randomized clinical trials that showed female colorectal cancer patients treated with irinotecan in the morning suffered from higher drug toxicities.
One confounding variable was the drug dose. Since the morning female patients suffered from greater adverse side effects, oftentimes the dose had to be reduced for these patients. On average, the drug dose was reduced by upto 10% compared to the dose intensity given to female patients receiving the afternoon treatment.
Unlike the female patients, it was found that male patients did not show a significant difference in white blood cell count and bone marrow cell proliferation activity throughout the day, which explains why the timing of the treatment had no impact.
Professor Youngil Koh said, “We plan to verify the conclusions of this study again with a large-scale follow-up study that completely controls for the confounding variables, and to confirm whether chrono-chemotherapy has similar effects on other cancers.”
CI Jae Kyoung Kim said, “Because the time of the internal circadian clock can vary greatly depending on the individual's sleep-wake patterns, we are currently developing a technology to estimate a patient’s circadian clock from their sleep pattern. We hope that this can be used to develop an individualized anti-cancer chronotherapy schedule.”
< Figure 2. Chemotherapy in the afternoon can improve treatment outcomes. >
The daily fluctuation of proliferative activity of bone marrow is larger in females than in males, and it becomes higher in the morning (left). Thus, chemotherapy in the morning strongly inhibits proliferative activity in female lymphoma patients, resulting in a higher incidence of adverse events such as neutropenia and infections. This forced the clinicians to reduce the dose intensity (center). Consequently, female patients undergoing the morning treatment showed a lower survival probability than those undergoing the afternoon treatment (right). Specifically, only ~13% of female patients treated in the afternoon had a worse outcome and ~2% of them died while ~37% of female patients treated in the morning had a worse outcome and ~25% of them died. Male patients did not show any difference in treatment outcomes depending on the chemotherapy delivery time.
2023.01.27 View 7915
Afternoon chemotherapy proved to deliver more desirable results for female lymphoma patients
Chemotherapy is a commonly used regimen for cancer treatment, but it is also a double-edged sword. While the drugs are highly effective at killing cancer cells, they are also notorious for killing healthy cells in the body. As such, minimizing the drug’s damage to the patient’s body is necessary for improving the prognosis of chemotherapy.
Recently, “chrono-chemotherapy” have been gaining interest in the research community. As the name suggests, the aim is timing the delivery of the drugs when the body is least vulnerable to their harmful effects and while the cancer cells are at their most vulnerable.
< Figure 1. Chrono-chemotherapy considering circadian rhythm >
Chrono-chemotherapy exploits the fact that human physiological processes, including cell proliferation and differentiation, are regulated by an endogenous timer called the circadian clock. However, this has not been widely exploited in real-world clinical settings because, as of now, there is no systematic method for finding the optimal chemotherapy delivery time.
This problem was tackled by an interdisciplinary team of researchers from South Korea. They were led by principal investigators Jae Kyoung Kim (a mathematician from the Biomedical Mathematics Group, Institute for Basic Science) and Youngil Koh (an oncologist at Seoul National University Hospital). The researchers studied a group of patients suffering from diffuse large B-cell lymphoma (DLBCL).
Terminology
* Diffuse large B-cell lymphoma (DLBCL): Lymphoma is a type of blood cancer caused by the malignant transformation of lymphoid tissue cells. Lymphoma is divided into Hodgkin's lymphoma and non-Hodgkin's lymphoma (malignant lymphoma), and diffuse large B-cell lymphoma accounts for about 30 to 40% of non-Hodgkin's lymphoma.
The research team noticed that DLBCL patients at Seoul National University Hospital received chemotherapy on two different schedules, with some patients receiving morning treatment (8:30 a.m.) and others taking the drugs in the afternoon (2:30 p.m.). All patients received the same cancer treatment (R-CHOP), which is a combination of targeted therapy and chemotherapy, four to six times in the morning or afternoon at intervals of about three weeks.
