Department+of+Biological+Science
-
 KAIST research team develops clathrin assembly for targeted protein delivery to cancer cells
In order to effectively treat cancer without additional side effects, we need a way to deliver drugs specifically to tumor cells. Protein assemblies have been widely used for drug delivery in the field of cancer treatment, but to use them for drug delivery they must first be functionalized, meaning they must be bound to the protein that recognizes the target tumor cell and deliver a drug that kills it. However, the functionalization process of protein assemblies is very complex, inefficient, and limited to small-sized chemical drugs, which limits their real-life applicability.
On March 14, a KAIST research team led by Professor Hak-Sung Kim from the KAIST Department of Biological Sciences reported the development of a clathrin assembly that can specifically deliver drugs to cancer cells.
Clathrin assemblies transport materials efficiently through endocytosis in living organisms. They are formed by the self-assembly of triskelion units, which are composed of three heavy chains bonded with three light chains. Inspired by this mechanism, the research team designed a clathrin chain to facilitate the functionalization of tumor cell recognition proteins and toxin proteins in order to deliver drugs specifically to tumor cells. From this, the team created a new type of clathrin assembly.
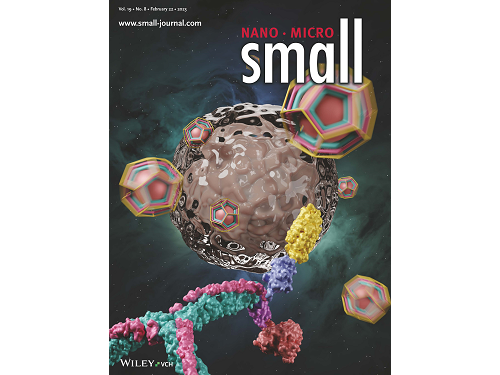
Figure 1. (Upper) Schematic diagram of the development of a new clathrin assembly that simultaneously functionalizes two types of proteins (cancer cell recognition protein and toxin protein) on heavy and light chains of clathrin in a one-pot reaction (bottom, left) Electron microscopy image of clathrin assembly: formation of an assembly with a diameter of about 28 nanometers (bottom, right) Cancer cell killing effect of CLA: CLA functionalized with epidermal growth factor receptor (EGFR) recognition protein and toxin protein kills only the cancer cells that overexpress EGFR.
The newly developed clathrin assembly requires a one-pot reaction, meaning both the toxin and tumor-recognition proteins can be functionalized simultaneously and show high efficiency. As a result, this technique is expected to be used in a wide variety of applications in the fields of biology and medicine including drug delivery, vaccine development, and diagnosing illnesses.
In this research, an epidermal growth factor receptor (EGFR), a common tumor marker, was used as the recognition protein, allowing drug delivery only to tumor cells. The clathrin assemblies that were functionalized to recognize EGFR showed a bonding strength 900-times stronger than it normally would due to the avidity effect. Based on this finding, the research team confirmed that treatment with toxin-functionalized clathrin assembly led to effective cell death for tumor cells, while it showed no such effect on healthy cells.
This research by Dr. Hong-Sik Kim and his colleagues was published in Small volume 19, issue 8 on February 22 under the title, "Construction and Functionalization of a Clathrin Assembly for a Targeted Protein Delivery", and it was selected as the cover paper.
Figure 2. Cover Paper: This study was published in the international journal 'Small' on February 22nd, Volume 19, No. 8, and was selected as the cover paper.
First author Dr. Hong-Sik Kim said, “Clathrin is difficult to functionalize, and since it is extracted from mammals, realistic applications have been limited.” He added, “But the new clathrin assembly we designed for this research can be functionalized with two different types of proteins through a single-step reaction, and can be produced from E. coli, meaning it can become an applicable protein assembly technology for a wide range of biomedical fields.”
This research was funded by the Global Ph.D. Fellowship and the Mid-career Researcher Grant of the National Research Foundation.
2023.03.22 View 8132
KAIST research team develops clathrin assembly for targeted protein delivery to cancer cells
In order to effectively treat cancer without additional side effects, we need a way to deliver drugs specifically to tumor cells. Protein assemblies have been widely used for drug delivery in the field of cancer treatment, but to use them for drug delivery they must first be functionalized, meaning they must be bound to the protein that recognizes the target tumor cell and deliver a drug that kills it. However, the functionalization process of protein assemblies is very complex, inefficient, and limited to small-sized chemical drugs, which limits their real-life applicability.
On March 14, a KAIST research team led by Professor Hak-Sung Kim from the KAIST Department of Biological Sciences reported the development of a clathrin assembly that can specifically deliver drugs to cancer cells.
Clathrin assemblies transport materials efficiently through endocytosis in living organisms. They are formed by the self-assembly of triskelion units, which are composed of three heavy chains bonded with three light chains. Inspired by this mechanism, the research team designed a clathrin chain to facilitate the functionalization of tumor cell recognition proteins and toxin proteins in order to deliver drugs specifically to tumor cells. From this, the team created a new type of clathrin assembly.
Figure 1. (Upper) Schematic diagram of the development of a new clathrin assembly that simultaneously functionalizes two types of proteins (cancer cell recognition protein and toxin protein) on heavy and light chains of clathrin in a one-pot reaction (bottom, left) Electron microscopy image of clathrin assembly: formation of an assembly with a diameter of about 28 nanometers (bottom, right) Cancer cell killing effect of CLA: CLA functionalized with epidermal growth factor receptor (EGFR) recognition protein and toxin protein kills only the cancer cells that overexpress EGFR.
The newly developed clathrin assembly requires a one-pot reaction, meaning both the toxin and tumor-recognition proteins can be functionalized simultaneously and show high efficiency. As a result, this technique is expected to be used in a wide variety of applications in the fields of biology and medicine including drug delivery, vaccine development, and diagnosing illnesses.
In this research, an epidermal growth factor receptor (EGFR), a common tumor marker, was used as the recognition protein, allowing drug delivery only to tumor cells. The clathrin assemblies that were functionalized to recognize EGFR showed a bonding strength 900-times stronger than it normally would due to the avidity effect. Based on this finding, the research team confirmed that treatment with toxin-functionalized clathrin assembly led to effective cell death for tumor cells, while it showed no such effect on healthy cells.
This research by Dr. Hong-Sik Kim and his colleagues was published in Small volume 19, issue 8 on February 22 under the title, "Construction and Functionalization of a Clathrin Assembly for a Targeted Protein Delivery", and it was selected as the cover paper.
Figure 2. Cover Paper: This study was published in the international journal 'Small' on February 22nd, Volume 19, No. 8, and was selected as the cover paper.
First author Dr. Hong-Sik Kim said, “Clathrin is difficult to functionalize, and since it is extracted from mammals, realistic applications have been limited.” He added, “But the new clathrin assembly we designed for this research can be functionalized with two different types of proteins through a single-step reaction, and can be produced from E. coli, meaning it can become an applicable protein assembly technology for a wide range of biomedical fields.”
