College+of+Natural+Sciences
-
 KAIST Succeeds in the Real-time Observation of Organoids using Holotomography
Organoids, which are 3D miniature organs that mimic the structure and function of human organs, play an essential role in disease research and drug development. A Korean research team has overcome the limitations of existing imaging technologies, succeeding in the real-time, high-resolution observation of living organoids.
KAIST (represented by President Kwang Hyung Lee) announced on the 14th of October that Professor YongKeun Park’s research team from the Department of Physics, in collaboration with the Genome Editing Research Center (Director Bon-Kyoung Koo) of the Institute for Basic Science (IBS President Do-Young Noh) and Tomocube Inc., has developed an imaging technology using holotomography to observe live, small intestinal organoids in real time at a high resolution.
Existing imaging techniques have struggled to observe living organoids in high resolution over extended periods and often required additional treatments like fluorescent staining.
< Figure 1. Overview of the low-coherence HT workflow. Using holotomography, 3D morphological restoration and quantitative analysis of organoids can be performed. In order to improve the limited field of view, which is a limitation of the microscope, our research team utilized a large-area field of view combination algorithm and made a 3D restoration by acquiring multi-focus holographic images for 3D measurements. After that, the organoids were compartmentalized to divide the parts necessary for analysis and quantitatively evaluated the protein concentration measurable from the refractive index and the survival rate of the organoids. >
The research team introduced holotomography technology to address these issues, which provides high-resolution images without the need for fluorescent staining and allows for the long-term observation of dynamic changes in real time without causing cell damage.
The team validated this technology using small intestinal organoids from experimental mice and were able to observe various cell structures inside the organoids in detail. They also captured dynamic changes such as growth processes, cell division, and cell death in real time using holotomography.
Additionally, the technology allowed for the precise analysis of the organoids' responses to drug treatments, verifying the survival of the cells.
The researchers believe that this breakthrough will open new horizons in organoid research, enabling the greater utilization of organoids in drug development, personalized medicine, and regenerative medicine.
Future research is expected to more accurately replicate the in vivo environment of organoids, contributing significantly to a more detailed understanding of various life phenomena at the cellular level through more precise 3D imaging.
< Figure 2. Real-time organoid morphology analysis. Using holotomography, it is possible to observe the lumen and villus development process of intestinal organoids in real time, which was difficult to observe with a conventional microscope. In addition, various information about intestinal organoids can be obtained by quantifying the size and protein amount of intestinal organoids through image analysis. >
Dr. Mahn Jae Lee, a graduate of KAIST's Graduate School of Medical Science and Engineering, currently at Chungnam National University Hospital and the first author of the paper, commented, "This research represents a new imaging technology that surpasses previous limitations and is expected to make a major contribution to disease modeling, personalized treatments, and drug development research using organoids."
The research results were published online in the international journal Experimental & Molecular Medicine on October 1, 2024, and the technology has been recognized for its applicability in various fields of life sciences. (Paper title: “Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography”)
This research was supported by the National Research Foundation of Korea, KAIST Institutes, and the Institute for Basic Science.
2024.10.14 View 5053
KAIST Succeeds in the Real-time Observation of Organoids using Holotomography
Organoids, which are 3D miniature organs that mimic the structure and function of human organs, play an essential role in disease research and drug development. A Korean research team has overcome the limitations of existing imaging technologies, succeeding in the real-time, high-resolution observation of living organoids.
KAIST (represented by President Kwang Hyung Lee) announced on the 14th of October that Professor YongKeun Park’s research team from the Department of Physics, in collaboration with the Genome Editing Research Center (Director Bon-Kyoung Koo) of the Institute for Basic Science (IBS President Do-Young Noh) and Tomocube Inc., has developed an imaging technology using holotomography to observe live, small intestinal organoids in real time at a high resolution.
Existing imaging techniques have struggled to observe living organoids in high resolution over extended periods and often required additional treatments like fluorescent staining.
< Figure 1. Overview of the low-coherence HT workflow. Using holotomography, 3D morphological restoration and quantitative analysis of organoids can be performed. In order to improve the limited field of view, which is a limitation of the microscope, our research team utilized a large-area field of view combination algorithm and made a 3D restoration by acquiring multi-focus holographic images for 3D measurements. After that, the organoids were compartmentalized to divide the parts necessary for analysis and quantitatively evaluated the protein concentration measurable from the refractive index and the survival rate of the organoids. >
The research team introduced holotomography technology to address these issues, which provides high-resolution images without the need for fluorescent staining and allows for the long-term observation of dynamic changes in real time without causing cell damage.
The team validated this technology using small intestinal organoids from experimental mice and were able to observe various cell structures inside the organoids in detail. They also captured dynamic changes such as growth processes, cell division, and cell death in real time using holotomography.
Additionally, the technology allowed for the precise analysis of the organoids' responses to drug treatments, verifying the survival of the cells.
The researchers believe that this breakthrough will open new horizons in organoid research, enabling the greater utilization of organoids in drug development, personalized medicine, and regenerative medicine.
Future research is expected to more accurately replicate the in vivo environment of organoids, contributing significantly to a more detailed understanding of various life phenomena at the cellular level through more precise 3D imaging.
< Figure 2. Real-time organoid morphology analysis. Using holotomography, it is possible to observe the lumen and villus development process of intestinal organoids in real time, which was difficult to observe with a conventional microscope. In addition, various information about intestinal organoids can be obtained by quantifying the size and protein amount of intestinal organoids through image analysis. >
Dr. Mahn Jae Lee, a graduate of KAIST's Graduate School of Medical Science and Engineering, currently at Chungnam National University Hospital and the first author of the paper, commented, "This research represents a new imaging technology that surpasses previous limitations and is expected to make a major contribution to disease modeling, personalized treatments, and drug development research using organoids."
The research results were published online in the international journal Experimental & Molecular Medicine on October 1, 2024, and the technology has been recognized for its applicability in various fields of life sciences. (Paper title: “Long-term three-dimensional high-resolution imaging of live unlabeled small intestinal organoids via low-coherence holotomography”)
This research was supported by the National Research Foundation of Korea, KAIST Institutes, and the Institute for Basic Science.
2024.10.14 View 5053 -
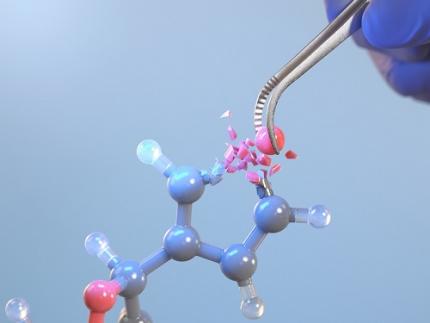 KAIST Changes the Paradigm of Drug Discovery with World's First Atomic Editing
In pioneering drug development, the new technology that enables the easy and rapid editing of key atoms responsible for drug efficacy has been regarded as a fundamental and "dream" technology, revolutionizing the process of discovering potential drug candidates. KAIST researchers have become the first in the world to successfully develop single-atom editing technology that maximizes drug efficacy.
On October 8th, KAIST (represented by President Kwang-Hyung Lee) announced that Professor Yoonsu Park’s research team from the Department of Chemistry successfully developed technology that enables the easy editing and correction of oxygen atoms in furan compounds into nitrogen atoms, directly converting them into pyrrole frameworks, which are widely used in pharmaceuticals.
< Image. Conceptual image illustrating the main idea of the research >
This research was published in the prestigious scientific journal Science on October 3rd under the title "Photocatalytic Furan-to-Pyrrole Conversion."
Many drugs have complex chemical structures, but their efficacy is often determined by a single critical atom. Atoms like oxygen and nitrogen play a central role in enhancing the pharmacological effects of these drugs, particularly against viruses.
This phenomenon, where the introduction of specific atoms into a drug molecule dramatically affects its efficacy, is known as the "Single Atom Effect." In leading-edge drug development, discovering atoms that maximize drug efficacy is key.
However, evaluating the Single Atom Effect has traditionally required multi-step, costly synthesis processes, as it has been difficult to selectively edit single atoms within stable ring structures containing oxygen or nitrogen.
Professor Park’s team overcame this challenge by introducing a photocatalyst that uses light energy. They developed a photocatalyst that acts as a “molecular scissor,” freely cutting and attaching five-membered rings, enabling single-atom editing at room temperature and atmospheric pressure—a world first.
The team discovered a new reaction mechanism in which the excited molecular scissor removes oxygen from furan via single-electron oxidation and then sequentially adds a nitrogen atom.
Donghyeon Kim and Jaehyun You, the study's first authors and candidates in KAIST’s integrated master's and doctoral program in the Department of Chemistry, explained that this technique offers high versatility by utilizing light energy to replace harsh conditions. They further noted that the technology enables selective editing, even when applied to complex natural products or pharmaceuticals. Professor Yoonsu Park, who led the research, remarked, "This breakthrough, which allows for the selective editing of five-membered organic ring structures, will open new doors for building libraries of drug candidates, a key challenge in pharmaceuticals. I hope this foundational technology will be used to revolutionize the drug development process."
