-
 KAIST Holds the 2014 System on Chip (SoC) Robot War in August and October
Domestic and international competitions for robots with artificial intelligence are organized by Professor Hoi-Jun Yoo of Electrical Engineering.
KAIST will host two robot competitions this year: The Robot Integration Festival will be held in August at the Convention Center in Daejeon and the International Robot Contest in October at the Kintex in Ilsan. Participating robots are developed based on the System on Chip (SoC).
SoC robots refer to an autonomous robot that has a processor, a memory, peripheral devices, logic, and other system components combined on a single chip, which enables the robots to handle tasks and make decisions without human intervention.
The competitions include three entries: Taekwon Robot, HURO-competition, and SoC Drone which was added for the first time this year.
The Taekwon Robot involves a one-on-one sparring match, using a Korean traditional martial art, between two robots. Competitors score points based on front and side kicks, as well as punching. The HURO-competition pits robots in a competition to perform assignments such as hurdling, barricade clearing, crossing bridges, and overcoming other obstacles. The SoC Drone evaluates robots' capability to track miniature cars and navigate between buildings while in flight. The drone should have two cameras and a SoC brainboard equipped to offer autonomous, remote-controlled flight.
The director of the competitions, Professor Hoi-Jun Yoo of Electrical Engineering at KAIST, commented that with the integration of Korea’s world-class semiconductor technology, the competitions would lead to improvements in robotics engineering and unmanned aerial vehicle technology.
The competitions are open to anyone interested in SoC robots and unmanned aerial vehicles. For more information about the competitions, please visit http://www.socrobotwar.org . The application deadline is April 15, 2014.
2014.03.11 View 12550
KAIST Holds the 2014 System on Chip (SoC) Robot War in August and October
Domestic and international competitions for robots with artificial intelligence are organized by Professor Hoi-Jun Yoo of Electrical Engineering.
KAIST will host two robot competitions this year: The Robot Integration Festival will be held in August at the Convention Center in Daejeon and the International Robot Contest in October at the Kintex in Ilsan. Participating robots are developed based on the System on Chip (SoC).
SoC robots refer to an autonomous robot that has a processor, a memory, peripheral devices, logic, and other system components combined on a single chip, which enables the robots to handle tasks and make decisions without human intervention.
The competitions include three entries: Taekwon Robot, HURO-competition, and SoC Drone which was added for the first time this year.
The Taekwon Robot involves a one-on-one sparring match, using a Korean traditional martial art, between two robots. Competitors score points based on front and side kicks, as well as punching. The HURO-competition pits robots in a competition to perform assignments such as hurdling, barricade clearing, crossing bridges, and overcoming other obstacles. The SoC Drone evaluates robots' capability to track miniature cars and navigate between buildings while in flight. The drone should have two cameras and a SoC brainboard equipped to offer autonomous, remote-controlled flight.
The director of the competitions, Professor Hoi-Jun Yoo of Electrical Engineering at KAIST, commented that with the integration of Korea’s world-class semiconductor technology, the competitions would lead to improvements in robotics engineering and unmanned aerial vehicle technology.
The competitions are open to anyone interested in SoC robots and unmanned aerial vehicles. For more information about the competitions, please visit http://www.socrobotwar.org . The application deadline is April 15, 2014.
2014.03.11 View 12550 -
 Book Announcement: Sound Visualization and Manipulation
The movie
Gravity
won seven Oscar awards this year, one of which was for its outstanding 3D sound mixing, immersing viewers in the full experience of the troubled space expedition.
3D audio effects are generated by manipulating the sound produced by speakers, speaker-arrays, or headphones to place a virtual sound source at a desired location in 3D space such as behind, above, or below the listener's head.
Two professors from the Department of Mechanical Engineering at KAIST have recently published a book that explains two important technologies related to 3D sound effects: sound visualization and manipulation.