They analyzed 210 patients to investigate whether there was any difference between morning and afternoon treatments. It was found that female patients who received the afternoon treatment had a 12.5 times reduced mortality rate (25% to 2%), while the cancer recurrence after 60 months decreased by 2.8 times (37% to 13%). In addition, chemotherapy side effects such as neutropenia were more common in female patients who received the morning treatment.
Surprisingly, there was no differences found in treatment efficiency depending on the treatment schedule in the cases of male patients.
To understand the cause of the gender differences, the research team analyzed upto 14,000 blood samples from the Seoul National University Hospital Health Examination Center. It was found that in females, white blood cell counts tended to decrease in the morning and increase in the afternoon. This indicates that the bone marrow proliferation rate was higher in the morning than in the afternoon because there is a upto 12 hour delay between bone marrow proliferation and blood cell production.
This means that if a female patient receives chemotherapy in the morning when bone marrow is actively producing blood cells, the possibility of adverse side effects becomes greater. These results are consistent with the findings from recent randomized clinical trials that showed female colorectal cancer patients treated with irinotecan in the morning suffered from higher drug toxicities.
One confounding variable was the drug dose. Since the morning female patients suffered from greater adverse side effects, oftentimes the dose had to be reduced for these patients. On average, the drug dose was reduced by upto 10% compared to the dose intensity given to female patients receiving the afternoon treatment.
Unlike the female patients, it was found that male patients did not show a significant difference in white blood cell count and bone marrow cell proliferation activity throughout the day, which explains why the timing of the treatment had no impact.
Professor Youngil Koh said, “We plan to verify the conclusions of this study again with a large-scale follow-up study that completely controls for the confounding variables, and to confirm whether chrono-chemotherapy has similar effects on other cancers.”
CI Jae Kyoung Kim said, “Because the time of the internal circadian clock can vary greatly depending on the individual's sleep-wake patterns, we are currently developing a technology to estimate a patient’s circadian clock from their sleep pattern. We hope that this can be used to develop an individualized anti-cancer chronotherapy schedule.”
< Figure 2. Chemotherapy in the afternoon can improve treatment outcomes. >
The daily fluctuation of proliferative activity of bone marrow is larger in females than in males, and it becomes higher in the morning (left). Thus, chemotherapy in the morning strongly inhibits proliferative activity in female lymphoma patients, resulting in a higher incidence of adverse events such as neutropenia and infections. This forced the clinicians to reduce the dose intensity (center). Consequently, female patients undergoing the morning treatment showed a lower survival probability than those undergoing the afternoon treatment (right). Specifically, only ~13% of female patients treated in the afternoon had a worse outcome and ~2% of them died while ~37% of female patients treated in the morning had a worse outcome and ~25% of them died. Male patients did not show any difference in treatment outcomes depending on the chemotherapy delivery time.
2023.01.27 View 7915 -
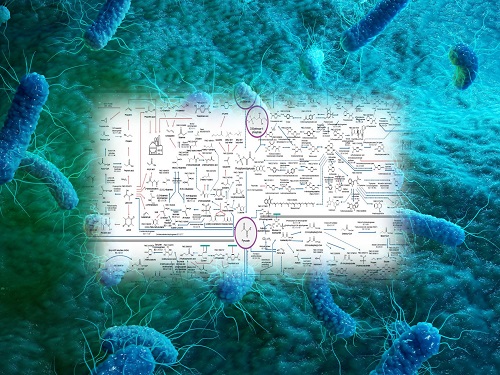 Interactive Map of Metabolical Synthesis of Chemicals
An interactive map that compiled the chemicals produced by biological, chemical and combined reactions has been distributed on the web
- A team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, organized and distributed an all-inclusive listing of chemical substances that can be synthesized using microorganisms
- It is expected to be used by researchers around the world as it enables easy assessment of the synthetic pathway through the web.
A research team comprised of Woo Dae Jang, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported an interactive metabolic map of bio-based chemicals. Their research paper “An interactive metabolic map of bio-based chemicals” was published online in Trends in Biotechnology on August 10, 2022.