The significance of this research was highlighted in the Perspective section of Science, a feature where a peer scientist of prominence outside of the project group provides commentary on an impactful research.
This research was supported by the National Research Foundation of Korea’s Creative Research Program, the Cross-Generation Collaborative Lab Project at KAIST, and the POSCO Science Fellowship of the POSCO TJ Park Foundation.
2024.10.11 View 5559
KAIST Changes the Paradigm of Drug Discovery with World's First Atomic Editing
In pioneering drug development, the new technology that enables the easy and rapid editing of key atoms responsible for drug efficacy has been regarded as a fundamental and "dream" technology, revolutionizing the process of discovering potential drug candidates. KAIST researchers have become the first in the world to successfully develop single-atom editing technology that maximizes drug efficacy.
On October 8th, KAIST (represented by President Kwang-Hyung Lee) announced that Professor Yoonsu Park’s research team from the Department of Chemistry successfully developed technology that enables the easy editing and correction of oxygen atoms in furan compounds into nitrogen atoms, directly converting them into pyrrole frameworks, which are widely used in pharmaceuticals.
< Image. Conceptual image illustrating the main idea of the research >
This research was published in the prestigious scientific journal Science on October 3rd under the title "Photocatalytic Furan-to-Pyrrole Conversion."
Many drugs have complex chemical structures, but their efficacy is often determined by a single critical atom. Atoms like oxygen and nitrogen play a central role in enhancing the pharmacological effects of these drugs, particularly against viruses.
This phenomenon, where the introduction of specific atoms into a drug molecule dramatically affects its efficacy, is known as the "Single Atom Effect." In leading-edge drug development, discovering atoms that maximize drug efficacy is key.
However, evaluating the Single Atom Effect has traditionally required multi-step, costly synthesis processes, as it has been difficult to selectively edit single atoms within stable ring structures containing oxygen or nitrogen.
Professor Park’s team overcame this challenge by introducing a photocatalyst that uses light energy. They developed a photocatalyst that acts as a “molecular scissor,” freely cutting and attaching five-membered rings, enabling single-atom editing at room temperature and atmospheric pressure—a world first.
The team discovered a new reaction mechanism in which the excited molecular scissor removes oxygen from furan via single-electron oxidation and then sequentially adds a nitrogen atom.
Donghyeon Kim and Jaehyun You, the study's first authors and candidates in KAIST’s integrated master's and doctoral program in the Department of Chemistry, explained that this technique offers high versatility by utilizing light energy to replace harsh conditions. They further noted that the technology enables selective editing, even when applied to complex natural products or pharmaceuticals. Professor Yoonsu Park, who led the research, remarked, "This breakthrough, which allows for the selective editing of five-membered organic ring structures, will open new doors for building libraries of drug candidates, a key challenge in pharmaceuticals. I hope this foundational technology will be used to revolutionize the drug development process."
The significance of this research was highlighted in the Perspective section of Science, a feature where a peer scientist of prominence outside of the project group provides commentary on an impactful research.
This research was supported by the National Research Foundation of Korea’s Creative Research Program, the Cross-Generation Collaborative Lab Project at KAIST, and the POSCO Science Fellowship of the POSCO TJ Park Foundation.
2024.10.11 View 5559 -
 KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5582
KAIST presents strategies for Holotomography in advanced bio research
Measuring and analyzing three-dimensional (3D) images of live cells and tissues is considered crucial in advanced fields of biology and medicine. Organoids, which are 3D structures that mimic organs, are particular examples that significantly benefits 3D live imaging. Organoids provide effective alternatives to animal testing in the drug development processes, and can rapidly determine personalized medicine. On the other hand, active researches are ongoing to utilize organoids for organ replacement.
< Figure 1. Schematic illustration of holotomography compared to X-ray CT. Similar to CT, they share the commonality of measuring the optical properties of an unlabeled specimen in three dimensions. Instead of X-rays, holotomography irradiates light in the visible range, and provides refractive index measurements of transparent specimens rather than absorptivity. While CT obtains three-dimensional information only through mechanical rotation of the irradiating light, holotomography can replace this by applying wavefront control technology in the visible range. >
Organelle-level observation of 3D biological specimens such as organoids and stem cell colonies without staining or preprocessing holds significant implications for both innovating basic research and bioindustrial applications related to regenerative medicine and bioindustrial applications.
Holotomography (HT) is a 3D optical microscopy that implements 3D reconstruction analogous to that of X-ray computed tomography (CT). Although HT and CT share a similar theoretical background, HT facilitates high-resolution examination inside cells and tissues, instead of the human body. HT obtains 3D images of cells and tissues at the organelle level without chemical or genetic labeling, thus overcomes various challenges of existing methods in bio research and industry. Its potential is highlighted in research fields where sample physiology must not be disrupted, such as regenerative medicine, personalized medicine, and infertility treatment.
< Figure 2. Label-free 3D imaging of diverse live cells. Time-lapse image of Hep3B cells illustrating subcellular morphology changes upon H2O2 treatment, followed by cellular recovery after returning to the regular cell culture medium. >
This paper introduces the advantages and broad applicability of HT to biomedical researchers, while presenting an overview of principles and future technical challenges to optical researchers. It showcases various cases of applying HT in studies such as 3D biology, regenerative medicine, and cancer research, as well as suggesting future optical development. Also, it categorizes HT based on the light source, to describe the principles, limitations, and improvements of each category in detail. Particularly, the paper addresses strategies for deepening cell and organoid studies by introducing artificial intelligence (AI) to HT.
Due to its potential to drive advanced bioindustry, HT is attracting interest and investment from universities and corporates worldwide. The KAIST research team has been leading this international field by developing core technologies and carrying out key application researches throughout the last decade.
< Figure 3. Various types of cells and organelles that make up the imaging barrier of a living intestinal organoid can be observed using holotomography. >
This paper, co-authored by Dr. Geon Kim from KAIST Research Center for Natural Sciences, Professor Ki-Jun Yoon's team from the Department of Biological Sciences, Director Bon-Kyoung Koo's team from the Institute for Basic Science (IBS) Center for Genome Engineering, and Dr. Seongsoo Lee's team from the Korea Basic Science Institute (KBSI), was published in 'Nature Reviews Methods Primers' on the 25th of July. This research was supported by the Leader Grant and Basic Science Research Program of the National Research Foundation, the Hologram Core Technology Development Grant of the Ministry of Science and ICT, the Nano and Material Technology Development Project, and the Health and Medical R&D Project of the Ministry of Health and Welfare.
2024.07.30 View 5582 -
 A 20-year-old puzzle solved: KAIST research team reveals the 'three-dimensional vortex' of zero-dimensional ferroelectrics
Materials that can maintain a magnetized state by themselves without an external magnetic field (i.e., permanent magnets) are called ferromagnets. Ferroelectrics can be thought of as the electric counterpart to ferromagnets, as they maintain a polarized state without an external electric field. It is well-known that ferromagnets lose their magnetic properties when reduced to nano sizes below a certain threshold. What happens when ferroelectrics are similarly made extremely small in all directions (i.e., into a zero-dimensional structure such as nanoparticles) has been a topic of controversy for a long time.
< (From left) Professor Yongsoo Yang, the corresponding author, and Chaehwa Jeong, the first author studying in the integrated master’s and doctoral program, of the KAIST Department of Physics >
The research team led by Dr. Yongsoo Yang from the Department of Physics at KAIST has, for the first time, experimentally clarified the three-dimensional, vortex-shaped polarization distribution inside ferroelectric nanoparticles through international collaborative research with POSTECH, SNU, KBSI, LBNL and University of Arkansas.
About 20 years ago, Prof. Laurent Bellaiche (currently at University of Arkansas) and his colleagues theoretically predicted that a unique form of polarization distribution, arranged in a toroidal vortex shape, could occur inside ferroelectric nanodots. They also suggested that if this vortex distribution could be properly controlled, it could be applied to ultra-high-density memory devices with capacities over 10,000 times greater than existing ones. However, experimental clarification had not been achieved due to the difficulty of measuring the three-dimensional polarization distribution within ferroelectric nanostructures.
The research team at KAIST successfully solved this 20-year-old challenge by implementing a technique called atomic electron tomography. This technique works by acquiring atomic-resolution transmission electron microscope images of the nanomaterials from multiple tilt angles, and then reconstructing them back into three-dimensional structures using advanced reconstruction algorithms. Electron tomography can be understood as essentially the same method with the CT scans used in hospitals to view internal organs in three dimensions; the KAIST team adapted it uniquely for nanomaterials, utilizing an electron microscope at the single-atom level.