This research was funded by the Global Ph.D. Fellowship and the Mid-career Researcher Grant of the National Research Foundation.
2023.03.22 View 8132 -
 Establishing a novel strategy to tackle Huntington’s disease
A platform to take on the Huntington’s disease via an innovative approach established by KAIST’s researchers through international collaboration with scientists in the Netherlands, France, and Sweden.
Through an international joint research effort involving ProQR Therapeutics of the Netherlands, Université Grenoble Alpes of France, and KTH Royal Institute of Technology of Sweden, Professor Ji-Soon Song's research team in the Department of Biological Sciences and KAIST Institute for BioCentury of KAIST, established a noble strategy to treat Huntington's disease. The new works showed that the protein converted from disease form to its disease-free form maintains its original function, providing new roadblocks to approach Huntington’s disease.
This research, titled, “A pathogenic-proteolysis resistant huntingtin isoform induced by an antisense oligonucleotide maintains huntingtin function”, co-authored by Hyeongju Kim, was published in the online edition of 'Journal of Clinical Investigation Insight' on August 9, 2022.
Huntington's disease is a dominantly inherited neurodegenerative disease and is caused by a mutation in a protein called ‘huntingtin’, which adds a distinctive feature of an expanded stretch of glutamine amino acids called polyglutamine to the protein. It is estimated that one in every 10,000 have Huntington's disease in United States. The patients would suffer a decade of regression before death, and, thus far, there is no known cure for the disease.
The cleavage near the stretched polyglutamine in mutated huntingtin is known to be the cause of the Huntington’s disease. However, as huntingtin protein is required for the development and normal function of the brain, it is critical to specifically eliminate the disease-causing protein while maintaining the ones that are still normally functioning.
The research team showed that huntingtin delta 12, the converted form of huntingtin that is resistant to developing cleavages at the ends of the protein, the known cause of the Huntington’s disease (HD), alleviated the disease’s symptoms while maintaining the functions of normal huntingtin.
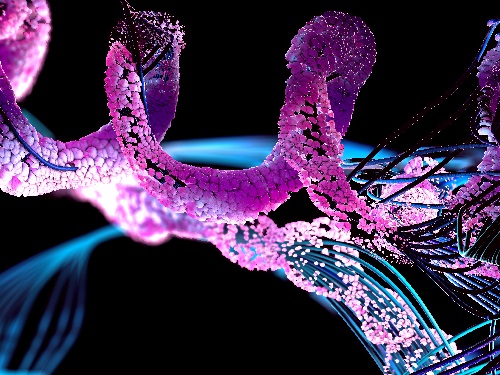
Figure. Huntington's disease resistance huntingtin protein induced by antisense oligonucleotide (AON) is resistant to Caspase-6 cleavage, therefore, does not cause Huntington’s disease while maintaining normal functions of huntingtin.
The research was welcomed as it is sure to fuel innovate strategies to tackle Huntington’s disease without altering the essential function of huntingtin.
This work was supported by a Global Research Lab grant from the National Research Foundation of Korea (NRF) and by a EUREKA Eurostars 2 grant from European Union Horizon 2020.
2022.09.02 View 8682
Establishing a novel strategy to tackle Huntington’s disease
A platform to take on the Huntington’s disease via an innovative approach established by KAIST’s researchers through international collaboration with scientists in the Netherlands, France, and Sweden.
Through an international joint research effort involving ProQR Therapeutics of the Netherlands, Université Grenoble Alpes of France, and KTH Royal Institute of Technology of Sweden, Professor Ji-Soon Song's research team in the Department of Biological Sciences and KAIST Institute for BioCentury of KAIST, established a noble strategy to treat Huntington's disease. The new works showed that the protein converted from disease form to its disease-free form maintains its original function, providing new roadblocks to approach Huntington’s disease.
This research, titled, “A pathogenic-proteolysis resistant huntingtin isoform induced by an antisense oligonucleotide maintains huntingtin function”, co-authored by Hyeongju Kim, was published in the online edition of 'Journal of Clinical Investigation Insight' on August 9, 2022.
Huntington's disease is a dominantly inherited neurodegenerative disease and is caused by a mutation in a protein called ‘huntingtin’, which adds a distinctive feature of an expanded stretch of glutamine amino acids called polyglutamine to the protein. It is estimated that one in every 10,000 have Huntington's disease in United States. The patients would suffer a decade of regression before death, and, thus far, there is no known cure for the disease.
The cleavage near the stretched polyglutamine in mutated huntingtin is known to be the cause of the Huntington’s disease. However, as huntingtin protein is required for the development and normal function of the brain, it is critical to specifically eliminate the disease-causing protein while maintaining the ones that are still normally functioning.
The research team showed that huntingtin delta 12, the converted form of huntingtin that is resistant to developing cleavages at the ends of the protein, the known cause of the Huntington’s disease (HD), alleviated the disease’s symptoms while maintaining the functions of normal huntingtin.
Figure. Huntington's disease resistance huntingtin protein induced by antisense oligonucleotide (AON) is resistant to Caspase-6 cleavage, therefore, does not cause Huntington’s disease while maintaining normal functions of huntingtin.
The research was welcomed as it is sure to fuel innovate strategies to tackle Huntington’s disease without altering the essential function of huntingtin.
This work was supported by a Global Research Lab grant from the National Research Foundation of Korea (NRF) and by a EUREKA Eurostars 2 grant from European Union Horizon 2020.
2022.09.02 View 8682 -
 A New Therapeutic Drug for Alzheimer’s Disease without Inflammatory Side Effects
Although Aduhelm, a monoclonal antibody targeting amyloid beta (Aβ), recently became the first US FDA approved drug for Alzheimer’s disease (AD) based on its ability to decrease Aβ plaque burden in AD patients, its effect on cognitive improvement is still controversial. Moreover, about 40% of the patients treated with this antibody experienced serious side effects including cerebral edemas (ARIA-E) and hemorrhages (ARIA-H) that are likely related to inflammatory responses in the brain when the Aβ antibody binds Fc receptors (FCR) of immune cells such as microglia and macrophages.
These inflammatory side effects can cause neuronal cell death and synapse elimination by activated microglia, and even have the potential to exacerbate cognitive impairment in AD patients. Thus, current Aβ antibody-based immunotherapy holds the inherent risk of doing more harm than good due to their inflammatory side effects.
To overcome these problems, a team of researchers at KAIST in South Korea has developed a novel fusion protein drug, αAβ-Gas6, which efficiently eliminates Aβ via an entirely different mechanism than Aβ antibody-based immunotherapy. In a mouse model of AD, αAβ-Gas6 not only removed Aβ with higher potency, but also circumvented the neurotoxic inflammatory side effects associated with conventional antibody treatments.
Their findings were published on August 4 in Nature Medicine.