As a response to rapid climate change and environmental pollution, research on the production of petrochemical products using microorganisms is receiving attention as a sustainable alternative to existing methods of productions. In order to synthesize various chemical substances, materials, and fuel using microorganisms, it is necessary to first construct the biosynthetic pathway toward desired product by exploration and discovery and introduce them into microorganisms. In addition, in order to efficiently synthesize various chemical substances, it is sometimes necessary to employ chemical methods along with bioengineering methods using microorganisms at the same time. For the production of non-native chemicals, novel pathways are designed by recruiting enzymes from heterologous sources or employing enzymes designed though rational engineering, directed evolution, or ab initio design.
The research team had completed a map of chemicals which compiled all available pathways of biological and/or chemical reactions that lead to the production of various bio-based chemicals back in 2019 and published the map in Nature Catalysis. The map was distributed in the form of a poster to industries and academia so that the synthesis paths of bio-based chemicals could be checked at a glance.
The research team has expanded the bio-based chemicals map this time in the form of an interactive map on the web so that anyone with internet access can quickly explore efficient paths to synthesize desired products. The web-based map provides interactive visual tools to allow interactive visualization, exploration, and analysis of complex networks of biological and/or chemical reactions toward the desired products. In addition, the reported paper also discusses the production of natural compounds that are used for diverse purposes such as food and medicine, which will help designing novel pathways through similar approaches or by exploiting the promiscuity of enzymes described in the map. The published bio-based chemicals map is also available at http://systemsbiotech.co.kr.
The co-first authors, Dr. Woo Dae Jang and Ph.D. student Gi Bae Kim, said, “We conducted this study to address the demand for updating the previously distributed chemicals map and enhancing its versatility.” “The map is expected to be utilized in a variety of research and in efforts to set strategies and prospects for chemical production incorporating bio and chemical methods that are detailed in the map.”
Distinguished Professor Sang Yup Lee said, “The interactive bio-based chemicals map is expected to help design and optimization of the metabolic pathways for the biosynthesis of target chemicals together with the strategies of chemical conversions, serving as a blueprint for developing further ideas on the production of desired chemicals through biological and/or chemical reactions.”
The interactive metabolic map of bio-based chemicals.
2022.08.11 View 16432
Interactive Map of Metabolical Synthesis of Chemicals
An interactive map that compiled the chemicals produced by biological, chemical and combined reactions has been distributed on the web
- A team led by Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, organized and distributed an all-inclusive listing of chemical substances that can be synthesized using microorganisms
- It is expected to be used by researchers around the world as it enables easy assessment of the synthetic pathway through the web.
A research team comprised of Woo Dae Jang, Gi Bae Kim, and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST reported an interactive metabolic map of bio-based chemicals. Their research paper “An interactive metabolic map of bio-based chemicals” was published online in Trends in Biotechnology on August 10, 2022.
As a response to rapid climate change and environmental pollution, research on the production of petrochemical products using microorganisms is receiving attention as a sustainable alternative to existing methods of productions. In order to synthesize various chemical substances, materials, and fuel using microorganisms, it is necessary to first construct the biosynthetic pathway toward desired product by exploration and discovery and introduce them into microorganisms. In addition, in order to efficiently synthesize various chemical substances, it is sometimes necessary to employ chemical methods along with bioengineering methods using microorganisms at the same time. For the production of non-native chemicals, novel pathways are designed by recruiting enzymes from heterologous sources or employing enzymes designed though rational engineering, directed evolution, or ab initio design.
The research team had completed a map of chemicals which compiled all available pathways of biological and/or chemical reactions that lead to the production of various bio-based chemicals back in 2019 and published the map in Nature Catalysis. The map was distributed in the form of a poster to industries and academia so that the synthesis paths of bio-based chemicals could be checked at a glance.
The research team has expanded the bio-based chemicals map this time in the form of an interactive map on the web so that anyone with internet access can quickly explore efficient paths to synthesize desired products. The web-based map provides interactive visual tools to allow interactive visualization, exploration, and analysis of complex networks of biological and/or chemical reactions toward the desired products. In addition, the reported paper also discusses the production of natural compounds that are used for diverse purposes such as food and medicine, which will help designing novel pathways through similar approaches or by exploiting the promiscuity of enzymes described in the map. The published bio-based chemicals map is also available at http://systemsbiotech.co.kr.