Professor Yang-Hann Kim, an eminent scholar in sound engineering, and Professor Jung-Woo Choi collaborated to write Sound Visualization and Manipulation (Wily 2013), which uniquely addresses the two most important problems in the field in a unified way.
The book introduces general concepts and theories and describes a number of techniques in sound visualization and manipulation, offering an interrelated approach to two very different topics: sound field visualization techniques based on microphone arrays and controlled sound field generation techniques using loudspeaker arrays.
The authors also display a solid understanding of the associated physical and mathematical concepts applied to solve the visualization and manipulation problems and provide extensive examples demonstrating the benefits and drawbacks of various applications, including beamforming and acoustic holography technology.
The book will be an excellent reference for graduate students, researchers, and professionals in acoustic engineering, as well as in audio and noise control system development.
For detailed descriptions of the book:
http://as.wiley.com/WileyCDA/WileyTitle/productCd-1118368479.html
2014.03.10 View 16691
Book Announcement: Sound Visualization and Manipulation
The movie
Gravity
won seven Oscar awards this year, one of which was for its outstanding 3D sound mixing, immersing viewers in the full experience of the troubled space expedition.
3D audio effects are generated by manipulating the sound produced by speakers, speaker-arrays, or headphones to place a virtual sound source at a desired location in 3D space such as behind, above, or below the listener's head.
Two professors from the Department of Mechanical Engineering at KAIST have recently published a book that explains two important technologies related to 3D sound effects: sound visualization and manipulation.
Professor Yang-Hann Kim, an eminent scholar in sound engineering, and Professor Jung-Woo Choi collaborated to write Sound Visualization and Manipulation (Wily 2013), which uniquely addresses the two most important problems in the field in a unified way.
The book introduces general concepts and theories and describes a number of techniques in sound visualization and manipulation, offering an interrelated approach to two very different topics: sound field visualization techniques based on microphone arrays and controlled sound field generation techniques using loudspeaker arrays.
The authors also display a solid understanding of the associated physical and mathematical concepts applied to solve the visualization and manipulation problems and provide extensive examples demonstrating the benefits and drawbacks of various applications, including beamforming and acoustic holography technology.
The book will be an excellent reference for graduate students, researchers, and professionals in acoustic engineering, as well as in audio and noise control system development.
For detailed descriptions of the book:
http://as.wiley.com/WileyCDA/WileyTitle/productCd-1118368479.html
2014.03.10 View 16691 -
 K-Glass: Korea's Answer to Google Glass
Wall Street Journal (blog) published an
article on the K-Glass developed by Professor Hoi-Jun Yoo of Electrical
Engineering at KAIST. For the article, please go to the link below:
K-Glass: Korea’s Answer to Google Glass, March 5, 2014
http://blogs.wsj.com/digits/2014/03/05/meet-k-glass-koreas-answer-to-google-glass/
2014.03.07 View 9130
K-Glass: Korea's Answer to Google Glass
Wall Street Journal (blog) published an
article on the K-Glass developed by Professor Hoi-Jun Yoo of Electrical
Engineering at KAIST. For the article, please go to the link below:
K-Glass: Korea’s Answer to Google Glass, March 5, 2014
http://blogs.wsj.com/digits/2014/03/05/meet-k-glass-koreas-answer-to-google-glass/
2014.03.07 View 9130 -
 Times Higher Education 2014 World Reputation Rankings
Times Higher Education released the 2014 World Reputation Rankings on March 6, 2014. KAIST moved from the 61-70 band in 2013 to the 51-60 place this year. For details, please visit the link below:
http://www.scoop.co.nz/stories/WO1403/S00091/times-higher-education-2014-world-reputation-rankings.htm
2014.03.07 View 8640
Times Higher Education 2014 World Reputation Rankings
Times Higher Education released the 2014 World Reputation Rankings on March 6, 2014. KAIST moved from the 61-70 band in 2013 to the 51-60 place this year. For details, please visit the link below:
http://www.scoop.co.nz/stories/WO1403/S00091/times-higher-education-2014-world-reputation-rankings.htm
2014.03.07 View 8640 -
 The 4th Meeting of Korea and Denmark Alliance for Green Growth
President Steve Kang attended the “Fourth Meeting of Korea and Denmark Alliance for Green Growth” which took place on March 6, 2014 at the Shilla Hotel in Seoul. President Kang was a keynote speaker at the meeting and gave a lecture on sustainable energy.