Schematic of a chimeric Gas6 fusion protein. A single chain variable fragment (scFv) of
an Amyloid β (Aβ)-targeting monoclonal antibody is fused with a truncated receptor binding
domain of Gas6, a bridging molecule for the clearance of dead cells via TAM (TYRO3, AXL,
and MERTK) receptors, which are expressed by microglia and astrocytes.
“FcR activation by Aβ targeting antibodies induces microglia-mediated Aβ phagocytosis, but it also produces inflammatory signals, inevitably damaging brain tissues,” said paper authors Chan Hyuk Kim and Won-Suk Chung, associate professors in the Department of Biological Sciences at KAIST.
“Therefore, we utilized efferocytosis, a cellular process by which dead cells are removed by phagocytes as an alternative pathway for the clearance of Aβ in the brain,” Prof. Kim and Chung said. “Efferocytosis is accompanied by anti-inflammatory responses to maintain tissue homeostasis. To exploit this process, we engineered Gas6, a soluble adaptor protein that mediates efferocytosis via TAM phagocytic receptors in such a way that its target specificity was redirected from dead cells to Aβ plaques.”
The professors and their team demonstrated that the resulting αAβ-Gas6 induced Aβ engulfment by activating not only microglial but also astrocytic phagocytosis since TAM phagocytic receptors are highly expressed by these two major phagocytes in the brain. Importantly, αAβ-Gas6 promoted the robust uptake of Aβ without showing any signs of inflammation and neurotoxicity, which contrasts sharply with the treatment using an Aβ monoclonal antibody. Moreover, they showed that αAβ-Gas6 substantially reduced excessive synapse elimination by microglia, consequently leading to better behavioral rescues in AD model mice.
“By using a mouse model of cerebral amyloid angiopathy (CAA), a cerebrovascular disorder caused by the deposition of Aβ within the walls of the brain’s blood vessels, we also showed that the intrathecal administration of Gas6 fusion protein significantly eliminated cerebrovascular amyloids, along with a reduction of microhemorrhages. These data demonstrate that aAb-Gas6 is a potent therapeutic agent in eliminating Aβ without exacerbating CAA-related microhemorrhages.”
The resulting αAβ-Gas6 clears Aβ oligomers and fibrils without causing neurotoxicity (a-b, neurons: red, and fragmented axons: yellow) and proinflammatory responses (c, TNF release), which are conversely exacerbated by the treatment of an Aβ-targeting monoclonal antibody (Aducanumab).
Professors Kim and Chung noted, “We believe our approach can be a breakthrough in treating AD without causing inflammatory side effects and synapse loss. Our approach holds promise as a novel therapeutic platform that is applicable to more than AD. By modifying the target-specificity of the fusion protein, the Gas6-fusion protein can be applied to various neurological disorders as well as autoimmune diseases affected by toxic molecules that should be removed without causing inflammatory responses.”
The number and total area of Aβ plaques (Thioflavin-T, green) were significantly reduced in αAβ-Gas6-treated AD mouse brains compared to Aducanumab-treated ones (a, b). The cognitive functions of AD model mice were significantly rescued by αAβ-Gas6 treatment, whereas Aducanumab-treated AD mice showed partial rescue in these cognitive tests (c-e).
Professors Kim and Chung founded “Illimis Therapeutics” based on this strategy of designing chimeric Gas6 fusion proteins that would remove toxic aggregates from the nervous system. Through this company, they are planning to further develop various Gas6-fusion proteins not only for Ab but also for Tau to treat AD symptoms.
This work was supported by KAIST and the Korea Health Technology R&D Project that was administered by the Korea Health Industry Development Institute (KHIDI) and the Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare (MOHW) and the Ministry of Science and ICT (MSIT), and KAIST.
Other contributors include Hyuncheol Jung and Se Young Lee, Sungjoon Lim, Hyeong Ryeol Choi, Yeseong Choi, Minjin Kim, Segi Kim, the Department of Biological Sciences, and the Korea Advanced Institute of Science and Technology (KAIST).
To receive more up-to-date information on this new development, follow “Illimis Therapeutics” on twitter @Illimistx.
2022.08.05 View 14498
A New Therapeutic Drug for Alzheimer’s Disease without Inflammatory Side Effects
Although Aduhelm, a monoclonal antibody targeting amyloid beta (Aβ), recently became the first US FDA approved drug for Alzheimer’s disease (AD) based on its ability to decrease Aβ plaque burden in AD patients, its effect on cognitive improvement is still controversial. Moreover, about 40% of the patients treated with this antibody experienced serious side effects including cerebral edemas (ARIA-E) and hemorrhages (ARIA-H) that are likely related to inflammatory responses in the brain when the Aβ antibody binds Fc receptors (FCR) of immune cells such as microglia and macrophages.
These inflammatory side effects can cause neuronal cell death and synapse elimination by activated microglia, and even have the potential to exacerbate cognitive impairment in AD patients. Thus, current Aβ antibody-based immunotherapy holds the inherent risk of doing more harm than good due to their inflammatory side effects.
To overcome these problems, a team of researchers at KAIST in South Korea has developed a novel fusion protein drug, αAβ-Gas6, which efficiently eliminates Aβ via an entirely different mechanism than Aβ antibody-based immunotherapy. In a mouse model of AD, αAβ-Gas6 not only removed Aβ with higher potency, but also circumvented the neurotoxic inflammatory side effects associated with conventional antibody treatments.
Their findings were published on August 4 in Nature Medicine.
Schematic of a chimeric Gas6 fusion protein. A single chain variable fragment (scFv) of
an Amyloid β (Aβ)-targeting monoclonal antibody is fused with a truncated receptor binding
domain of Gas6, a bridging molecule for the clearance of dead cells via TAM (TYRO3, AXL,
and MERTK) receptors, which are expressed by microglia and astrocytes.
“FcR activation by Aβ targeting antibodies induces microglia-mediated Aβ phagocytosis, but it also produces inflammatory signals, inevitably damaging brain tissues,” said paper authors Chan Hyuk Kim and Won-Suk Chung, associate professors in the Department of Biological Sciences at KAIST.
“Therefore, we utilized efferocytosis, a cellular process by which dead cells are removed by phagocytes as an alternative pathway for the clearance of Aβ in the brain,” Prof. Kim and Chung said. “Efferocytosis is accompanied by anti-inflammatory responses to maintain tissue homeostasis. To exploit this process, we engineered Gas6, a soluble adaptor protein that mediates efferocytosis via TAM phagocytic receptors in such a way that its target specificity was redirected from dead cells to Aβ plaques.”
The professors and their team demonstrated that the resulting αAβ-Gas6 induced Aβ engulfment by activating not only microglial but also astrocytic phagocytosis since TAM phagocytic receptors are highly expressed by these two major phagocytes in the brain. Importantly, αAβ-Gas6 promoted the robust uptake of Aβ without showing any signs of inflammation and neurotoxicity, which contrasts sharply with the treatment using an Aβ monoclonal antibody. Moreover, they showed that αAβ-Gas6 substantially reduced excessive synapse elimination by microglia, consequently leading to better behavioral rescues in AD model mice.