< Figure 1. Three-dimensional polarization distribution of BaTiO3 nanoparticles revealed by atomic electron tomography. >(Left) Schematic of the electron tomography technique, which involves acquiring transmission electron microscope images at multiple tilt angles and reconstructing them into 3D atomic structures.(Center) Experimentally determined three-dimensional polarization distribution inside a BaTiO3 nanoparticle via atomic electron tomography. A vortex-like structure is clearly visible near the bottom (blue dot).(Right) A two-dimensional cross-section of the polarization distribution, thinly sliced at the center of the vortex, with the color and arrows together indicating the direction of the polarization. A distinct vortex structure can be observed.
Using atomic electron tomography, the team completely measured the positions of cation atoms inside barium titanate (BaTiO3) nanoparticles, a well-known ferroelectric material, in three dimensions. From the precisely determined 3D atomic arrangements, they were able to further calculate the internal three-dimensional polarization distribution at the single-atom level. The analysis of the polarization distribution revealed, for the first time experimentally, that topological polarization orderings including vortices, anti-vortices, skyrmions, and a Bloch point occur inside the 0-dimensional ferroelectrics, as theoretically predicted 20 years ago. Furthermore, it was also found that the number of internal vortices can be controlled depending on their sizes.
Prof. Sergey Prosandeev and Prof. Bellaiche (who proposed with other co-workers the polar vortex ordering theoretically 20 years ago), joined this collaboration and further proved that the vortex distribution results obtained from experiments are consistent with theoretical calculations.
By controlling the number and orientation of these polarization distributions, it is expected that this can be utilized into next-generation high-density memory device that can store more than 10,000 times the amount of information in the same-sized device compared to existing ones.
Dr. Yang, who led the research, explained the significance of the results: “This result suggests that controlling the size and shape of ferroelectrics alone, without needing to tune the substrate or surrounding environmental effects such as epitaxial strain, can manipulate ferroelectric vortices or other topological orderings at the nano-scale. Further research could then be applied to the development of next-generation ultra-high-density memory.”
This research, with Chaehwa Jeong from the Department of Physics at KAIST as the first author, was published online in Nature Communications on May 8th (Title: Revealing the Three-Dimensional Arrangement of Polar Topology in Nanoparticles).
The study was mainly supported by the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIT).
2024.05.31 View 7621
A 20-year-old puzzle solved: KAIST research team reveals the 'three-dimensional vortex' of zero-dimensional ferroelectrics
Materials that can maintain a magnetized state by themselves without an external magnetic field (i.e., permanent magnets) are called ferromagnets. Ferroelectrics can be thought of as the electric counterpart to ferromagnets, as they maintain a polarized state without an external electric field. It is well-known that ferromagnets lose their magnetic properties when reduced to nano sizes below a certain threshold. What happens when ferroelectrics are similarly made extremely small in all directions (i.e., into a zero-dimensional structure such as nanoparticles) has been a topic of controversy for a long time.
< (From left) Professor Yongsoo Yang, the corresponding author, and Chaehwa Jeong, the first author studying in the integrated master’s and doctoral program, of the KAIST Department of Physics >
The research team led by Dr. Yongsoo Yang from the Department of Physics at KAIST has, for the first time, experimentally clarified the three-dimensional, vortex-shaped polarization distribution inside ferroelectric nanoparticles through international collaborative research with POSTECH, SNU, KBSI, LBNL and University of Arkansas.
About 20 years ago, Prof. Laurent Bellaiche (currently at University of Arkansas) and his colleagues theoretically predicted that a unique form of polarization distribution, arranged in a toroidal vortex shape, could occur inside ferroelectric nanodots. They also suggested that if this vortex distribution could be properly controlled, it could be applied to ultra-high-density memory devices with capacities over 10,000 times greater than existing ones. However, experimental clarification had not been achieved due to the difficulty of measuring the three-dimensional polarization distribution within ferroelectric nanostructures.
The research team at KAIST successfully solved this 20-year-old challenge by implementing a technique called atomic electron tomography. This technique works by acquiring atomic-resolution transmission electron microscope images of the nanomaterials from multiple tilt angles, and then reconstructing them back into three-dimensional structures using advanced reconstruction algorithms. Electron tomography can be understood as essentially the same method with the CT scans used in hospitals to view internal organs in three dimensions; the KAIST team adapted it uniquely for nanomaterials, utilizing an electron microscope at the single-atom level.
< Figure 1. Three-dimensional polarization distribution of BaTiO3 nanoparticles revealed by atomic electron tomography. >(Left) Schematic of the electron tomography technique, which involves acquiring transmission electron microscope images at multiple tilt angles and reconstructing them into 3D atomic structures.(Center) Experimentally determined three-dimensional polarization distribution inside a BaTiO3 nanoparticle via atomic electron tomography. A vortex-like structure is clearly visible near the bottom (blue dot).(Right) A two-dimensional cross-section of the polarization distribution, thinly sliced at the center of the vortex, with the color and arrows together indicating the direction of the polarization. A distinct vortex structure can be observed.
Using atomic electron tomography, the team completely measured the positions of cation atoms inside barium titanate (BaTiO3) nanoparticles, a well-known ferroelectric material, in three dimensions. From the precisely determined 3D atomic arrangements, they were able to further calculate the internal three-dimensional polarization distribution at the single-atom level. The analysis of the polarization distribution revealed, for the first time experimentally, that topological polarization orderings including vortices, anti-vortices, skyrmions, and a Bloch point occur inside the 0-dimensional ferroelectrics, as theoretically predicted 20 years ago. Furthermore, it was also found that the number of internal vortices can be controlled depending on their sizes.
Prof. Sergey Prosandeev and Prof. Bellaiche (who proposed with other co-workers the polar vortex ordering theoretically 20 years ago), joined this collaboration and further proved that the vortex distribution results obtained from experiments are consistent with theoretical calculations.
By controlling the number and orientation of these polarization distributions, it is expected that this can be utilized into next-generation high-density memory device that can store more than 10,000 times the amount of information in the same-sized device compared to existing ones.
Dr. Yang, who led the research, explained the significance of the results: “This result suggests that controlling the size and shape of ferroelectrics alone, without needing to tune the substrate or surrounding environmental effects such as epitaxial strain, can manipulate ferroelectric vortices or other topological orderings at the nano-scale. Further research could then be applied to the development of next-generation ultra-high-density memory.”
This research, with Chaehwa Jeong from the Department of Physics at KAIST as the first author, was published online in Nature Communications on May 8th (Title: Revealing the Three-Dimensional Arrangement of Polar Topology in Nanoparticles).
The study was mainly supported by the National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIT).
2024.05.31 View 7621 -
 Revolutionary 'scLENS' Unveiled to Decode Complex Single-Cell Genomic Data
Unlocking biological information from complex single-cell genomic data has just become easier and more precise, thanks to the innovative 'scLENS' tool developed by the Biomedical Mathematics Group within the IBS Center for Mathematical and Computational Sciences led by Chief Investigator Jae Kyoung Kim, who is also a professor at KAIST. This new finding represents a significant leap forward in the field of single-cell transcriptomics.
Single-cell genomic analysis is an advanced technique that measures gene expression at the individual cell level, revealing cellular changes and interactions that are not observable with traditional genomic analysis methods. When applied to cancer tissues, this analysis can delineate the composition of diverse cell types within a tumor, providing insights into how cancer progresses and identifying key genes involved during each stage of progression.
Despite the immense potential of single-cell genomic analysis, handling the vast amount of data that it generates has always been challenging. The amount of data covers the expression of tens of thousands of genes across hundreds to thousands of individual cells. This not only results in large datasets but also introduces noise-related distortions, which arise in part due to current measurement limitations.
< Figure 1. Overview of scLENS (single-cell Low-dimensional embedding using the effective Noise Subtract) >
(Left) Current dimensionality reduction methods for scRNA-seq data involve conventional data preprocessing steps, such as log normalization, followed by manual selection of signals from the scaled data. However, this study reveals that the high levels of sparsity and variability in scRNA-seq data can lead to signal distortion during the data preprocessing, compromising the accuracy of downstream analyses.
(Right) To address this issue, the researchers integrated L2 normalization into the conventional preprocessing pipeline, effectively mitigating signal distortion. Moreover, they developed a novel signal detection algorithm that eliminates the need for user intervention by leveraging random matrix theory-based noise filtering and signal robustness testing. By incorporating these techniques, scLENS enables accurate and automated analysis of scRNA-seq data, overcoming the limitations of existing dimensionality reduction methods.
Corresponding author Jae Kyoung Kim highlighted, “There has been a remarkable advancement in experimental technologies for analyzing single-cell transcriptomes over the past decade. However, due to limitations in data analysis methods, there has been a struggle to fully utilize valuable data obtained through extensive cost and time."
Researchers have developed numerous analysis methods over the years to discern biological signals from this noise. However, the accuracy of these methods has been less than satisfactory. A critical issue is that determining signal and noise thresholds often depends on subjective decisions from the users.
The newly developed scLENS tool harnesses Random Matrix Theory and Signal robustness test to automatically differentiate signals from noise without relying on subjective user input.
First author Hyun Kim stated, "Previously, users had to arbitrarily decide the threshold for signal and noise, which compromised the reproducibility of analysis results and introduced subjectivity. scLENS eliminates this problem by automatically detecting signals using only the inherent structure of the data."