The co-first authors, Dr. Woo Dae Jang and Ph.D. student Gi Bae Kim, said, “We conducted this study to address the demand for updating the previously distributed chemicals map and enhancing its versatility.” “The map is expected to be utilized in a variety of research and in efforts to set strategies and prospects for chemical production incorporating bio and chemical methods that are detailed in the map.”
Distinguished Professor Sang Yup Lee said, “The interactive bio-based chemicals map is expected to help design and optimization of the metabolic pathways for the biosynthesis of target chemicals together with the strategies of chemical conversions, serving as a blueprint for developing further ideas on the production of desired chemicals through biological and/or chemical reactions.”
The interactive metabolic map of bio-based chemicals.
2022.08.11 View 16432 -
 VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 11779
VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 11779 -
 The Dynamic Tracking of Tissue-Specific Secretory Proteins
Researchers develop a versatile and powerful tool for studying the spatiotemporal dynamics of secretory proteins, a valuable class of biomarkers and therapeutic targets
Researchers have presented a method for profiling tissue-specific secretory proteins in live mice. This method is expected to be applicable to various tissues or disease models for investigating biomarkers or therapeutic targets involved in disease progression. This research was reported in Nature Communications on September 1.
Secretory proteins released into the blood play essential roles in physiological systems. They are core mediators of interorgan communication, while serving as biomarkers and therapeutic targets.
Previous studies have analyzed conditioned media from culture models to identify cell type-specific secretory proteins, but these models often fail to fully recapitulate the intricacies of multi-organ systems and thus do not sufficiently reflect biological realities.
These limitations provided compelling motivation for the research team led by Jae Myoung Suh and his collaborators to develop techniques that could identify and resolve characteristics of tissue-specific secretory proteins along time and space dimensions.
For addressing this gap in the current methodology, the research team utilized proximity-labeling enzymes such as TurboID to label secretory proteins in endoplasmic reticulum lumen using biotin. Thereafter, the biotin-labeled secretory proteins were readily enriched through streptavidin affinity purification and could be identified through mass spectrometry.
To demonstrate its functionality in live mice, research team delivered TurboID to mouse livers via an adenovirus. After administering the biotin, only liver-derived secretory proteins were successfully detected in the plasma of the mice. Interestingly, the pattern of biotin-labeled proteins secreted from the liver was clearly distinctive from those of hepatocyte cell lines.
First author Kwang-eun Kim from the Graduate School of Medical Science and Engineering explained, “The proteins secreted by the liver were significantly different from the results of cell culture models. This data shows the limitations of cell culture models for secretory protein study, and this technique can overcome those limitations. It can be further used to discover biomarkers and therapeutic targets that can more fully reflect the physiological state.”
This work research was supported by the National Research Foundation of Korea, the KAIST Key Research Institutes Project (Interdisciplinary Research Group), and the Institute for Basic Science in Korea.
-PublicationKwang-eun Kim, Isaac Park et al., “Dynamic tracking and identification of tissue-specific secretory proteins in the circulation of live mice,” Nature Communications on Sept.1,
2021(https://doi.org/10.1038/s41467-021-25546-y)
-ProfileProfessor Jae Myoung Suh Integrated Lab of Metabolism, Obesity and Diabetes Researchhttps://imodkaist.wixsite.com/home
Graduate School of Medical Science and Engineering College of Life Science and BioengineeringKAIST
2021.09.14 View 10394
The Dynamic Tracking of Tissue-Specific Secretory Proteins
Researchers develop a versatile and powerful tool for studying the spatiotemporal dynamics of secretory proteins, a valuable class of biomarkers and therapeutic targets
Researchers have presented a method for profiling tissue-specific secretory proteins in live mice. This method is expected to be applicable to various tissues or disease models for investigating biomarkers or therapeutic targets involved in disease progression. This research was reported in Nature Communications on September 1.