“By using a mouse model of cerebral amyloid angiopathy (CAA), a cerebrovascular disorder caused by the deposition of Aβ within the walls of the brain’s blood vessels, we also showed that the intrathecal administration of Gas6 fusion protein significantly eliminated cerebrovascular amyloids, along with a reduction of microhemorrhages. These data demonstrate that aAb-Gas6 is a potent therapeutic agent in eliminating Aβ without exacerbating CAA-related microhemorrhages.”
The resulting αAβ-Gas6 clears Aβ oligomers and fibrils without causing neurotoxicity (a-b, neurons: red, and fragmented axons: yellow) and proinflammatory responses (c, TNF release), which are conversely exacerbated by the treatment of an Aβ-targeting monoclonal antibody (Aducanumab).
Professors Kim and Chung noted, “We believe our approach can be a breakthrough in treating AD without causing inflammatory side effects and synapse loss. Our approach holds promise as a novel therapeutic platform that is applicable to more than AD. By modifying the target-specificity of the fusion protein, the Gas6-fusion protein can be applied to various neurological disorders as well as autoimmune diseases affected by toxic molecules that should be removed without causing inflammatory responses.”
The number and total area of Aβ plaques (Thioflavin-T, green) were significantly reduced in αAβ-Gas6-treated AD mouse brains compared to Aducanumab-treated ones (a, b). The cognitive functions of AD model mice were significantly rescued by αAβ-Gas6 treatment, whereas Aducanumab-treated AD mice showed partial rescue in these cognitive tests (c-e).
Professors Kim and Chung founded “Illimis Therapeutics” based on this strategy of designing chimeric Gas6 fusion proteins that would remove toxic aggregates from the nervous system. Through this company, they are planning to further develop various Gas6-fusion proteins not only for Ab but also for Tau to treat AD symptoms.
This work was supported by KAIST and the Korea Health Technology R&D Project that was administered by the Korea Health Industry Development Institute (KHIDI) and the Korea Dementia Research Center (KDRC) funded by the Ministry of Health & Welfare (MOHW) and the Ministry of Science and ICT (MSIT), and KAIST.
Other contributors include Hyuncheol Jung and Se Young Lee, Sungjoon Lim, Hyeong Ryeol Choi, Yeseong Choi, Minjin Kim, Segi Kim, the Department of Biological Sciences, and the Korea Advanced Institute of Science and Technology (KAIST).
To receive more up-to-date information on this new development, follow “Illimis Therapeutics” on twitter @Illimistx.
2022.08.05 View 14498 -
 A Genetic Change for Achieving a Long and Healthy Life
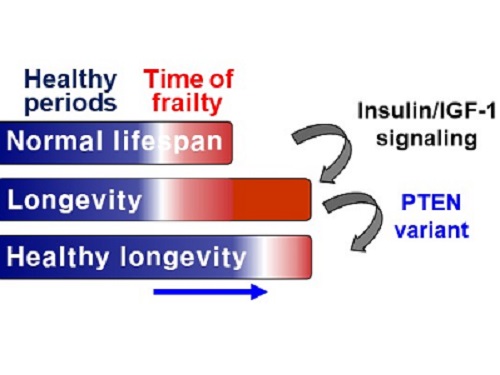
Researchers identified a single amino acid change in the tumor suppressor protein in PTEN that extends healthy periods while maintaining longevity
Living a long, healthy life is everyone’s wish, but it is not an easy one to achieve. Many aging studies are developing strategies to increase health spans, the period of life spent with good health, without chronic diseases and disabilities. Researchers at KAIST presented new insights for improving the health span by just regulating the activity of a protein.
A research group under Professor Seung-Jae V. Lee from the Department of Biological Sciences identified a single amino acid change in the tumor suppressor protein phosphatase and tensin homolog (PTEN) that dramatically extends healthy periods while maintaining longevity. This study highlights the importance of the well-conserved tumor suppressor protein PTEN in health span regulation, which can be targeted to develop therapies for promoting healthy longevity in humans. The research was published in Nature Communications on September 24, 2021.
Insulin and insulin-like growth factor-1 (IGF-1) signaling (IIS) is one of the evolutionarily conserved aging-modulatory pathways present in life forms ranging from tiny roundworms to humans. The proper reduction of IIS leads to longevity in animals but often causes defects in multiple health parameters including impaired motility, reproduction, and growth.
The research team found that a specific amino acid change in the PTEN protein improves health status while retaining the longevity conferred by reduced IIS. They used the roundworm C. elegans, an excellent model animal that has been widely used for aging research, mainly because of its very short normal lifespan of about two to three weeks. The PTEN protein is a phosphatase that removes phosphate from lipids as well as proteins. Interestingly, the newly identified amino acid change delicately recalibrated the IIS by partially maintaining protein phosphatase activity while reducing lipid phosphatase activity.
As a result, the amino acid change in the PTEN protein maintained the activity of the longevity-promoting transcription factor Forkhead Box O (FOXO) protein while restricting the detrimental upregulation of another transcription factor, NRF2, leading to long and healthy life in animals with reduced IIS.
Professor Lee said, “Our study raises the exciting possibility of simultaneously promoting longevity and health in humans by slightly tweaking the activity of one protein, PTEN.”
This work was supported by the MInistry of Science and ICT through the National Research Foundation of Korea.
-Publication:Hae-Eun H. Park, Wooseon Hwang, Seokjin Ham, Eunah Kim, Ozlem Altintas, Sangsoon Park, Heehwa G. Son, Yujin Lee, Dongyeop Lee, Won Do Heo, and Seung-Jae V. Lee. 2021. “A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling,” Nature Communications, 12(1), 5631. (https://doi.org/10.1038/s41467-021-25920-w)
-ProfileProfessor Seung-Jae V. LeeMolecular Genetics of Aging LaboratoryDepartment of Biological Sciences KAIST
2021.11.19 View 10223
A Genetic Change for Achieving a Long and Healthy Life
Researchers identified a single amino acid change in the tumor suppressor protein in PTEN that extends healthy periods while maintaining longevity
Living a long, healthy life is everyone’s wish, but it is not an easy one to achieve. Many aging studies are developing strategies to increase health spans, the period of life spent with good health, without chronic diseases and disabilities. Researchers at KAIST presented new insights for improving the health span by just regulating the activity of a protein.
A research group under Professor Seung-Jae V. Lee from the Department of Biological Sciences identified a single amino acid change in the tumor suppressor protein phosphatase and tensin homolog (PTEN) that dramatically extends healthy periods while maintaining longevity. This study highlights the importance of the well-conserved tumor suppressor protein PTEN in health span regulation, which can be targeted to develop therapies for promoting healthy longevity in humans. The research was published in Nature Communications on September 24, 2021.
Insulin and insulin-like growth factor-1 (IGF-1) signaling (IIS) is one of the evolutionarily conserved aging-modulatory pathways present in life forms ranging from tiny roundworms to humans. The proper reduction of IIS leads to longevity in animals but often causes defects in multiple health parameters including impaired motility, reproduction, and growth.