During the development of scLENS, researchers identified the fundamental reasons for inaccuracies in existing analysis methods. They found that commonly used data preprocessing methods distort both biological signals and noise. The new preprocessing approach that scLENS offers is free from such distortions.
By resolving issues related to noise threshold determined by subjective user choice and signal distortion in conventional data preprocessing, scLENS significantly outperforms existing methods in accuracy. Additionally, scLENS automates the laborious process of signal dimension selection, allowing researchers to extract biological signals conveniently and automatically.
CI Kim added, "scLENS solves major issues in single-cell transcriptome data analysis, substantially improving the accuracy and efficiency throughout the analysis process. This is a prime example of how fundamental mathematical theories can drive innovation in life sciences research, allowing researchers to more quickly and accurately answer biological questions and uncover secrets of life that were previously hidden."
This research was published in the international journal 'Nature Communications' on April 27.
Terminology
* Single-cell RNA sequencing (scRNA-seq): A technique used to measure gene expression levels in individual cells, providing insights into cell heterogeneity and rare cell types.
* Dimensionality reduction: A method to reduce the number of features or variables in a dataset while preserving the most important information, making data analysis more manageable and interpretable.
* Random matrix theory: A mathematical framework used to model and analyze the properties of large, random matrices, which can be applied to filter out noise in high-dimensional data.
* Signal robustness test: Among the signals, this test selects signals that are robust to the slight perturbation in data because real biological signals should be invariant for such slight modification in the data.
2024.05.09 View 6086
Revolutionary 'scLENS' Unveiled to Decode Complex Single-Cell Genomic Data
Unlocking biological information from complex single-cell genomic data has just become easier and more precise, thanks to the innovative 'scLENS' tool developed by the Biomedical Mathematics Group within the IBS Center for Mathematical and Computational Sciences led by Chief Investigator Jae Kyoung Kim, who is also a professor at KAIST. This new finding represents a significant leap forward in the field of single-cell transcriptomics.
Single-cell genomic analysis is an advanced technique that measures gene expression at the individual cell level, revealing cellular changes and interactions that are not observable with traditional genomic analysis methods. When applied to cancer tissues, this analysis can delineate the composition of diverse cell types within a tumor, providing insights into how cancer progresses and identifying key genes involved during each stage of progression.
Despite the immense potential of single-cell genomic analysis, handling the vast amount of data that it generates has always been challenging. The amount of data covers the expression of tens of thousands of genes across hundreds to thousands of individual cells. This not only results in large datasets but also introduces noise-related distortions, which arise in part due to current measurement limitations.
< Figure 1. Overview of scLENS (single-cell Low-dimensional embedding using the effective Noise Subtract) >
(Left) Current dimensionality reduction methods for scRNA-seq data involve conventional data preprocessing steps, such as log normalization, followed by manual selection of signals from the scaled data. However, this study reveals that the high levels of sparsity and variability in scRNA-seq data can lead to signal distortion during the data preprocessing, compromising the accuracy of downstream analyses.
(Right) To address this issue, the researchers integrated L2 normalization into the conventional preprocessing pipeline, effectively mitigating signal distortion. Moreover, they developed a novel signal detection algorithm that eliminates the need for user intervention by leveraging random matrix theory-based noise filtering and signal robustness testing. By incorporating these techniques, scLENS enables accurate and automated analysis of scRNA-seq data, overcoming the limitations of existing dimensionality reduction methods.
Corresponding author Jae Kyoung Kim highlighted, “There has been a remarkable advancement in experimental technologies for analyzing single-cell transcriptomes over the past decade. However, due to limitations in data analysis methods, there has been a struggle to fully utilize valuable data obtained through extensive cost and time."
Researchers have developed numerous analysis methods over the years to discern biological signals from this noise. However, the accuracy of these methods has been less than satisfactory. A critical issue is that determining signal and noise thresholds often depends on subjective decisions from the users.
The newly developed scLENS tool harnesses Random Matrix Theory and Signal robustness test to automatically differentiate signals from noise without relying on subjective user input.
First author Hyun Kim stated, "Previously, users had to arbitrarily decide the threshold for signal and noise, which compromised the reproducibility of analysis results and introduced subjectivity. scLENS eliminates this problem by automatically detecting signals using only the inherent structure of the data."
During the development of scLENS, researchers identified the fundamental reasons for inaccuracies in existing analysis methods. They found that commonly used data preprocessing methods distort both biological signals and noise. The new preprocessing approach that scLENS offers is free from such distortions.
By resolving issues related to noise threshold determined by subjective user choice and signal distortion in conventional data preprocessing, scLENS significantly outperforms existing methods in accuracy. Additionally, scLENS automates the laborious process of signal dimension selection, allowing researchers to extract biological signals conveniently and automatically.
CI Kim added, "scLENS solves major issues in single-cell transcriptome data analysis, substantially improving the accuracy and efficiency throughout the analysis process. This is a prime example of how fundamental mathematical theories can drive innovation in life sciences research, allowing researchers to more quickly and accurately answer biological questions and uncover secrets of life that were previously hidden."
This research was published in the international journal 'Nature Communications' on April 27.
Terminology
* Single-cell RNA sequencing (scRNA-seq): A technique used to measure gene expression levels in individual cells, providing insights into cell heterogeneity and rare cell types.
* Dimensionality reduction: A method to reduce the number of features or variables in a dataset while preserving the most important information, making data analysis more manageable and interpretable.
* Random matrix theory: A mathematical framework used to model and analyze the properties of large, random matrices, which can be applied to filter out noise in high-dimensional data.
* Signal robustness test: Among the signals, this test selects signals that are robust to the slight perturbation in data because real biological signals should be invariant for such slight modification in the data.
2024.05.09 View 6086 -
 KAIST Research Team Breaks Down Musical Instincts with AI
Music, often referred to as the universal language, is known to be a common component in all cultures. Then, could ‘musical instinct’ be something that is shared to some degree despite the extensive environmental differences amongst cultures?
On January 16, a KAIST research team led by Professor Hawoong Jung from the Department of Physics announced to have identified the principle by which musical instincts emerge from the human brain without special learning using an artificial neural network model.
Previously, many researchers have attempted to identify the similarities and differences between the music that exist in various different cultures, and tried to understand the origin of the universality. A paper published in Science in 2019 had revealed that music is produced in all ethnographically distinct cultures, and that similar forms of beats and tunes are used. Neuroscientist have also previously found out that a specific part of the human brain, namely the auditory cortex, is responsible for processing musical information.
Professor Jung’s team used an artificial neural network model to show that cognitive functions for music forms spontaneously as a result of processing auditory information received from nature, without being taught music. The research team utilized AudioSet, a large-scale collection of sound data provided by Google, and taught the artificial neural network to learn the various sounds. Interestingly, the research team discovered that certain neurons within the network model would respond selectively to music. In other words, they observed the spontaneous generation of neurons that reacted minimally to various other sounds like those of animals, nature, or machines, but showed high levels of response to various forms of music including both instrumental and vocal.
The neurons in the artificial neural network model showed similar reactive behaviours to those in the auditory cortex of a real brain. For example, artificial neurons responded less to the sound of music that was cropped into short intervals and were rearranged. This indicates that the spontaneously-generated music-selective neurons encode the temporal structure of music. This property was not limited to a specific genre of music, but emerged across 25 different genres including classic, pop, rock, jazz, and electronic.
< Figure 1. Illustration of the musicality of the brain and artificial neural network (created with DALL·E3 AI based on the paper content) >
Furthermore, suppressing the activity of the music-selective neurons was found to greatly impede the cognitive accuracy for other natural sounds. That is to say, the neural function that processes musical information helps process other sounds, and that ‘musical ability’ may be an instinct formed as a result of an evolutionary adaptation acquired to better process sounds from nature.
Professor Hawoong Jung, who advised the research, said, “The results of our study imply that evolutionary pressure has contributed to forming the universal basis for processing musical information in various cultures.” As for the significance of the research, he explained, “We look forward for this artificially built model with human-like musicality to become an original model for various applications including AI music generation, musical therapy, and for research in musical cognition.” He also commented on its limitations, adding, “This research however does not take into consideration the developmental process that follows the learning of music, and it must be noted that this is a study on the foundation of processing musical information in early development.”
< Figure 2. The artificial neural network that learned to recognize non-musical natural sounds in the cyber space distinguishes between music and non-music. >
This research, conducted by first author Dr. Gwangsu Kim of the KAIST Department of Physics (current affiliation: MIT Department of Brain and Cognitive Sciences) and Dr. Dong-Kyum Kim (current affiliation: IBS) was published in Nature Communications under the title, “Spontaneous emergence of rudimentary music detectors in deep neural networks”.
This research was supported by the National Research Foundation of Korea.
2024.01.23 View 7776
KAIST Research Team Breaks Down Musical Instincts with AI
Music, often referred to as the universal language, is known to be a common component in all cultures. Then, could ‘musical instinct’ be something that is shared to some degree despite the extensive environmental differences amongst cultures?