Secretory proteins released into the blood play essential roles in physiological systems. They are core mediators of interorgan communication, while serving as biomarkers and therapeutic targets.
Previous studies have analyzed conditioned media from culture models to identify cell type-specific secretory proteins, but these models often fail to fully recapitulate the intricacies of multi-organ systems and thus do not sufficiently reflect biological realities.
These limitations provided compelling motivation for the research team led by Jae Myoung Suh and his collaborators to develop techniques that could identify and resolve characteristics of tissue-specific secretory proteins along time and space dimensions.
For addressing this gap in the current methodology, the research team utilized proximity-labeling enzymes such as TurboID to label secretory proteins in endoplasmic reticulum lumen using biotin. Thereafter, the biotin-labeled secretory proteins were readily enriched through streptavidin affinity purification and could be identified through mass spectrometry.
To demonstrate its functionality in live mice, research team delivered TurboID to mouse livers via an adenovirus. After administering the biotin, only liver-derived secretory proteins were successfully detected in the plasma of the mice. Interestingly, the pattern of biotin-labeled proteins secreted from the liver was clearly distinctive from those of hepatocyte cell lines.
First author Kwang-eun Kim from the Graduate School of Medical Science and Engineering explained, “The proteins secreted by the liver were significantly different from the results of cell culture models. This data shows the limitations of cell culture models for secretory protein study, and this technique can overcome those limitations. It can be further used to discover biomarkers and therapeutic targets that can more fully reflect the physiological state.”
This work research was supported by the National Research Foundation of Korea, the KAIST Key Research Institutes Project (Interdisciplinary Research Group), and the Institute for Basic Science in Korea.
-PublicationKwang-eun Kim, Isaac Park et al., “Dynamic tracking and identification of tissue-specific secretory proteins in the circulation of live mice,” Nature Communications on Sept.1,
2021(https://doi.org/10.1038/s41467-021-25546-y)
-ProfileProfessor Jae Myoung Suh Integrated Lab of Metabolism, Obesity and Diabetes Researchhttps://imodkaist.wixsite.com/home
Graduate School of Medical Science and Engineering College of Life Science and BioengineeringKAIST
2021.09.14 View 10394 -
 Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11935
Wearable Device to Monitor Sweat in Real Time
An on-skin platform for the wireless monitoring of flow rate, cumulative loss, and temperature of sweat in real time
An electronic patch can monitor your sweating and check your health status. Even more, the soft microfluidic device that adheres to the surface of the skin, captures, stores, and performs biomarker analysis of sweat as it is released through the eccrine glands.
This wearable and wireless electronic device developed by Professor Kyeongha Kwon and her collaborators is a digital and wireless platform that could help track the so-called ‘filling process’ of sweat without having to visually examine the device. The platform was integrated with microfluidic systems to analyze the sweat’s components.
To monitor the sweat release rate in real time, the researchers created a ‘thermal flow sensing module.’ They designed a sophisticated microfluidic channel to allow the collected sweat to flow through a narrow passage and a heat source was placed on the outer surface of the channel to induce a heat exchange between the sweat and the heated channel.
As a result, the researchers could develop a wireless electronic patch that can measure the temperature difference in a specific location upstream and downstream of the heat source with an electronic circuit and convert it into a digital signal to measure the sweat release rate in real time. The patch accurately measured the perspiration rate in the range of 0-5 microliters/minute (μl/min), which was considered physiologically significant. The sensor can measure the flow of sweat directly and then use the information it collected to quantify total sweat loss. Moreover, the device features advanced microfluidic systems and colorimetric chemical reagents to gather pH measurements and determine the concentration of chloride, creatinine, and glucose in a user's sweat.
Professor Kwon said that these indicators could be used to diagnose various diseases related with sweating such as cystic fibrosis, diabetes, kidney dysfunction, and metabolic alkalosis. “As the sweat flowing in the microfluidic channel is completely separated from the electronic circuit, the new patch overcame the shortcomings of existing flow rate measuring devices, which were vulnerable to corrosion and aging,” she explained.