KAIST and the Technical University of Denmark (DTU) signed a memorandum of understanding (MOU) on the “Cooperation for Innovation and Entrepreneurship” at the meeting.
In the MOU, KAIST and DTU agreed to post the information on their websites regarding the patents acquired through the implementation of joint research programs. In addition, KAIST students will attend conferences and idea competitions organized by DTU, e.g., the Green Challenges. DTU students will participate in KAIST’s conferences and competitions including “Startup KAIST Global Idea Competition.”
2014.03.07 View 9524
The 4th Meeting of Korea and Denmark Alliance for Green Growth
President Steve Kang attended the “Fourth Meeting of Korea and Denmark Alliance for Green Growth” which took place on March 6, 2014 at the Shilla Hotel in Seoul. President Kang was a keynote speaker at the meeting and gave a lecture on sustainable energy.
KAIST and the Technical University of Denmark (DTU) signed a memorandum of understanding (MOU) on the “Cooperation for Innovation and Entrepreneurship” at the meeting.
In the MOU, KAIST and DTU agreed to post the information on their websites regarding the patents acquired through the implementation of joint research programs. In addition, KAIST students will attend conferences and idea competitions organized by DTU, e.g., the Green Challenges. DTU students will participate in KAIST’s conferences and competitions including “Startup KAIST Global Idea Competition.”
2014.03.07 View 9524 -
 KAIST Holds Open Lecture For Daejeon Residents
Free of cost for any Korean citizen, the registration for the new course opens on the official website from 5th March KAIST’s Department of Humanities and Social Science is currently operating free humanities and liberal arts classes for Daejeon residents.
The theme of the course for this semester is “World and Politics,” which will begin on 13th March and run every Thursday for 6 weeks at KAIST’s International Seminar Room.
This course has been organized to introduce the general public to the current political situation with neighboring countries such as China, Japan and North Korea, as well as the characteristics of multinational companies.
Top experts in the related fields will give lectures. First, Professor Ha-Yong Jung from Kyunghee University will talk on “American liberalism and democracy”; Professor Gyeong-Mo An from Korea National Defense University on “Kim Jeong-Eun and the Future of North Korea--Is the Collapse of North Korea A Reality?” and Ja-Seon Koo, a visiting professor at Korea National Diplomatic Academy on “The Chinese Communist Party during the Xi Jinping Period.”
“With the era of globalization, the political situations in the neighboring countries have both direct and indirect effects on our lives,” said Professor Hyeon-Seok Park who has organized the courses. "These classes will be an opportunity for our citizens to understand and learn about the current affairs in the world.”
Anyone can attend the course, and registration is from March 5th to 9th at the official webpage of KAIST’s Humanities and Social Sciences Department (http://hss.kaist.ac.kr). All the courses are free of charge.
Contact: Department of Humanities and Social Science Research (Tel. 350-4687, E-mail: baobab@kaist.ac.kr)
2014.03.06 View 7767
KAIST Holds Open Lecture For Daejeon Residents
Free of cost for any Korean citizen, the registration for the new course opens on the official website from 5th March KAIST’s Department of Humanities and Social Science is currently operating free humanities and liberal arts classes for Daejeon residents.
The theme of the course for this semester is “World and Politics,” which will begin on 13th March and run every Thursday for 6 weeks at KAIST’s International Seminar Room.