On January 16, a KAIST research team led by Professor Hawoong Jung from the Department of Physics announced to have identified the principle by which musical instincts emerge from the human brain without special learning using an artificial neural network model.
Previously, many researchers have attempted to identify the similarities and differences between the music that exist in various different cultures, and tried to understand the origin of the universality. A paper published in Science in 2019 had revealed that music is produced in all ethnographically distinct cultures, and that similar forms of beats and tunes are used. Neuroscientist have also previously found out that a specific part of the human brain, namely the auditory cortex, is responsible for processing musical information.
Professor Jung’s team used an artificial neural network model to show that cognitive functions for music forms spontaneously as a result of processing auditory information received from nature, without being taught music. The research team utilized AudioSet, a large-scale collection of sound data provided by Google, and taught the artificial neural network to learn the various sounds. Interestingly, the research team discovered that certain neurons within the network model would respond selectively to music. In other words, they observed the spontaneous generation of neurons that reacted minimally to various other sounds like those of animals, nature, or machines, but showed high levels of response to various forms of music including both instrumental and vocal.
The neurons in the artificial neural network model showed similar reactive behaviours to those in the auditory cortex of a real brain. For example, artificial neurons responded less to the sound of music that was cropped into short intervals and were rearranged. This indicates that the spontaneously-generated music-selective neurons encode the temporal structure of music. This property was not limited to a specific genre of music, but emerged across 25 different genres including classic, pop, rock, jazz, and electronic.
< Figure 1. Illustration of the musicality of the brain and artificial neural network (created with DALL·E3 AI based on the paper content) >
Furthermore, suppressing the activity of the music-selective neurons was found to greatly impede the cognitive accuracy for other natural sounds. That is to say, the neural function that processes musical information helps process other sounds, and that ‘musical ability’ may be an instinct formed as a result of an evolutionary adaptation acquired to better process sounds from nature.
Professor Hawoong Jung, who advised the research, said, “The results of our study imply that evolutionary pressure has contributed to forming the universal basis for processing musical information in various cultures.” As for the significance of the research, he explained, “We look forward for this artificially built model with human-like musicality to become an original model for various applications including AI music generation, musical therapy, and for research in musical cognition.” He also commented on its limitations, adding, “This research however does not take into consideration the developmental process that follows the learning of music, and it must be noted that this is a study on the foundation of processing musical information in early development.”
< Figure 2. The artificial neural network that learned to recognize non-musical natural sounds in the cyber space distinguishes between music and non-music. >
This research, conducted by first author Dr. Gwangsu Kim of the KAIST Department of Physics (current affiliation: MIT Department of Brain and Cognitive Sciences) and Dr. Dong-Kyum Kim (current affiliation: IBS) was published in Nature Communications under the title, “Spontaneous emergence of rudimentary music detectors in deep neural networks”.
This research was supported by the National Research Foundation of Korea.
2024.01.23 View 7776 -
 KAIST builds a high-resolution 3D holographic sensor using a single mask
Holographic cameras can provide more realistic images than ordinary cameras thanks to their ability to acquire 3D information about objects. However, existing holographic cameras use interferometers that measure the wavelength and refraction of light through the interference of light waves, which makes them complex and sensitive to their surrounding environment.
On August 23, a KAIST research team led by Professor YongKeun Park from the Department of Physics announced a new leap forward in 3D holographic imaging sensor technology.
The team proposed an innovative holographic camera technology that does not use complex interferometry. Instead, it uses a mask to precisely measure the phase information of light and reconstruct the 3D information of an object with higher accuracy.
< Figure 1. Structure and principle of the proposed holographic camera. The amplitude and phase information of light scattered from a holographic camera can be measured. >
The team used a mask that fulfills certain mathematical conditions and incorporated it into an ordinary camera, and the light scattered from a laser is measured through the mask and analyzed using a computer. This does not require a complex interferometer and allows the phase information of light to be collected through a simplified optical system. With this technique, the mask that is placed between the two lenses and behind an object plays an important role. The mask selectively filters specific parts of light,, and the intensity of the light passing through the lens can be measured using an ordinary commercial camera. This technique combines the image data received from the camera with the unique pattern received from the mask and reconstructs an object’s precise 3D information using an algorithm.
This method allows a high-resolution 3D image of an object to be captured in any position. In practical situations, one can construct a laser-based holographic 3D image sensor by adding a mask with a simple design to a general image sensor. This makes the design and construction of the optical system much easier. In particular, this novel technology can capture high-resolution holographic images of objects moving at high speeds, which widens its potential field of application.
< Figure 2. A moving doll captured by a conventional camera and the proposed holographic camera. When taking a picture without focusing on the object, only a blurred image of the doll can be obtained from a general camera, but the proposed holographic camera can restore the blurred image of the doll into a clear image. >
The results of this study, conducted by Dr. Jeonghun Oh from the KAIST Department of Physics as the first author, were published in Nature Communications on August 12 under the title, "Non-interferometric stand-alone single-shot holographic camera using reciprocal diffractive imaging".
Dr. Oh said, “The holographic camera module we are suggesting can be built by adding a filter to an ordinary camera, which would allow even non-experts to handle it easily in everyday life if it were to be commercialized.” He added, “In particular, it is a promising candidate with the potential to replace existing remote sensing technologies.”
This research was supported by the National Research Foundation’s Leader Research Project, the Korean Ministry of Science and ICT’s Core Hologram Technology Support Project, and the Nano and Material Technology Development Project.
2023.09.05 View 8879
KAIST builds a high-resolution 3D holographic sensor using a single mask
Holographic cameras can provide more realistic images than ordinary cameras thanks to their ability to acquire 3D information about objects. However, existing holographic cameras use interferometers that measure the wavelength and refraction of light through the interference of light waves, which makes them complex and sensitive to their surrounding environment.
On August 23, a KAIST research team led by Professor YongKeun Park from the Department of Physics announced a new leap forward in 3D holographic imaging sensor technology.
The team proposed an innovative holographic camera technology that does not use complex interferometry. Instead, it uses a mask to precisely measure the phase information of light and reconstruct the 3D information of an object with higher accuracy.
< Figure 1. Structure and principle of the proposed holographic camera. The amplitude and phase information of light scattered from a holographic camera can be measured. >
The team used a mask that fulfills certain mathematical conditions and incorporated it into an ordinary camera, and the light scattered from a laser is measured through the mask and analyzed using a computer. This does not require a complex interferometer and allows the phase information of light to be collected through a simplified optical system. With this technique, the mask that is placed between the two lenses and behind an object plays an important role. The mask selectively filters specific parts of light,, and the intensity of the light passing through the lens can be measured using an ordinary commercial camera. This technique combines the image data received from the camera with the unique pattern received from the mask and reconstructs an object’s precise 3D information using an algorithm.
This method allows a high-resolution 3D image of an object to be captured in any position. In practical situations, one can construct a laser-based holographic 3D image sensor by adding a mask with a simple design to a general image sensor. This makes the design and construction of the optical system much easier. In particular, this novel technology can capture high-resolution holographic images of objects moving at high speeds, which widens its potential field of application.
< Figure 2. A moving doll captured by a conventional camera and the proposed holographic camera. When taking a picture without focusing on the object, only a blurred image of the doll can be obtained from a general camera, but the proposed holographic camera can restore the blurred image of the doll into a clear image. >
The results of this study, conducted by Dr. Jeonghun Oh from the KAIST Department of Physics as the first author, were published in Nature Communications on August 12 under the title, "Non-interferometric stand-alone single-shot holographic camera using reciprocal diffractive imaging".
Dr. Oh said, “The holographic camera module we are suggesting can be built by adding a filter to an ordinary camera, which would allow even non-experts to handle it easily in everyday life if it were to be commercialized.” He added, “In particular, it is a promising candidate with the potential to replace existing remote sensing technologies.”
This research was supported by the National Research Foundation’s Leader Research Project, the Korean Ministry of Science and ICT’s Core Hologram Technology Support Project, and the Nano and Material Technology Development Project.
2023.09.05 View 8879 -
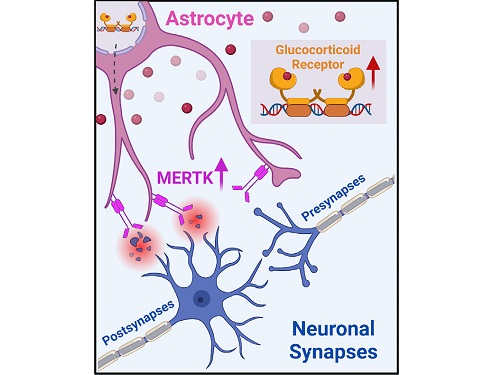 A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 8175
A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 8175 -
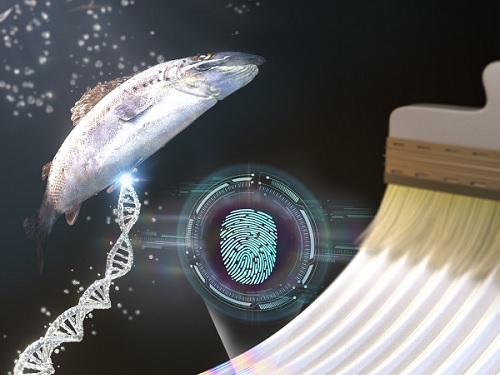 KAIST research team develops a forgery prevention technique using salmon DNA
The authenticity scandal that plagued the artwork “Beautiful Woman” by Kyung-ja Chun for 30 years shows how concerns about replicas can become a burden to artists, as most of them are not experts in the field of anti-counterfeiting. To solve this problem, artist-friendly physical unclonable functions (PUFs) based on optical techniques instead of electronic ones, which can be applied immediately onto artwork through brushstrokes are needed.
