BIO
-
 13 KAIST Faculty Named as Inaugural Members of Y-KAST
The Korean Academy of Science and Technology (KAST) launched the Young Korean Academy of Science and Technology (Y-KAST) and selected 73 scientists as its inaugural members on February 24. Among them, 13 KAIST faculty were recognized as the inaugural members of Y-KAST.
Y-KAIST, made up of distinguished mid-career scientists under the age of 45, will take the leading role in international collaboration as well as innovative agenda-making in science and technology.
The inaugural members include Professor Hyotcherl Ihee of the Department of Chemistry and Dr. Sung-Jin Oh of the Center for Mathematical Challenges at the Korea Institute for Advanced Study (KIAS), affiliated with KAIST. Professor Ihee is gaining wide acclaim in the fields of physics and chemistry, and in 2016, Dr. Oh was the youngest ever awardee of the Presidential Award of Young Scientist.
The other Y-KAIST members are as follows: Professors Haeshin Lee of the Department of Chemistry; Mi Young Kim, Byung-Kwan Cho, and Ji-Joon Song of the Department of Biological Sciences; Song-Yong Kim of the Department of Mechanical Engineering; Sang-il Oum of the Department of Mathematical Sciences; Jung Kyoon Choi of the Department of Bio and Brain Engineering; Seokwoo Jeon, Sang Ouk Kim, and Il-Doo Kim of the Department of Materials Science and Engineering; Jang Wook Choi of the Graduate School of EEWS (Energy, Environment, Water and Sustainability); and Jeong Ho Lee of the Graduate School of Medical Science and Engineering.
The leading countries of the Academy of Science, which include Germany, Sweden, Belgium, Canada, and Japan, have established the Young Academy of Science since 2010 in order to encourage the research activities of their young scientists and to establish a global platform for collaborative research projects through their active networking at home and abroad.
President Myung-Chul Lee of KAST said, “We will spare no effort to connect these outstanding mid-career researchers for their future collaboration. Their networking will make significant impacts toward their own research activities as well as the global stature of Korea’s science and technology R&D.
(Photo caption: Members of Y-KAST pose at the inaugural ceremony of Y-KAST on February 24.)
2017.03.02 View 19734
13 KAIST Faculty Named as Inaugural Members of Y-KAST
The Korean Academy of Science and Technology (KAST) launched the Young Korean Academy of Science and Technology (Y-KAST) and selected 73 scientists as its inaugural members on February 24. Among them, 13 KAIST faculty were recognized as the inaugural members of Y-KAST.
Y-KAIST, made up of distinguished mid-career scientists under the age of 45, will take the leading role in international collaboration as well as innovative agenda-making in science and technology.
The inaugural members include Professor Hyotcherl Ihee of the Department of Chemistry and Dr. Sung-Jin Oh of the Center for Mathematical Challenges at the Korea Institute for Advanced Study (KIAS), affiliated with KAIST. Professor Ihee is gaining wide acclaim in the fields of physics and chemistry, and in 2016, Dr. Oh was the youngest ever awardee of the Presidential Award of Young Scientist.
The other Y-KAIST members are as follows: Professors Haeshin Lee of the Department of Chemistry; Mi Young Kim, Byung-Kwan Cho, and Ji-Joon Song of the Department of Biological Sciences; Song-Yong Kim of the Department of Mechanical Engineering; Sang-il Oum of the Department of Mathematical Sciences; Jung Kyoon Choi of the Department of Bio and Brain Engineering; Seokwoo Jeon, Sang Ouk Kim, and Il-Doo Kim of the Department of Materials Science and Engineering; Jang Wook Choi of the Graduate School of EEWS (Energy, Environment, Water and Sustainability); and Jeong Ho Lee of the Graduate School of Medical Science and Engineering.
The leading countries of the Academy of Science, which include Germany, Sweden, Belgium, Canada, and Japan, have established the Young Academy of Science since 2010 in order to encourage the research activities of their young scientists and to establish a global platform for collaborative research projects through their active networking at home and abroad.
President Myung-Chul Lee of KAST said, “We will spare no effort to connect these outstanding mid-career researchers for their future collaboration. Their networking will make significant impacts toward their own research activities as well as the global stature of Korea’s science and technology R&D.
(Photo caption: Members of Y-KAST pose at the inaugural ceremony of Y-KAST on February 24.)