This course has been organized to introduce the general public to the current political situation with neighboring countries such as China, Japan and North Korea, as well as the characteristics of multinational companies.
Top experts in the related fields will give lectures. First, Professor Ha-Yong Jung from Kyunghee University will talk on “American liberalism and democracy”; Professor Gyeong-Mo An from Korea National Defense University on “Kim Jeong-Eun and the Future of North Korea--Is the Collapse of North Korea A Reality?” and Ja-Seon Koo, a visiting professor at Korea National Diplomatic Academy on “The Chinese Communist Party during the Xi Jinping Period.”
“With the era of globalization, the political situations in the neighboring countries have both direct and indirect effects on our lives,” said Professor Hyeon-Seok Park who has organized the courses. "These classes will be an opportunity for our citizens to understand and learn about the current affairs in the world.”
Anyone can attend the course, and registration is from March 5th to 9th at the official webpage of KAIST’s Humanities and Social Sciences Department (http://hss.kaist.ac.kr). All the courses are free of charge.
Contact: Department of Humanities and Social Science Research (Tel. 350-4687, E-mail: baobab@kaist.ac.kr)
2014.03.06 View 7767 -
 Seo-Eun Lee, an undergaruate student receives the Best Paper Award from Optical Society of Korea
Seo-Eun Lee, a student studying at KAIST’s Department of Biological Sciences, has won the Best Paper Award from Bio-Photonics Division at the 2014 Optical Society of Korea Winter Conference, held on 19th February at Daejeon Convention Center.
Only one outstanding paper per division is given an award among the total of 270 papers, and it is very unusual for an undergraduate student to win the award in the field that is not her major.
Lee has studied cell imaging using holography technology since June 2013 under the supervision of Professor Yong-Geun Park from the Department of Physics.
The Optical Society of Korea was founded in 1989, and as the largest academy in the field of optics in Korea, it holds academic presentations, seminars and lectures every year.
2014.03.06 View 12015
Seo-Eun Lee, an undergaruate student receives the Best Paper Award from Optical Society of Korea
Seo-Eun Lee, a student studying at KAIST’s Department of Biological Sciences, has won the Best Paper Award from Bio-Photonics Division at the 2014 Optical Society of Korea Winter Conference, held on 19th February at Daejeon Convention Center.
Only one outstanding paper per division is given an award among the total of 270 papers, and it is very unusual for an undergraduate student to win the award in the field that is not her major.
Lee has studied cell imaging using holography technology since June 2013 under the supervision of Professor Yong-Geun Park from the Department of Physics.
The Optical Society of Korea was founded in 1989, and as the largest academy in the field of optics in Korea, it holds academic presentations, seminars and lectures every year.
2014.03.06 View 12015 -
 Welcoming the Class of 2014
“The four years from today will go quickly, and I urge you to make the most of your time in KAIST, a great educational and research institution where you will explore the frontiers of science and technology and take part in the creation of new knowledge,” President Kang told the freshmen at the convocation ceremony.
Freshmen Convocation for the Class of 2014 took place on March 3, 2014 at the auditorium on the main campus. Members of the KAIST community, along with hundreds of parents and guests, welcomed the incoming 800 freshmen, celebrating the beginning of their four-year college life.
Kwang-Joon Ahn, a graduate of the Korea Science Academy, and Ha-Rim Jin, a graduate of Daegu Il Science High School, were representatives of the incoming students, and they took the “Class of 2014 Pledge,” a commitment to uphold KAIST’s core values, which is "creativity and challenge (endeavoring spirit)," and to pursue intellectual passion and discovery.
President Steve Kang delivered congratulatory remarks, encouraging students to use their opportunities to the fullest while at KAIST to broaden their knowledge and experience. He also stressed the following four important principles they should cultivate to become the leaders of tomorrow: be grateful, excel in their field, keep open minds about what the globalized world would bring, and never give up on their dreams and belief.