On May 23, a KAIST research team led by Professor Dong Ki Yoon in the Department of Chemistry revealed the development of a proprietary technology for security and certification using random patterns that occur during the self-assembly of soft materials.
With the development of the Internet of Things in recent years, various electronic devices and services can now be connected to the internet and carry out new innovative functions. However, counterfeiting technologies that infringe on individuals’ privacy have also entered the marketplace.
The technique developed by the research team involves random and spontaneous patterns that naturally occur during the self-assembly of two different types of soft materials, which can be used in the same way as human fingerprints for non-replicable security. This is very significant in that even non-experts in the field of security can construct anti-counterfeiting systems through simple actions like drawing a picture.
The team developed two unique methods. The first method uses liquid crystals. When liquid crystals become trapped in patterned substrates, they induce the symmetrical destruction of the structure and create a maze-like topology (Figure 1). The research team defined the pathways open to the right as 0 (blue), and those open to the left as 1 (red), and confirmed that the structure could be converted into a digital code composed of 0’s and 1’s that can serve as a type of fingerprint through object recognition using machine learning. This groundbreaking technique can be utilized by non-experts, as it does not require complex semiconductor patterns that are required by existing technology, and can be observed through the level of resolution of a smartphone camera. In particular, this technique can reconstruct information more easily than conventional methods that use semiconductor chips.
< Figure 1. Security technology using the maze made up of magnetically-assembled structures formed on a substrate patterned with liquid crystal materials. >
The second method uses DNA extracted from salmon. The DNA can be dissolved in water and applied with a brush to induce bulking instability, which forms random patterns similar to a zebra’s stripes. Here, the patterns create ridge endings and bifurcation, which are characteristics in fingerprints, and these can also be digitalized into 0’s and 1’s through machine learning. The research team applied conventional fingerprint recognition technology to this patterning technique and demonstrated its use as an artificial fingerprint. This method can be easily carried out using a brush, and the solution can be mixed into various colors and used as a new security ink.
< Figure 2. Technology to produce security ink using DNA polymers extracted from salmon >
This new security technology developed by the research team uses only simple organic materials and requires basic manufacturing processes, making it possible to enhance security at a low cost. In addition, users can produce patterns in the shapes and sizes they want, and even if the patterns are made in the same way, their randomness makes each individual pattern different. This provides high levels of security and gives the technique enhanced marketability.
Professor Dong Ki Yoon said, “These studies have taken the randomness that naturally occurs during self-assembly to create non-replicable patterns that can act like human fingerprints.” He added, “These ideas will be the cornerstone of technology that applies the many randomities that exist in nature to security systems.”
The two studies were published in the journal Advanced Materials under the titles “1Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media” and “2Paintable Physical Unclonable Function Using DNA” on May 6 and 5, respectively.
Author Information: 1Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon*, and Dong Ki Yoon*/ 2Soon Mo Park†, Geonhyeong Park†, Dong Ki Yoon*: †co-first authors, *corresponding author
This research was funded by the Center for Multiscale Chiral Architectures and supported by the Ministry of Science and ICT-Korea Research Foundation, BRIDGE Convergent Research and Development Program, the Running Together Project, and the Samsung Future Technology Development Program.
< Figure 1-1. A scene from the schematic animation of the process of Blues (0) and Reds (1) forming the PUF by exploring the maze. From "Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media" by Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon, Dong Ki Yoon. https://doi.org/10.1002/adma.202303077 >
< Figure 2-1. A schematic diagram of the formation of digital fingerprints formed using the DNA ink. From "Paintable Physical Unclonable Function Using DNA" by Soon Mo Park, Geonhyeong Park, Dong Ki Yoon. https://doi.org/10.1002/adma.202302135 >
2023.06.08 View 8496
KAIST research team develops a forgery prevention technique using salmon DNA
The authenticity scandal that plagued the artwork “Beautiful Woman” by Kyung-ja Chun for 30 years shows how concerns about replicas can become a burden to artists, as most of them are not experts in the field of anti-counterfeiting. To solve this problem, artist-friendly physical unclonable functions (PUFs) based on optical techniques instead of electronic ones, which can be applied immediately onto artwork through brushstrokes are needed.
On May 23, a KAIST research team led by Professor Dong Ki Yoon in the Department of Chemistry revealed the development of a proprietary technology for security and certification using random patterns that occur during the self-assembly of soft materials.
With the development of the Internet of Things in recent years, various electronic devices and services can now be connected to the internet and carry out new innovative functions. However, counterfeiting technologies that infringe on individuals’ privacy have also entered the marketplace.
The technique developed by the research team involves random and spontaneous patterns that naturally occur during the self-assembly of two different types of soft materials, which can be used in the same way as human fingerprints for non-replicable security. This is very significant in that even non-experts in the field of security can construct anti-counterfeiting systems through simple actions like drawing a picture.
The team developed two unique methods. The first method uses liquid crystals. When liquid crystals become trapped in patterned substrates, they induce the symmetrical destruction of the structure and create a maze-like topology (Figure 1). The research team defined the pathways open to the right as 0 (blue), and those open to the left as 1 (red), and confirmed that the structure could be converted into a digital code composed of 0’s and 1’s that can serve as a type of fingerprint through object recognition using machine learning. This groundbreaking technique can be utilized by non-experts, as it does not require complex semiconductor patterns that are required by existing technology, and can be observed through the level of resolution of a smartphone camera. In particular, this technique can reconstruct information more easily than conventional methods that use semiconductor chips.
< Figure 1. Security technology using the maze made up of magnetically-assembled structures formed on a substrate patterned with liquid crystal materials. >
The second method uses DNA extracted from salmon. The DNA can be dissolved in water and applied with a brush to induce bulking instability, which forms random patterns similar to a zebra’s stripes. Here, the patterns create ridge endings and bifurcation, which are characteristics in fingerprints, and these can also be digitalized into 0’s and 1’s through machine learning. The research team applied conventional fingerprint recognition technology to this patterning technique and demonstrated its use as an artificial fingerprint. This method can be easily carried out using a brush, and the solution can be mixed into various colors and used as a new security ink.
< Figure 2. Technology to produce security ink using DNA polymers extracted from salmon >
This new security technology developed by the research team uses only simple organic materials and requires basic manufacturing processes, making it possible to enhance security at a low cost. In addition, users can produce patterns in the shapes and sizes they want, and even if the patterns are made in the same way, their randomness makes each individual pattern different. This provides high levels of security and gives the technique enhanced marketability.
Professor Dong Ki Yoon said, “These studies have taken the randomness that naturally occurs during self-assembly to create non-replicable patterns that can act like human fingerprints.” He added, “These ideas will be the cornerstone of technology that applies the many randomities that exist in nature to security systems.”
The two studies were published in the journal Advanced Materials under the titles “1Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media” and “2Paintable Physical Unclonable Function Using DNA” on May 6 and 5, respectively.
Author Information: 1Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon*, and Dong Ki Yoon*/ 2Soon Mo Park†, Geonhyeong Park†, Dong Ki Yoon*: †co-first authors, *corresponding author
This research was funded by the Center for Multiscale Chiral Architectures and supported by the Ministry of Science and ICT-Korea Research Foundation, BRIDGE Convergent Research and Development Program, the Running Together Project, and the Samsung Future Technology Development Program.
< Figure 1-1. A scene from the schematic animation of the process of Blues (0) and Reds (1) forming the PUF by exploring the maze. From "Planar Spin Glass with Topologically-Protected Mazes in the Liquid Crystal Targeting for Reconfigurable Micro Security Media" by Geonhyeong Park, Yun-Seok Choi, S. Joon Kwon, Dong Ki Yoon. https://doi.org/10.1002/adma.202303077 >
< Figure 2-1. A schematic diagram of the formation of digital fingerprints formed using the DNA ink. From "Paintable Physical Unclonable Function Using DNA" by Soon Mo Park, Geonhyeong Park, Dong Ki Yoon. https://doi.org/10.1002/adma.202302135 >
2023.06.08 View 8496 -
 KAIST researchers find the key to overcome the limits in X-ray microscopy
X-ray microscopes have the advantage of penetrating most substances, so internal organs and skeletons can be observed non-invasively through chest X-rays or CT scans. Recently, studies to increase the resolution of X-ray imaging technology are being actively conducted in order to precisely observe the internal structure of semiconductors and batteries at the nanoscale.