2017.03.02 View 19734 -
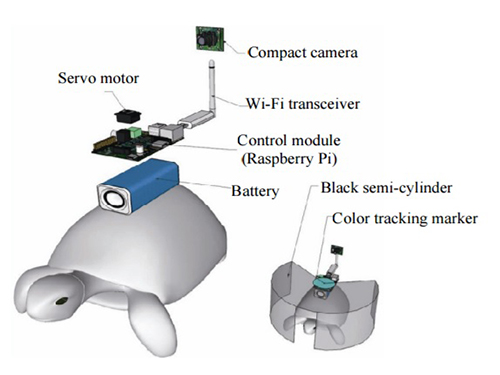 Controlling Turtle Motion with Human Thought
KAIST researchers have developed a technology that can remotely control an animal’s movement with human thought.
In the 2009 blockbuster “Avatar,” a human remotely controls the body of an alien. It does so by injecting human intelligence into a remotely located, biological body. Although still in the realm of science fiction, researchers are nevertheless developing so-called ‘brain-computer interfaces’ (BCIs) following recent advances in electronics and computing. These technologies can ‘read’ and use human thought to control machines, for example, humanoid robots.
New research has demonstrated the possibility of combining a BCI with a device that transmits information from a computer to a brain, or known as a ‘computer-to-brain interface’ (CBI). The combination of these devices could be used to establish a functional link between the brains of different species. Now, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have developed a human-turtle interaction system in which a signal originating from a human brain can affect where a turtle moves.
Unlike previous research that has tried to control animal movement by applying invasive methods, most notably in insects, Professors Phill-Seung Lee of the Mechanical Engineering Department and Sungho Jo of the Computing School propose a conceptual system that can guide an animal’s moving path by controlling its instinctive escape behavior. They chose a turtle because of its cognitive abilities as well as its ability to distinguish different wavelengths of light. Specifically, turtles can recognize a white light source as an open space and so move toward it. They also show specific avoidance behavior to things that might obstruct their view. Turtles also move toward and away from obstacles in their environment in a predictable manner. It was this instinctive, predictable behavior that the researchers induced using the BCI.
The entire human-turtle setup is as follows: A head-mounted display (HMD) is combined with a BCI to immerse the human user in the turtle’s environment. The human operator wears the BCI-HMD system, while the turtle has a 'cyborg system'—consisting of a camera, Wi-Fi transceiver, computer control module, and battery—all mounted on the turtle’s upper shell. Also included on the turtle’s shell is a black semi-cylinder with a slit, which forms the ‘stimulation device.’ This can be turned ±36 degrees via the BCI.
The entire process works like this: the human operator receives images from the camera mounted on the turtle. These real-time video images allow the human operator to decide where the turtle should move. The human provides thought commands that are recognized by the wearable BCI system as electroencephalography (EEG) signals. The BCI can distinguish between three mental states: left, right, and idle. The left and right commands activate the turtle’s stimulation device via Wi-Fi, turning it so that it obstructs the turtle’s view. This invokes its natural instinct to move toward light and change its direction. Finally, the human acquires updated visual feedback from the camera mounted on the shell and in this way continues to remotely navigate the turtle’s trajectory.
The research demonstrates that the animal guiding scheme via BCI can be used in a variety of environments with turtles moving indoors and outdoors on many different surfaces, like gravel and grass, and tackling a range of obstacles, such as shallow water and trees. This technology could be developed to integrate positioning systems and improved augmented and virtual reality techniques, enabling various applications, including devices for military reconnaissance and surveillance.
***
Reference: “Remote Navigation of Turtle by Controlling Instinct Behavior via Human Brain-computer Interface,” Journal of Bionic Engineering, July 2016 (DOI: 10.1016/S1672-6529(16)60322-0)
Depiction of Cyborg System
A human controller influences the turtle’s escape behavior by sending left and right signals via Wi-Fi to a control system on the back of the turtle.
2017.02.21 View 16192
Controlling Turtle Motion with Human Thought
KAIST researchers have developed a technology that can remotely control an animal’s movement with human thought.
In the 2009 blockbuster “Avatar,” a human remotely controls the body of an alien. It does so by injecting human intelligence into a remotely located, biological body. Although still in the realm of science fiction, researchers are nevertheless developing so-called ‘brain-computer interfaces’ (BCIs) following recent advances in electronics and computing. These technologies can ‘read’ and use human thought to control machines, for example, humanoid robots.
New research has demonstrated the possibility of combining a BCI with a device that transmits information from a computer to a brain, or known as a ‘computer-to-brain interface’ (CBI). The combination of these devices could be used to establish a functional link between the brains of different species. Now, researchers from the Korea Advanced Institute of Science and Technology (KAIST) have developed a human-turtle interaction system in which a signal originating from a human brain can affect where a turtle moves.