President Kang said:
“Probably, many of you, the graduates of the best high schools in Korea, will find KAIST a tougher place to be in than you imagined. But challenges, particularly intellectual challenges, should be viewed as an opportunity to grow. It is ok to fail. In fact, without risking failures, there won’t be a meaningful growth because the real growth comes from overcoming challenges.”
“You can’t avoid failing in the course of your college life, but your perseverance to do it over will allow you to develop the skills and passion needed to become a leader who will contribute to the local community, as well as to the betterment of humanity.”
The KAIST Alumni Scholarship Foundation presented a scholarship of USD 3,700 to 24 freshmen.
The convocation ended with music performances by members of the student clubs at KAIST.
2014.03.04 View 10026
Welcoming the Class of 2014
“The four years from today will go quickly, and I urge you to make the most of your time in KAIST, a great educational and research institution where you will explore the frontiers of science and technology and take part in the creation of new knowledge,” President Kang told the freshmen at the convocation ceremony.
Freshmen Convocation for the Class of 2014 took place on March 3, 2014 at the auditorium on the main campus. Members of the KAIST community, along with hundreds of parents and guests, welcomed the incoming 800 freshmen, celebrating the beginning of their four-year college life.
Kwang-Joon Ahn, a graduate of the Korea Science Academy, and Ha-Rim Jin, a graduate of Daegu Il Science High School, were representatives of the incoming students, and they took the “Class of 2014 Pledge,” a commitment to uphold KAIST’s core values, which is "creativity and challenge (endeavoring spirit)," and to pursue intellectual passion and discovery.
President Steve Kang delivered congratulatory remarks, encouraging students to use their opportunities to the fullest while at KAIST to broaden their knowledge and experience. He also stressed the following four important principles they should cultivate to become the leaders of tomorrow: be grateful, excel in their field, keep open minds about what the globalized world would bring, and never give up on their dreams and belief.
President Kang said:
“Probably, many of you, the graduates of the best high schools in Korea, will find KAIST a tougher place to be in than you imagined. But challenges, particularly intellectual challenges, should be viewed as an opportunity to grow. It is ok to fail. In fact, without risking failures, there won’t be a meaningful growth because the real growth comes from overcoming challenges.”
“You can’t avoid failing in the course of your college life, but your perseverance to do it over will allow you to develop the skills and passion needed to become a leader who will contribute to the local community, as well as to the betterment of humanity.”
The KAIST Alumni Scholarship Foundation presented a scholarship of USD 3,700 to 24 freshmen.
The convocation ended with music performances by members of the student clubs at KAIST.
2014.03.04 View 10026 -
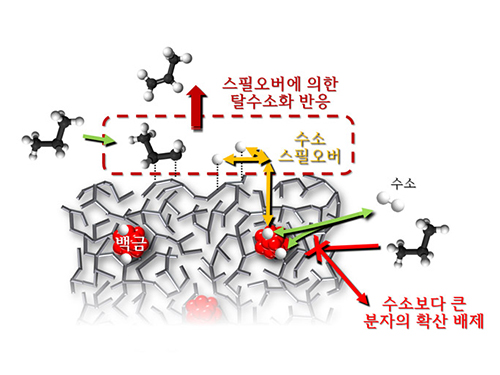 Spillover Phenomenon Identified Using Model Catalyst System
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 12195
Spillover Phenomenon Identified Using Model Catalyst System
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 12195 -
 Seung-Han Lee, a doctoral student in electrical engineering, receives the best paper award from ISQED 2014
Seung-Han
Lee, a doctoral candidate in the department of electrical engineering at KAIST,
received a Best Paper Award from the International Symposium on Quality
Electronic Design (ISQED), a high-profile international conference started in 2000 to promote innovation and quality in electronic and
engineering designs through inter- and multidisciplinary approaches. The
award ceremony will take place at the 2014 ISQED on March 3-5, 2014 at the Convention
Center in Santa Clara, CA, USA.