KAIST (President Kwang Hyung Lee) announced on April 12th that a joint research team led by Professor YongKeun Park of the Department of Physics and Dr. Jun Lim of the Pohang Accelerator Laboratory has succeeded in developing a core technology that can overcome the resolution limitations of existing X-ray microscopes.
d
This study, in which Dr. KyeoReh Lee participated as the first author, was published on 6th of April in “Light: Science and Application”, a world-renowned academic journal in optics and photonics. (Paper title: Direct high-resolution X-ray imaging exploiting pseudorandomness).
X-ray nanomicroscopes do not have refractive lenses. In an X-ray microscope, a circular grating called a concentric zone plate is used instead of a lens. The resolution of an image obtained using the zone plate is determined by the quality of the nanostructure that comprises the plate. There are several difficulties in fabricating and maintaining these nanostructures, which set the limit to the level of resolution for X-ray microscopy.
The research team developed a new X-ray nanomicroscopy technology to overcome this problem. The X-ray lens proposed by the research team is in the form of numerous holes punched in a thin tungsten film, and generates random diffraction patterns by diffracting incident X-rays. The research team mathematically identified that, paradoxically, the high-resolution information of the sample was fully contained in these random diffraction patterns, and actually succeeded in extracting the information and imaging the internal states of the samples.
The imaging method using the mathematical properties of random diffraction was proposed and implemented in the visible light band for the first time by Dr. KyeoReh Lee and Professor YongKeun Park in 2016*. This study uses the results of previous studies to solve the difficult, lingering problem in the field of the X-ray imaging. ※ "Exploiting the speckle-correlation scattering matrix for a compact reference-free holographic image sensor." Nature communications 7.1 (2016): 13359.
The resolution of the image of the constructed sample has no direct correlation with the size of the pattern etched on the random lens used. Based on this idea, the research team succeeded in acquiring images with 14 nm resolution (approximately 1/7 the size of the coronavirus) by using random lenses made in a circular pattern with a diameter of 300 nm.
The imaging technology developed by this research team is a key fundamental technology that can enhance the resolution of X-ray nanomicroscopy, which has been blocked by limitations of the production of existing zone plates.
The first author and one of the co-corresponding author, Dr. KyeoReh Lee of KAIST Department of Physics, said, “In this study, the resolution was limited to 14 nm, but if the next-generation X-ray light source and high-performance X-ray detector are used, the resolution would exceed that of the conventional X-ray nano-imaging and approach the resolution of an electron microscope.” and added, “Unlike an electron microscope, X-rays can observe the internal structure without damaging the sample, so it will be able to present a new standard for non-invasive nanostructure observation processes such as quality inspections for semiconductors.”.
The co-corresponding author, Dr. Jun Lim of the Pohang Accelerator Laboratory, said, “In the same context, the developed image technology is expected to greatly increase the performance in the 4th generation multipurpose radiation accelerator which is set to be established in Ochang of the Northern Chungcheong Province.”
This research was conducted with the support through the Research Leader Program and the Sejong Science Fellowship of the National Research Foundation of Korea.
Fig. 1. Designed diffuser as X-ray imaging lens. a, Schematic of full-field transmission X-ray microscopy. The attenuation (amplitude) map of a sample is measured. The image resolution (dx) is limited by the outermost zone width of the zone plate (D). b, Schematic of the proposed method. A designed diffuser is used instead of a zone plate. The image resolution is finer than the hole size of the diffuser (dx << D).
Fig. 2. The left panel is a surface electron microscopy (SEM) image of the X-ray diffuser used in the experiment. The middle panel shows the design of the X-ray diffuser, and there is an inset in the middle of the panel that shows a corresponding part of the SEM image. The right panel shows an experimental random X-ray diffraction pattern, also known as a speckle pattern, obtained from the X-ray diffuser.
Fig. 3. Images taken from the proposed randomness-based X-ray imaging (bottom) and the corresponding surface electron microscope (SEM) images (top).
2023.04.12 View 8297
KAIST researchers find the key to overcome the limits in X-ray microscopy
X-ray microscopes have the advantage of penetrating most substances, so internal organs and skeletons can be observed non-invasively through chest X-rays or CT scans. Recently, studies to increase the resolution of X-ray imaging technology are being actively conducted in order to precisely observe the internal structure of semiconductors and batteries at the nanoscale.
KAIST (President Kwang Hyung Lee) announced on April 12th that a joint research team led by Professor YongKeun Park of the Department of Physics and Dr. Jun Lim of the Pohang Accelerator Laboratory has succeeded in developing a core technology that can overcome the resolution limitations of existing X-ray microscopes.
d
This study, in which Dr. KyeoReh Lee participated as the first author, was published on 6th of April in “Light: Science and Application”, a world-renowned academic journal in optics and photonics. (Paper title: Direct high-resolution X-ray imaging exploiting pseudorandomness).
X-ray nanomicroscopes do not have refractive lenses. In an X-ray microscope, a circular grating called a concentric zone plate is used instead of a lens. The resolution of an image obtained using the zone plate is determined by the quality of the nanostructure that comprises the plate. There are several difficulties in fabricating and maintaining these nanostructures, which set the limit to the level of resolution for X-ray microscopy.
The research team developed a new X-ray nanomicroscopy technology to overcome this problem. The X-ray lens proposed by the research team is in the form of numerous holes punched in a thin tungsten film, and generates random diffraction patterns by diffracting incident X-rays. The research team mathematically identified that, paradoxically, the high-resolution information of the sample was fully contained in these random diffraction patterns, and actually succeeded in extracting the information and imaging the internal states of the samples.
The imaging method using the mathematical properties of random diffraction was proposed and implemented in the visible light band for the first time by Dr. KyeoReh Lee and Professor YongKeun Park in 2016*. This study uses the results of previous studies to solve the difficult, lingering problem in the field of the X-ray imaging. ※ "Exploiting the speckle-correlation scattering matrix for a compact reference-free holographic image sensor." Nature communications 7.1 (2016): 13359.
The resolution of the image of the constructed sample has no direct correlation with the size of the pattern etched on the random lens used. Based on this idea, the research team succeeded in acquiring images with 14 nm resolution (approximately 1/7 the size of the coronavirus) by using random lenses made in a circular pattern with a diameter of 300 nm.
The imaging technology developed by this research team is a key fundamental technology that can enhance the resolution of X-ray nanomicroscopy, which has been blocked by limitations of the production of existing zone plates.
The first author and one of the co-corresponding author, Dr. KyeoReh Lee of KAIST Department of Physics, said, “In this study, the resolution was limited to 14 nm, but if the next-generation X-ray light source and high-performance X-ray detector are used, the resolution would exceed that of the conventional X-ray nano-imaging and approach the resolution of an electron microscope.” and added, “Unlike an electron microscope, X-rays can observe the internal structure without damaging the sample, so it will be able to present a new standard for non-invasive nanostructure observation processes such as quality inspections for semiconductors.”.
The co-corresponding author, Dr. Jun Lim of the Pohang Accelerator Laboratory, said, “In the same context, the developed image technology is expected to greatly increase the performance in the 4th generation multipurpose radiation accelerator which is set to be established in Ochang of the Northern Chungcheong Province.”
This research was conducted with the support through the Research Leader Program and the Sejong Science Fellowship of the National Research Foundation of Korea.
Fig. 1. Designed diffuser as X-ray imaging lens. a, Schematic of full-field transmission X-ray microscopy. The attenuation (amplitude) map of a sample is measured. The image resolution (dx) is limited by the outermost zone width of the zone plate (D). b, Schematic of the proposed method. A designed diffuser is used instead of a zone plate. The image resolution is finer than the hole size of the diffuser (dx << D).
Fig. 2. The left panel is a surface electron microscopy (SEM) image of the X-ray diffuser used in the experiment. The middle panel shows the design of the X-ray diffuser, and there is an inset in the middle of the panel that shows a corresponding part of the SEM image. The right panel shows an experimental random X-ray diffraction pattern, also known as a speckle pattern, obtained from the X-ray diffuser.
Fig. 3. Images taken from the proposed randomness-based X-ray imaging (bottom) and the corresponding surface electron microscope (SEM) images (top).
2023.04.12 View 8297 -
 KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7638
KAIST research team develops a cheap and safe redox flow battery
Redox flow batteries, one of the potential replacements for the widely used lithium-ion secondary batteries, can be utilized as new and renewable energy as well as for energy storage systems (ESS) thanks to their low cost, low flammability, and long lifetime of over 20 years. Since the price of vanadium, the most widely used active material for redox flow batteries, has been rising in recent years, scientists have been actively searching for redox materials to replace it.