The patch can be easily attached to the skin with flexible circuit board printing technology and silicone sealing technology. It has an additional sensor that detects changes in skin temperature. Using a smartphone app, a user can check the data measured by the wearable patch in real time.
Professor Kwon added, “This patch can be widely used for personal hydration strategies, the detection of dehydration symptoms, and other health management purposes. It can also be used in a systematic drug delivery system, such as for measuring the blood flow rate in blood vessels near the skin’s surface or measuring a drug’s release rate in real time to calculate the exact dosage.”
-PublicationKyeongha Kwon, Jong Uk Kim, John A. Rogers, et al. “An on-skin platform for wireless monitoring of flow rate, cumulative loss and temperature of sweat in real time.” Nature Electronics (doi.org/10.1038/s41928-021-00556-2)
-ProfileProfessor Kyeongha KwonSchool of Electrical EngineeringKAIST
2021.06.25 View 11935 -
 Professor Mu-Hyun Baik Honored with the POSCO TJ Park Prize
Professor Mu-Hyun Baik at the Department of Chemistry was honored to be the recipient of the 2021 POSCO TJ Park Prize in Science. The POSCO TJ Park Foundation awards every year the individual or organization which made significant contribution in science, education, community development, philanthropy, and technology.
Professor Baik, a renowned computational chemist in analyzing complicated chemical reactions to understand how molecules behave and how they change. Professor Baik was awarded in recognition of his pioneering research in designing numerous organometallic catalysts with using computational molecular modelling. In 2016, he published in Science on the catalytic borylation of methane that showed how chemical reactions can be carried out using the natural gas methane as a substrate.
In 2020, he reported in Science that electrodes can be used as functional groups with adjustable inductive effects to change the chemical reactivity of molecules that are attached to them, closely mimicking the inductive effect of conventional functional groups. This constitutes a potentially powerful new way of controlling chemical reactions, offering an alternative to preparing derivatives to install electron-withdrawing functional groups.
Joined at KAIST in 2015, Professor Baik also serves as associate director at the Center for Catalytic Hydrocarbon Functionalization at the Institute for Basic Science (IBS) since 2015. Among the many recognitions and awards that he received include the Kavli Fellowship by the Kavli Foundation and the National Academy of Science in the US in 2019 and the 2018 Friedrich Wilhelm Bessel Award by the Alexander von Humboldt Foundation in Germany.
2021.03.11 View 10081
Professor Mu-Hyun Baik Honored with the POSCO TJ Park Prize
Professor Mu-Hyun Baik at the Department of Chemistry was honored to be the recipient of the 2021 POSCO TJ Park Prize in Science. The POSCO TJ Park Foundation awards every year the individual or organization which made significant contribution in science, education, community development, philanthropy, and technology.
Professor Baik, a renowned computational chemist in analyzing complicated chemical reactions to understand how molecules behave and how they change. Professor Baik was awarded in recognition of his pioneering research in designing numerous organometallic catalysts with using computational molecular modelling. In 2016, he published in Science on the catalytic borylation of methane that showed how chemical reactions can be carried out using the natural gas methane as a substrate.
In 2020, he reported in Science that electrodes can be used as functional groups with adjustable inductive effects to change the chemical reactivity of molecules that are attached to them, closely mimicking the inductive effect of conventional functional groups. This constitutes a potentially powerful new way of controlling chemical reactions, offering an alternative to preparing derivatives to install electron-withdrawing functional groups.
Joined at KAIST in 2015, Professor Baik also serves as associate director at the Center for Catalytic Hydrocarbon Functionalization at the Institute for Basic Science (IBS) since 2015. Among the many recognitions and awards that he received include the Kavli Fellowship by the Kavli Foundation and the National Academy of Science in the US in 2019 and the 2018 Friedrich Wilhelm Bessel Award by the Alexander von Humboldt Foundation in Germany.
2021.03.11 View 10081