The research team found that a specific amino acid change in the PTEN protein improves health status while retaining the longevity conferred by reduced IIS. They used the roundworm C. elegans, an excellent model animal that has been widely used for aging research, mainly because of its very short normal lifespan of about two to three weeks. The PTEN protein is a phosphatase that removes phosphate from lipids as well as proteins. Interestingly, the newly identified amino acid change delicately recalibrated the IIS by partially maintaining protein phosphatase activity while reducing lipid phosphatase activity.
As a result, the amino acid change in the PTEN protein maintained the activity of the longevity-promoting transcription factor Forkhead Box O (FOXO) protein while restricting the detrimental upregulation of another transcription factor, NRF2, leading to long and healthy life in animals with reduced IIS.
Professor Lee said, “Our study raises the exciting possibility of simultaneously promoting longevity and health in humans by slightly tweaking the activity of one protein, PTEN.”
This work was supported by the MInistry of Science and ICT through the National Research Foundation of Korea.
-Publication:Hae-Eun H. Park, Wooseon Hwang, Seokjin Ham, Eunah Kim, Ozlem Altintas, Sangsoon Park, Heehwa G. Son, Yujin Lee, Dongyeop Lee, Won Do Heo, and Seung-Jae V. Lee. 2021. “A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling,” Nature Communications, 12(1), 5631. (https://doi.org/10.1038/s41467-021-25920-w)
-ProfileProfessor Seung-Jae V. LeeMolecular Genetics of Aging LaboratoryDepartment of Biological Sciences KAIST
2021.11.19 View 10223 -
 Two Researchers Designated as SUHF Fellows
Professor Taeyun Ku from the Graduate School of Medical Science and Engineering and Professor Hanseul Yang from the Department of Biological Sciences were nominated as 2021 fellows of the Suh Kyungbae Foundation (SUHF).
SUHF selected three young promising scientists from 53 researchers who are less than five years into their careers. A panel of judges comprised of scholars from home and abroad made the final selection based on the candidates’ innovativeness and power to influence. Professor You-Bong Hyun from Seoul National University also won the fellowship.
Professor Ku’s main topic is opto-connectomics. He will study ways to visualize the complex brain network using innovative technology that transforms neurons into optical elements.
Professor Yang will research the possibility of helping patients recover from skin diseases or injuries without scars by studying spiny mouse genes.
SUHF was established by Amorepacific Group Chairman Suh Kyungbae in 2016 with 300 billion KRW of his private funds. Under the vision of ‘contributing to humanity by supporting innovative discoveries of bioscience researchers,’ the foundation supports promising Korean scientists who pioneer new fields of research in biological sciences.
From 2017 to this year, SUHF has selected 20 promising scientists in the field of biological sciences. Selected scientists are provided with up to KRW 500 million each year for five years. The foundation has provided a total of KRW 48.5 billion in research funds to date.
2021.09.15 View 10589
Two Researchers Designated as SUHF Fellows
Professor Taeyun Ku from the Graduate School of Medical Science and Engineering and Professor Hanseul Yang from the Department of Biological Sciences were nominated as 2021 fellows of the Suh Kyungbae Foundation (SUHF).
SUHF selected three young promising scientists from 53 researchers who are less than five years into their careers. A panel of judges comprised of scholars from home and abroad made the final selection based on the candidates’ innovativeness and power to influence. Professor You-Bong Hyun from Seoul National University also won the fellowship.
Professor Ku’s main topic is opto-connectomics. He will study ways to visualize the complex brain network using innovative technology that transforms neurons into optical elements.
Professor Yang will research the possibility of helping patients recover from skin diseases or injuries without scars by studying spiny mouse genes.
SUHF was established by Amorepacific Group Chairman Suh Kyungbae in 2016 with 300 billion KRW of his private funds. Under the vision of ‘contributing to humanity by supporting innovative discoveries of bioscience researchers,’ the foundation supports promising Korean scientists who pioneer new fields of research in biological sciences.
From 2017 to this year, SUHF has selected 20 promising scientists in the field of biological sciences. Selected scientists are provided with up to KRW 500 million each year for five years. The foundation has provided a total of KRW 48.5 billion in research funds to date.
2021.09.15 View 10589 -
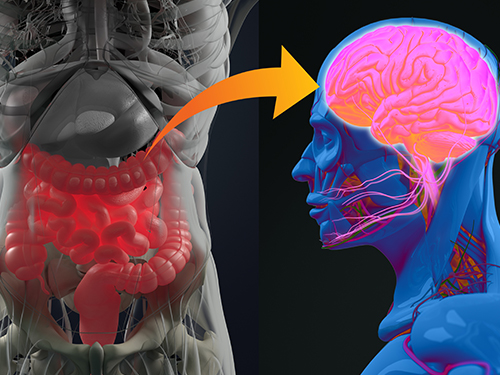 Gut Hormone Triggers Craving for More Proteins
- Revelations from a fly study could improve our understanding of protein malnutrition in humans. -
A new study led by KAIST researchers using fruit flies reveals how protein deficiency in the diet triggers cross talk between the gut and brain to induce a desire to eat foods rich in proteins or essential amino acids. This finding reported in the May 5 issue of Nature can lead to a better understanding of malnutrition in humans.
“All organisms require a balanced intake of carbohydrates, proteins, and fats for their well being,” explained KAIST neuroscientist and professor Greg Seong-Bae Suh. “Taking in sufficient calories alone won’t do the job, as it can still lead to severe forms of malnutrition including kwashiorkor, if the diet does not include enough proteins,” he added.
Scientists already knew that inadequate protein intake in organisms causes a preferential choice of foods rich in proteins or essential amino acids but they didn’t know precisely how this happens. A group of researchers led by Professor Suh at KAIST and Professor Won-Jae Lee at Seoul National University (SNU) investigated this process in flies by examining the effects of different genes on food preference following protein deprivation.
The group found that protein deprivation triggered the release of a gut hormone called neuropeptide CNMamide (CNMa) from a specific population of enterocytes - the intestine lining cells. Until now, scientists have known that enterocytes release digestive enzymes into the intestine to help digest and absorb nutrients in the gut. “Our study showed that enterocytes have a more complex role than we previously thought,” said Professor Suh.
Enterocytes respond to protein deprivation by releasing CNMa that conveys the nutrient status in the gut to the CNMa receptors on nerve cells in the brain. This then triggers a desire to eat foods containing essential amino acids.
Interestingly, the KAIST-SNU team also found that the microbiome - Acetobacter bacteria - present in the gut produces amino acids that can compensate for mild protein deficit in the diet. This basal level of amino acids provided by the microbiome modifies CNMa release and tempers the flies’ compensatory desire to ingest more proteins.
The research team was able to further clarify two signalling pathways that respond to protein loss from the diet and ultimately produce the CNMa hormone in these specific enterocytes.