On March 23, a joint research team led by Professors Hye Ryung Byon and Mu-Hyun Baik from the KAIST Department of Chemistry, and Professor Jongcheol Seo from the POSTECH Department of Chemistry announced that they had developed a highly soluble and stable organic redox-active molecule for use in aqueous redox flow batteries.
The research team focused on developing aqueous redox flow batteries by redesigning an organic molecule. It is possible to control the solubility and electrochemical redox potential of organic molecules by engineering their design, which makes them a promising active material candidate with possibly higher energy storage capabilities than vanadium. Most organic redox-active molecules have low solubilities or have slow chemical stability during redox reactions. Low solubility means low energy storage capacity and low chemical stability leads to reduced cycle performance. For this research, the team chose naphthalene diimide (NDI) as their active molecule. Until now, there was little research done on NDI despite its high chemical stability, as it shows low solubility in aqueous electrolyte solutions.
Although NDI molecules are almost insoluble in water, the research team tethered four ammonium functionalities and achieved a solubility as high as 1.5M* in water. In addition, they confirmed that when a 1M solution of NDI was used in neutral redox flow batteries for 500 cycles, 98% of its capacity was maintained. This means 0.004% capacity decay per cycle, and only 2% of its capacity would be lost if the battery were to be operated for 45 days.
Furthermore, the developed NDI molecule can save two electrons per molecule, and the team proved that 2M of electrons could be stored in every 1M of NDI solution used. For reference, vanadium used in vanadium redox flow batteries, which require a highly concentrated sulfuric acid solution, has a solubility of about 1.6M and can only hold one electron per molecule, meaning it can store a total of 1.6M of electrons. Therefore, the newly developed NDI active molecule shows a higher storage capacity compared to existing vanadium devices.
*1M (mol/L): 6.022 x 1023 active molecules are present in 1L of solution
This paper, written by co-first authors Research Professor Vikram Singh, and Ph.D. candidates Seongyeon Kwon and Yunseop Choi, was published in the online version of Advanced Materials on February 7 under the title, Controlling π-π interactions of highly soluble naphthalene diimide derivatives for neutral pH aqueous redox flow batteries. Ph.D. Candidate Yelim Yi and Professor Mi Hee Lee’s team from the KAIST Department of Chemistry also contributed to the study by conducting electron paramagnetic resonance analyses.
Professor Hye Ryung Byon said, “We have demonstrated the principles of molecular design by modifying an existing organic active molecule with low solubility and utilizing it as an active molecule for redox flow batteries. We have also shown that during a redox reaction, we can use molecular interactions to suppress the chemical reactivity of radically formed molecules.”
She added, “Should this be used later for aqueous redox flow batteries, along with its high energy density and high solubility, it would also have the advantage of being available for use in neutral pH electrolytes. Vanadium redox flow batteries currently use acidic solutions, which cause corrosion, and we expect our molecule to solve this issue. Since existing lithium ion-based ESS are flammable, we must develop safer and cheaper next-generation ESS, and our research has shown great promise in addressing this.”
This research was funded by Samsung Research Funding & Incubation Center, the Institute for Basic Science, and the National Research Foundation.
Figure 1. (a) Structures of various NDI molecules. (b) Solubility of NDI molecules in water (black bars) and aqueous electrolytes including KCl electrolyte (blue bars). (c–d) Structural changes of the molecules as the developed NDI molecule stores two electrons. (c) Illustration of cluster combination and separation of NDI molecules developed during redox reaction and (d) Snapshot of the MD simulation. NDI molecules prepared from the left, formation of bimolecular sieve and tetramolecular sieve clusters after the first reductive reaction, and a single molecule with a three-dimensional structure after the second reduction.
Figure 2. Performance results of an aqueous redox flow battery using 1M of the developed NDI molecule as the cathode electrolyte and 3.1M of ammonium iodine as the anode electrolyte. Using 1.5 M KCl solution. (a) A schematic diagram of a redox flow battery. (b) Voltage-capacity graph according to cycle in a redox flow battery. (c) Graphs of capacity and coulombs, voltage, and energy efficiency maintained at 500 cycles.
2023.04.03 View 7638 -
 Using light to throw and catch atoms to open up a new chapter for quantum computing
The technology to move and arrange atoms, the most basic component of a quantum computer, is very important to Rydberg quantum computing research. However, to place the atoms at the desired location, the atoms must be captured and transported one by one using a highly focused laser beam, commonly referred to as an optical tweezer. and, the quantum information of the atoms is likely to change midway.
KAIST (President Kwang Hyung Lee) announced on the 27th that a research team led by Professor Jaewook Ahn of the Department of Physics developed a technology to throw and receive rubidium atoms one by one using a laser beam.
The research team developed a method to throw and receive atoms which would minimize the time the optical tweezers are in contact with the atoms in which the quantum information the atoms carry may change. The research team used the characteristic that the rubidium atoms, which are kept at a very low temperature of 40μK below absolute zero, move very sensitively to the electromagnetic force applied by light along the focal point of the light tweezers.
The research team accelerated the laser of an optical tweezer to give an optical kick to an atom to send it to a target, then caught the flying atom with another optical tweezer to stop it. The atom flew at a speed of 65 cm/s, and traveled up to 4.2 μm. Compared to the existing technique of guiding the atoms with the optical tweezers, the technique of throwing and receiving atoms eliminates the need to calculate the transporting path for the tweezers, and makes it easier to fix the defects in the atomic arrangement. As a result, it is effective in generating and maintaining a large number of atomic arrangements, and when the technology is used to throw and receive flying atom qubits, it will be used in studying new and more powerful quantum computing methods that presupposes the structural changes in quantum arrangements.
"This technology will be used to develop larger and more powerful Rydberg quantum computers," says Professor Jaewook Ahn. “In a Rydberg quantum computer,” he continues, “atoms are arranged to store quantum information and interact with neighboring atoms through electromagnetic forces to perform quantum computing. The method of throwing an atom away for quick reconstruction the quantum array can be an effective way to fix an error in a quantum computer that requires a removal or replacement of an atom.”
The research, which was conducted by doctoral students Hansub Hwang and Andrew Byun of the Department of Physics at KAIST and Sylvain de Léséleuc, a researcher at the National Institute of Natural Sciences in Japan, was published in the international journal, Optica, 0n March 9th. (Paper title: Optical tweezers throw and catch single atoms).
This research was carried out with the support of the Samsung Science & Technology Foundation.
<Figure 1> A schematic diagram of the atom catching and throwing technique. The optical tweezer on the left kicks the atom to throw it into a trajectory to have the tweezer on the right catch it to stop it.
2023.03.28 View 7808
Using light to throw and catch atoms to open up a new chapter for quantum computing
The technology to move and arrange atoms, the most basic component of a quantum computer, is very important to Rydberg quantum computing research. However, to place the atoms at the desired location, the atoms must be captured and transported one by one using a highly focused laser beam, commonly referred to as an optical tweezer. and, the quantum information of the atoms is likely to change midway.
KAIST (President Kwang Hyung Lee) announced on the 27th that a research team led by Professor Jaewook Ahn of the Department of Physics developed a technology to throw and receive rubidium atoms one by one using a laser beam.
The research team developed a method to throw and receive atoms which would minimize the time the optical tweezers are in contact with the atoms in which the quantum information the atoms carry may change. The research team used the characteristic that the rubidium atoms, which are kept at a very low temperature of 40μK below absolute zero, move very sensitively to the electromagnetic force applied by light along the focal point of the light tweezers.
The research team accelerated the laser of an optical tweezer to give an optical kick to an atom to send it to a target, then caught the flying atom with another optical tweezer to stop it. The atom flew at a speed of 65 cm/s, and traveled up to 4.2 μm. Compared to the existing technique of guiding the atoms with the optical tweezers, the technique of throwing and receiving atoms eliminates the need to calculate the transporting path for the tweezers, and makes it easier to fix the defects in the atomic arrangement. As a result, it is effective in generating and maintaining a large number of atomic arrangements, and when the technology is used to throw and receive flying atom qubits, it will be used in studying new and more powerful quantum computing methods that presupposes the structural changes in quantum arrangements.
"This technology will be used to develop larger and more powerful Rydberg quantum computers," says Professor Jaewook Ahn. “In a Rydberg quantum computer,” he continues, “atoms are arranged to store quantum information and interact with neighboring atoms through electromagnetic forces to perform quantum computing. The method of throwing an atom away for quick reconstruction the quantum array can be an effective way to fix an error in a quantum computer that requires a removal or replacement of an atom.”
The research, which was conducted by doctoral students Hansub Hwang and Andrew Byun of the Department of Physics at KAIST and Sylvain de Léséleuc, a researcher at the National Institute of Natural Sciences in Japan, was published in the international journal, Optica, 0n March 9th. (Paper title: Optical tweezers throw and catch single atoms).
This research was carried out with the support of the Samsung Science & Technology Foundation.
<Figure 1> A schematic diagram of the atom catching and throwing technique. The optical tweezer on the left kicks the atom to throw it into a trajectory to have the tweezer on the right catch it to stop it.
2023.03.28 View 7808