Professor
Chong-Min Kyung, an advisor to Seung-Han, expressed his excitement about his student's achievement.
“This is
the first time a Korean has ever received the best paper award at this academic
conference. It’s great news to our student as well as to KAIST.”
The topic
of Lee’s research paper was dynamic cache data management for minimizing the
energy consumption of three-dimensional multi-processor semiconductor chips.
2014.03.03 View 13137
Seung-Han Lee, a doctoral student in electrical engineering, receives the best paper award from ISQED 2014
Seung-Han
Lee, a doctoral candidate in the department of electrical engineering at KAIST,
received a Best Paper Award from the International Symposium on Quality
Electronic Design (ISQED), a high-profile international conference started in 2000 to promote innovation and quality in electronic and
engineering designs through inter- and multidisciplinary approaches. The
award ceremony will take place at the 2014 ISQED on March 3-5, 2014 at the Convention
Center in Santa Clara, CA, USA.
Professor
Chong-Min Kyung, an advisor to Seung-Han, expressed his excitement about his student's achievement.
“This is
the first time a Korean has ever received the best paper award at this academic
conference. It’s great news to our student as well as to KAIST.”
The topic
of Lee’s research paper was dynamic cache data management for minimizing the
energy consumption of three-dimensional multi-processor semiconductor chips.
2014.03.03 View 13137 -
 Quacquarelli Symonds (QS) World University Rankings by Subject 2014
The QS
World University Rankings are annual university rankings published by
Quacquarelli Symonds (QS) which provides the overall rankings of top global
universities as well as the rankings for individual subjects. The 2014 QS World
University Rankings by Subject is a comprehensive guide to the world’s best universities
in 30 popular subjects of 5 academic disciplines: arts & humanities,
engineering & technology, life sciences & medicine, natural sciences,
and social sciences.
According
to the 2014 subject rankings, released on February 26, KAIST made the list of top
50 universities in 9 subjects: physics & astronomy; materials sciences;
chemistry; chemical engineering; mechanical, aeronautical & manufacturing
engineering; electrical & electronic engineering; civil & structural engineering;
computer science & information systems; and biological sciences.
Among
them, KAIST was ranked number one in Korea for 5 subjects: materials sciences
(16th); mechanical, aeronautical & manufacturing engineering (21st);
civil & structural engineering (32nd); computer science &
information systems (36th), and biological sciences (43rd).
For basic sciences, KAIST has made good progress as well. For example, in
mathematics, KAIST took first place in Korea and was ranked in the 51st-100th
of the world’s top universities. Another notable result was that its business college
in Seoul campus, a relatively new addition to KAIST, made the rankings list of
51st-100th in accounting & finance.
The 2014
QS subject rankings used the following criteria for its evaluation of
university performance: a survey of academic and employer reputation, citations
per paper, inclusion of specialists, and the h-index, known as the Hirsch index or Hirsch number, which was
suggested by Jorge E. Hirsch, a physicist at the University of California in
San Diego, as a tool for determining theoretical physicists’ relative quality.
Today, the h-index is used to measure
both the productivity and impact of the published work of a scientist or
scholar.
2014.02.28 View 12454
Quacquarelli Symonds (QS) World University Rankings by Subject 2014
The QS
World University Rankings are annual university rankings published by
Quacquarelli Symonds (QS) which provides the overall rankings of top global
universities as well as the rankings for individual subjects. The 2014 QS World
University Rankings by Subject is a comprehensive guide to the world’s best universities
in 30 popular subjects of 5 academic disciplines: arts & humanities,
engineering & technology, life sciences & medicine, natural sciences,
and social sciences.
According
to the 2014 subject rankings, released on February 26, KAIST made the list of top
50 universities in 9 subjects: physics & astronomy; materials sciences;
chemistry; chemical engineering; mechanical, aeronautical & manufacturing
engineering; electrical & electronic engineering; civil & structural engineering;
computer science & information systems; and biological sciences.