The team said that further studies are still needed to understand how CNMa communicates with its receptors in the brain, and whether this happens by directly activating nerve cells that link the gut to the brain or by indirectly activating the brain through blood circulation. Their research could provide insights into the understanding of similar process in mammals including humans.
“We chose to investigate a simple organism, the fly, which would make it easier for us to identify and characterize key nutrient sensors. Because all organisms have cravings for needed nutrients, the nutrient sensors and their pathways we identified in flies would also be relevant to those in mammals. We believe that this research will greatly advance our understanding of the causes of metabolic disease and eating-related disorders,” Professor Suh added.
This work was supported by the Samsung Science and Technology Foundation (SSTF) and the National Research Foundation (NRF) of Korea.
Publication:
Kim, B., et al. (2021) Response of the Drosophila microbiome– gut–brain axis to amino acid deficit. Nature. Available online at https://doi.org/10.1038/s41586-021-03522-2
Profile:
Greg Seong-Bae Suh, Ph.D
Associate Professor
seongbaesuh@kaist.ac.krLab of Neural Interoception
https://www.suhlab-neuralinteroception.kaist.ac.kr/Department of Biological Sciences
https://bio.kaist.ac.kr/
Korea Advanced Institute of Science and Technology (KAIST)
https:/kaist.ac.kr/en/
Daejeon 34141, Korea
(END)
2021.05.17 View 9178
Gut Hormone Triggers Craving for More Proteins
- Revelations from a fly study could improve our understanding of protein malnutrition in humans. -
A new study led by KAIST researchers using fruit flies reveals how protein deficiency in the diet triggers cross talk between the gut and brain to induce a desire to eat foods rich in proteins or essential amino acids. This finding reported in the May 5 issue of Nature can lead to a better understanding of malnutrition in humans.
“All organisms require a balanced intake of carbohydrates, proteins, and fats for their well being,” explained KAIST neuroscientist and professor Greg Seong-Bae Suh. “Taking in sufficient calories alone won’t do the job, as it can still lead to severe forms of malnutrition including kwashiorkor, if the diet does not include enough proteins,” he added.
Scientists already knew that inadequate protein intake in organisms causes a preferential choice of foods rich in proteins or essential amino acids but they didn’t know precisely how this happens. A group of researchers led by Professor Suh at KAIST and Professor Won-Jae Lee at Seoul National University (SNU) investigated this process in flies by examining the effects of different genes on food preference following protein deprivation.
The group found that protein deprivation triggered the release of a gut hormone called neuropeptide CNMamide (CNMa) from a specific population of enterocytes - the intestine lining cells. Until now, scientists have known that enterocytes release digestive enzymes into the intestine to help digest and absorb nutrients in the gut. “Our study showed that enterocytes have a more complex role than we previously thought,” said Professor Suh.
Enterocytes respond to protein deprivation by releasing CNMa that conveys the nutrient status in the gut to the CNMa receptors on nerve cells in the brain. This then triggers a desire to eat foods containing essential amino acids.
Interestingly, the KAIST-SNU team also found that the microbiome - Acetobacter bacteria - present in the gut produces amino acids that can compensate for mild protein deficit in the diet. This basal level of amino acids provided by the microbiome modifies CNMa release and tempers the flies’ compensatory desire to ingest more proteins.
The research team was able to further clarify two signalling pathways that respond to protein loss from the diet and ultimately produce the CNMa hormone in these specific enterocytes.
The team said that further studies are still needed to understand how CNMa communicates with its receptors in the brain, and whether this happens by directly activating nerve cells that link the gut to the brain or by indirectly activating the brain through blood circulation. Their research could provide insights into the understanding of similar process in mammals including humans.
“We chose to investigate a simple organism, the fly, which would make it easier for us to identify and characterize key nutrient sensors. Because all organisms have cravings for needed nutrients, the nutrient sensors and their pathways we identified in flies would also be relevant to those in mammals. We believe that this research will greatly advance our understanding of the causes of metabolic disease and eating-related disorders,” Professor Suh added.
This work was supported by the Samsung Science and Technology Foundation (SSTF) and the National Research Foundation (NRF) of Korea.
Publication:
Kim, B., et al. (2021) Response of the Drosophila microbiome– gut–brain axis to amino acid deficit. Nature. Available online at https://doi.org/10.1038/s41586-021-03522-2
Profile:
Greg Seong-Bae Suh, Ph.D
Associate Professor
seongbaesuh@kaist.ac.krLab of Neural Interoception
https://www.suhlab-neuralinteroception.kaist.ac.kr/Department of Biological Sciences
https://bio.kaist.ac.kr/
Korea Advanced Institute of Science and Technology (KAIST)
https:/kaist.ac.kr/en/
Daejeon 34141, Korea
(END)
2021.05.17 View 9178 -
 Astrocytes Eat Connections to Maintain Plasticity in Adult Brains
Developing brains constantly sprout new neuronal connections called synapses as they learn and remember. Important connections — the ones that are repeatedly introduced, such as how to avoid danger — are nurtured and reinforced, while connections deemed unnecessary are pruned away. Adult brains undergo similar pruning, but it was unclear how or why synapses in the adult brain get eliminated.
Now, a team of KAIST researchers has found the mechanism underlying plasticity and, potentially, neurological disorders in adult brains. They published their findings on December 23 in Nature.
“Our findings have profound implications for our understanding of how neural circuits change during learning and memory, as well as in diseases,” said paper author Won-Suk Chung, an assistant professor in the Department of Biological Sciences at KAIST. “Changes in synapse number have strong association with the prevalence of various neurological disorders, such as autism spectrum disorder, schizophrenia, frontotemporal dementia, and several forms of seizures.”
Gray matter in the brain contains microglia and astrocytes, two complementary cells that, among other things, support neurons and synapses. Microglial are a frontline immunity defense, responsible for eating pathogens and dead cells, and astrocytes are star-shaped cells that help structure the brain and maintain homeostasis by helping to control signaling between neurons. According to Professor Chung, it is generally thought that microglial eat synapses as part of its clean-up effort in a process known as phagocytosis.
“Using novel tools, we show that, for the first time, it is astrocytes and not microglia that constantly eliminate excessive and unnecessary adult excitatory synaptic connections in response to neuronal activity,” Professor Chung said. “Our paper challenges the general consensus in this field that microglia are the primary synapse phagocytes that control synapse numbers in the brain.”
Professor Chung and his team developed a molecular sensor to detect synapse elimination by glial cells and quantified how often and by which type of cell synapses were eliminated. They also deployed it in a mouse model without MEGF10, the gene that allows astrocytes to eliminate synapses. Adult animals with this defective astrocytic phagocytosis had unusually increased excitatory synapse numbers in the hippocampus. Through a collaboration with Dr. Hyungju Park at KBRI, they showed that these increased excitatory synapses are functionally impaired, which cause defective learning and memory formation in MEGF10 deleted animals.