Among
them, KAIST was ranked number one in Korea for 5 subjects: materials sciences
(16th); mechanical, aeronautical & manufacturing engineering (21st);
civil & structural engineering (32nd); computer science &
information systems (36th), and biological sciences (43rd).
For basic sciences, KAIST has made good progress as well. For example, in
mathematics, KAIST took first place in Korea and was ranked in the 51st-100th
of the world’s top universities. Another notable result was that its business college
in Seoul campus, a relatively new addition to KAIST, made the rankings list of
51st-100th in accounting & finance.
The 2014
QS subject rankings used the following criteria for its evaluation of
university performance: a survey of academic and employer reputation, citations
per paper, inclusion of specialists, and the h-index, known as the Hirsch index or Hirsch number, which was
suggested by Jorge E. Hirsch, a physicist at the University of California in
San Diego, as a tool for determining theoretical physicists’ relative quality.
Today, the h-index is used to measure
both the productivity and impact of the published work of a scientist or
scholar.
2014.02.28 View 12454 -
 Festival Featuring Asia's Best Science Students to be Held
The first Electronic Olympics, which will host students from five top Asian research-centered universities, will be held in August at KAIST. Students will take part in competitive events and explore cultural diversity. Student representatives of HKUST, NTU, TITECH, Tsinghua University, and KAIST gathered on February 20 to begin planning the tentatively named “ASPIRE E-Olympics.”
The key words of this Olympics are "Harmony" and "Competition." The events will be composed of an AI programming contest, SEM (Scanning Electron Microscope) picture contest, and the other technology-based contests. Cultural events, where each university’s students can interact, will also be prepared.
ASPIRE (Asian Science and Technology Pioneering Institutes of Research and Education) events have been held from 2009. Previously, the ASPIRE forum has been an exchange event for groups of vice presidents and graduate school students from the five schools to exchange achievements in education and research. This year, it has been extended to undergraduates.
Yoseop Kim, KAIST’s student body vice president, said that he wants to make a MOU with some of Asia’s best research-centered universities and develop it into something similar to the Davos Forum. His intention is to support the E-Olympics in the hope that ASPIRE will become a top university consortium.
From left, HKUST, KAIST, NTU, TITECH, Tsinghua University Logos
Student representative group photo of Top Asian Research-Centered Universities
Electronic Olympics for students from five top Asian science and engineering universities to be held in August
2014.02.27 View 10154
Festival Featuring Asia's Best Science Students to be Held
The first Electronic Olympics, which will host students from five top Asian research-centered universities, will be held in August at KAIST. Students will take part in competitive events and explore cultural diversity. Student representatives of HKUST, NTU, TITECH, Tsinghua University, and KAIST gathered on February 20 to begin planning the tentatively named “ASPIRE E-Olympics.”
The key words of this Olympics are "Harmony" and "Competition." The events will be composed of an AI programming contest, SEM (Scanning Electron Microscope) picture contest, and the other technology-based contests. Cultural events, where each university’s students can interact, will also be prepared.
ASPIRE (Asian Science and Technology Pioneering Institutes of Research and Education) events have been held from 2009. Previously, the ASPIRE forum has been an exchange event for groups of vice presidents and graduate school students from the five schools to exchange achievements in education and research. This year, it has been extended to undergraduates.
Yoseop Kim, KAIST’s student body vice president, said that he wants to make a MOU with some of Asia’s best research-centered universities and develop it into something similar to the Davos Forum. His intention is to support the E-Olympics in the hope that ASPIRE will become a top university consortium.
From left, HKUST, KAIST, NTU, TITECH, Tsinghua University Logos
Student representative group photo of Top Asian Research-Centered Universities
Electronic Olympics for students from five top Asian science and engineering universities to be held in August
2014.02.27 View 10154