“Through this process, we show that, at least in the adult hippocampal CA1 region, astrocytes are the major player in eliminating synapses, and this astrocytic function is essential for controlling synapse number and plasticity,” Chung said.
Professor Chung noted that researchers are only beginning to understand how synapse elimination affects maturation and homeostasis in the brain. In his group’s preliminary data in other brain regions, it appears that each region has different rates of synaptic elimination by astrocytes. They suspect a variety of internal and external factors are influencing how astrocytes modulate each regional circuit, and plan to elucidate these variables.
“Our long-term goal is understanding how astrocyte-mediated synapse turnover affects the initiation and progression of various neurological disorders,” Professor Chung said. “It is intriguing to postulate that modulating astrocytic phagocytosis to restore synaptic connectivity may be a novel strategy in treating various brain disorders.”
This work was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea, and the Korea Brain Research Institute basic research program.
Other contributors include Joon-Hyuk Lee and Se Young Lee, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST); Ji-young Kim, Hyoeun Lee and Hyungju Park; Research Group for Neurovascular Unit, Korea Brain Research Institute (KBRI); Seulgi Noh, and Ji Young Mun, Research Group for Neural Circuit, KBRI. Kim, Noh and Park are also affiliated with the Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST).
-Profile
Professor Won-Suk Chung
Department of Biological Sciences
Gliabiology Lab (https://www.kaistglia.org/)
KAIST
-Publication
"Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis"
https://doi.org/10.1038/s41586-020-03060-3
2020.12.24 View 13429
Astrocytes Eat Connections to Maintain Plasticity in Adult Brains
Developing brains constantly sprout new neuronal connections called synapses as they learn and remember. Important connections — the ones that are repeatedly introduced, such as how to avoid danger — are nurtured and reinforced, while connections deemed unnecessary are pruned away. Adult brains undergo similar pruning, but it was unclear how or why synapses in the adult brain get eliminated.
Now, a team of KAIST researchers has found the mechanism underlying plasticity and, potentially, neurological disorders in adult brains. They published their findings on December 23 in Nature.
“Our findings have profound implications for our understanding of how neural circuits change during learning and memory, as well as in diseases,” said paper author Won-Suk Chung, an assistant professor in the Department of Biological Sciences at KAIST. “Changes in synapse number have strong association with the prevalence of various neurological disorders, such as autism spectrum disorder, schizophrenia, frontotemporal dementia, and several forms of seizures.”
Gray matter in the brain contains microglia and astrocytes, two complementary cells that, among other things, support neurons and synapses. Microglial are a frontline immunity defense, responsible for eating pathogens and dead cells, and astrocytes are star-shaped cells that help structure the brain and maintain homeostasis by helping to control signaling between neurons. According to Professor Chung, it is generally thought that microglial eat synapses as part of its clean-up effort in a process known as phagocytosis.
“Using novel tools, we show that, for the first time, it is astrocytes and not microglia that constantly eliminate excessive and unnecessary adult excitatory synaptic connections in response to neuronal activity,” Professor Chung said. “Our paper challenges the general consensus in this field that microglia are the primary synapse phagocytes that control synapse numbers in the brain.”
Professor Chung and his team developed a molecular sensor to detect synapse elimination by glial cells and quantified how often and by which type of cell synapses were eliminated. They also deployed it in a mouse model without MEGF10, the gene that allows astrocytes to eliminate synapses. Adult animals with this defective astrocytic phagocytosis had unusually increased excitatory synapse numbers in the hippocampus. Through a collaboration with Dr. Hyungju Park at KBRI, they showed that these increased excitatory synapses are functionally impaired, which cause defective learning and memory formation in MEGF10 deleted animals.
“Through this process, we show that, at least in the adult hippocampal CA1 region, astrocytes are the major player in eliminating synapses, and this astrocytic function is essential for controlling synapse number and plasticity,” Chung said.
Professor Chung noted that researchers are only beginning to understand how synapse elimination affects maturation and homeostasis in the brain. In his group’s preliminary data in other brain regions, it appears that each region has different rates of synaptic elimination by astrocytes. They suspect a variety of internal and external factors are influencing how astrocytes modulate each regional circuit, and plan to elucidate these variables.
“Our long-term goal is understanding how astrocyte-mediated synapse turnover affects the initiation and progression of various neurological disorders,” Professor Chung said. “It is intriguing to postulate that modulating astrocytic phagocytosis to restore synaptic connectivity may be a novel strategy in treating various brain disorders.”
This work was supported by the Samsung Science & Technology Foundation, the National Research Foundation of Korea, and the Korea Brain Research Institute basic research program.
Other contributors include Joon-Hyuk Lee and Se Young Lee, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST); Ji-young Kim, Hyoeun Lee and Hyungju Park; Research Group for Neurovascular Unit, Korea Brain Research Institute (KBRI); Seulgi Noh, and Ji Young Mun, Research Group for Neural Circuit, KBRI. Kim, Noh and Park are also affiliated with the Department of Brain and Cognitive Sciences, Daegu Gyeongbuk Institute of Science and Technology (DGIST).
-Profile
Professor Won-Suk Chung
Department of Biological Sciences
Gliabiology Lab (https://www.kaistglia.org/)
KAIST
-Publication
"Astrocytes phagocytose adult hippocampal synapses for circuit homeostasis"
https://doi.org/10.1038/s41586-020-03060-3
2020.12.24 View 13429 -
 Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 16040
Professor Won-Ki Cho Selected as the 2020 SUHF Young Investigator
Professor Won-Ki Cho from the Department of Biological Sciences was named one of three recipients of the 2020 Suh Kyung-Bae Science Foundation (SUHF) Young Investigator Award.
The SUHF is a non-profit organization established in 2016 and funded by a personal donation of 300 billion KRW in shares from Chairman and CEO Kyung-Bae Suh of the Amorepacific Group. The primary purpose of the foundation is to serve as a platform to nurture and provide comprehensive long-term support for creative and passionate young Korean scientists committed to pursuing research in the field of life sciences. The SUHF selects three to five scientists through an open recruiting process every year and grants each scientist a maximum of 2.5 billion KRW over a period of up to five years.
Since January this year, the foundation received 67 research proposals from scientists across the nation, especially from those who had less than five years of experience as professors, and selected the three recipients.
Professor Cho proposed research on how to observe the interactions between nuclear structures and constantly-changing chromatin monomers in four dimensions through ultra-high-resolution imaging of single living cells. This proposal was recognized as one that could help us better understand the process of transcription regulation, which remains a long-standing question in biology.
The other awards were given to Professor Soung-hun Roh of Seoul National University and Professor Joo-Hyeon Lee of the University of Cambridge.
With these three new awardees, a total of 17 scientists have been named SUHF Young Investigators to date, and the funding to support these scientists now totals 42.5 billion KRW.
Professor Inkyung Jung and Professor Ki-Jun Yoon from the Department of Biological Sciences, and Professor Young Seok Ju and Professor Jeong Ho Lee from the Graduate School of Medical Science and Engineering are the four previous winners from KAIST in the years 2017 through 2019.
(END)
2020.10.15 View 16040 -
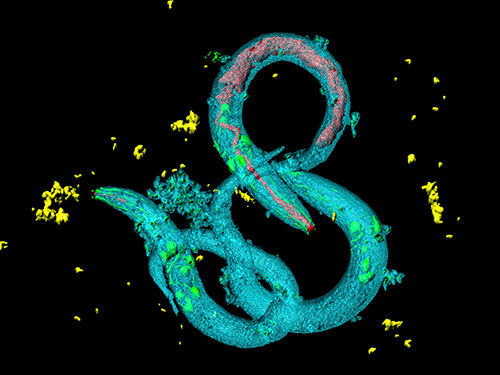 Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13926
Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13926 -
 A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 15322
A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 15322 -
 Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 12593
Scientists Discover the Mechanism of DNA High-Order Structure Formation
(Molecular structures of Abo1 in different energy states (left), Demonstration of an Abo1-assisted histone loading onto DNA by the DNA curtain assay. )
The genetic material of our cells—DNA—exists in a high-order structure called “chromatin”. Chromatin consists of DNA wrapped around histone proteins and efficiently packs DNA into a small volume. Moreover, using a spool and thread analogy, chromatin allows DNA to be locally wound or unwound, thus enabling genes to be enclosed or exposed. The misregulation of chromatin structures results in aberrant gene expression and can ultimately lead to developmental disorders or cancers. Despite the importance of DNA high-order structures, the complexity of the underlying machinery has circumvented molecular dissection.
For the first time, molecular biologists have uncovered how one particular mechanism uses energy to ensure proper histone placement onto DNA to form chromatin. They published their results on Dec. 17 in Nature Communications.
The study focused on proteins called histone chaperones. Histone chaperones are responsible for adding and removing specific histones at specific times during the DNA packaging process. The wrong histone at the wrong time and place could result in the misregulation of gene expression or aberrant DNA replication. Thus, histone chaperones are key players in the assembly and disassembly of chromatin.
“In order to carefully control the assembly and disassembly of chromatin units, histone chaperones act as molecular escorts that prevent histone aggregation and undesired interactions,” said Professor Ji-Joon Song in the Department of Biological Sciences at KAIST. “We set out to understand how a unique histone chaperone uses chemical energy to assemble or disassemble chromatin.”
Song and his team looked to Abo1, the only known histone chaperone that utilizes cellular energy (ATP). While Abo1 is found in yeast, it has an analogous partner in other organisms, including humans, called ATAD2. Both use ATP, which is produced through a cellular process where enzymes break down a molecule’s phosphate bond. ATP energy is typically used to power other cellular processes, but it is a rare partner for histone chaperones.
“This was an interesting problem in the field because all other histone chaperones studied to date do not use ATP,” Song said.
By imaging Abo1 with a single-molecule fluorescence imaging technique known as the DNA curtain assay, the researchers could examine the protein interactions at the single-molecule level. The technique allows scientists to arrange the DNA molecules and proteins on a single layer of a microfluidic chamber and examine the layer with fluorescence microscopy.
The researchers found through real-time observation that Abo1 is ring-shaped and changes its structure to accommodate a specific histone and deposit it on DNA. Moreover, they found that the accommodating structural changes are powered by ADP.
“We discovered a mechanism by which Abo1 accommodates histone substrates, ultimately allowing it to function as a unique energy-dependent histone chaperone,” Song said. “We also found that despite looking like a protein disassembly machine, Abo1 actually loads histone substrates onto DNA to facilitate chromatin assembly.”
The researchers plan to continue exploring how energy-dependent histone chaperones bind and release histones, with the ultimate goal of developing therapeutics that can target cancer-causing misbehavior by Abo1’s analogous human counterpart, ATAD2.
-Profile
Professor Ji-Joon Song
Department of Biological Sciences KI for the BioCentury (https://kis.kaist.ac.kr/index.php?mid=KIB_O) KAIST
2020.01.07 View 12593 -
 A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 19331
A Single, Master Switch for Sugar Levels?
When a fly eats sugar, a single brain cell sends simultaneous messages to stimulate one hormone and inhibit another to control glucose levels in the body. Further research into this control system with remarkable precision could shed light on the neural mechanisms of diabetes and obesity in humans .
A single neuron appears to monitor and control sugar levels in the fly body, according to research published this week in Nature. This new insight into the mechanisms in the fly brain that maintain a balance of two key hormones controlling glucose levels, insulin and glucagon, can provide a framework for understanding diabetes and obesity in humans.
Neurons that sense and respond to glucose were identified more than 50 years ago, but what they do in our body has remained unclear. Researchers at the Korea Advanced Institute of Science and Technology (KAIST) and New York University School of Medicine have now found a single “glucose-sensing neuron” that appears to be the master controller in Drosophila, the vinegar fly, for maintaining an ideal glucose balance, called homeostasis.
Professor Greg Seong-Bae Suh, Dr. Yangkyun Oh and colleagues identified a key neuron that is excited by glucose, which they called CN neuron. This CN neuron has a unique shape – it has an axon (which is used to transmit information to downstream cells) that is bifurcated. One branch projects to insulin-producing cells, and sends a signal triggering the secretion of the insulin equivalent in flies. The other branch projects to glucagon-producing cells and sends a signal inhibiting the secretion of the glucagon equivalent.
When flies consume food, the levels of glucose in their body increase; this excites the CN neuron, which fires the simultaneous signals to stimulate insulin and inhibit glucagon secretion, thereby maintaining the appropriate balance between the hormones and sugar in the blood. The researchers were able to see this happening in the brain in real time by using a combination of cutting-edge fluorescent calcium imaging technology, as well as measuring hormone and sugar levels and applying highly sophisticated molecular genetic techniques.
When flies were not fed, however, the researchers observed a reduction in the activity of CN neuron, a reduction in insulin secretion and an increase in glucagon secretion. These findings indicate that these key hormones are under the direct control of the glucose-sensing neuron. Furthermore, when they silenced the CN neuron rendering dysfunctional CN neuron in flies, these animals experienced an imbalance, resulting in hyperglycemia – high levels of sugars in the blood, similar to what is observed in diabetes in humans. This further suggests that the CN neuron is critical to maintaining glucose homeostasis in animals.
While further research is required to investigate this process in humans, Suh notes this is a significant step forward in the fields of both neurobiology and endocrinology.
“This work lays the foundation for translational research to better understand how this delicate regulatory process is affected by diabetes, obesity, excessive nutrition and diets high in sugar,” Suh said.
Profile: Greg Seong-Bae Suh
seongbaesuh@kaist.ac.kr
Professor Department of Biological Sciences
KAIST
(Figure: A single glucose-excited CN neuron extends bifurcated axonal branches,
one of which innervates insulin producing cells and stimulates their activity an the other axonal branch projects to glucagon producing cells and inhibits their activity.)
2019.10.24 View 19331