Unlike previous research that has tried to control animal movement by applying invasive methods, most notably in insects, Professors Phill-Seung Lee of the Mechanical Engineering Department and Sungho Jo of the Computing School propose a conceptual system that can guide an animal’s moving path by controlling its instinctive escape behavior. They chose a turtle because of its cognitive abilities as well as its ability to distinguish different wavelengths of light. Specifically, turtles can recognize a white light source as an open space and so move toward it. They also show specific avoidance behavior to things that might obstruct their view. Turtles also move toward and away from obstacles in their environment in a predictable manner. It was this instinctive, predictable behavior that the researchers induced using the BCI.
The entire human-turtle setup is as follows: A head-mounted display (HMD) is combined with a BCI to immerse the human user in the turtle’s environment. The human operator wears the BCI-HMD system, while the turtle has a 'cyborg system'—consisting of a camera, Wi-Fi transceiver, computer control module, and battery—all mounted on the turtle’s upper shell. Also included on the turtle’s shell is a black semi-cylinder with a slit, which forms the ‘stimulation device.’ This can be turned ±36 degrees via the BCI.
The entire process works like this: the human operator receives images from the camera mounted on the turtle. These real-time video images allow the human operator to decide where the turtle should move. The human provides thought commands that are recognized by the wearable BCI system as electroencephalography (EEG) signals. The BCI can distinguish between three mental states: left, right, and idle. The left and right commands activate the turtle’s stimulation device via Wi-Fi, turning it so that it obstructs the turtle’s view. This invokes its natural instinct to move toward light and change its direction. Finally, the human acquires updated visual feedback from the camera mounted on the shell and in this way continues to remotely navigate the turtle’s trajectory.
The research demonstrates that the animal guiding scheme via BCI can be used in a variety of environments with turtles moving indoors and outdoors on many different surfaces, like gravel and grass, and tackling a range of obstacles, such as shallow water and trees. This technology could be developed to integrate positioning systems and improved augmented and virtual reality techniques, enabling various applications, including devices for military reconnaissance and surveillance.
***
Reference: “Remote Navigation of Turtle by Controlling Instinct Behavior via Human Brain-computer Interface,” Journal of Bionic Engineering, July 2016 (DOI: 10.1016/S1672-6529(16)60322-0)
Depiction of Cyborg System
A human controller influences the turtle’s escape behavior by sending left and right signals via Wi-Fi to a control system on the back of the turtle.
2017.02.21 View 16192 -
 Mystery of Biological Plastic Synthesis Machinery Unveiled
Plastics and other polymers are used every day. These polymers are mostly made from fossil resources by refining petrochemicals. On the other hand, many microorganisms naturally synthesize polyesters known as polyhydroxyalkanoates (PHAs) as distinct granules inside cells.
PHAs are a family of microbial polyesters that have attracted much attention as biodegradable and biocompatible plastics and elastomers that can substitute petrochemical counterparts. There have been numerous papers and patents on gene cloning and metabolic engineering of PHA biosynthetic machineries, biochemical studies, and production of PHAs; simple Google search with “polyhydroxyalkanoates” yielded returns of 223,000 document pages. PHAs have always been considered amazing examples of biological polymer synthesis. It is astounding to see PHAs of 500 kDa to sometimes as high as 10,000 kDa can be synthesized in vivo by PHA synthase, the key polymerizing enzyme in PHA biosynthesis. They have attracted great interest in determining the crystal structure of PHA synthase over the last 30 years, but unfortunately without success. Thus, the characteristics and molecular mechanisms of PHA synthase were under a dark veil.
In two papers published back-to-back in Biotechnology Journal online on November 30, 2016, a Korean research team led by Professor Kyung-Jin Kim at Kyungpook National University and Distinguished Professor Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) described the crystal structure of PHA synthase from Ralstonia eutropha, the best studied bacterium for PHA production, and reported the structural basis for the detailed molecular mechanisms of PHA biosynthesis. The crystal structure has been deposited to Protein Data Bank in February 2016. After deciphering the crystal structure of the catalytic domain of PHA synthase, in addition to other structural studies on whole enzyme and related proteins, the research team also performed experiments to elucidate the mechanisms of the enzyme reaction, validating detailed structures, enzyme engineering, and also N-terminal domain studies among others.
Through several biochemical studies based on crystal structure, the authors show that PHA synthase exists as a dimer and is divided into two distinct domains, the N-terminal domain (RePhaC1ND) and the C-terminal domain (RePhaC1CD). The RePhaC1CD catalyzes the polymerization reaction via a non-processive ping-pong mechanism using a Cys-His-Asp catalytic triad. The two catalytic sites of the RePhaC1CD dimer are positioned 33.4 Å apart, suggesting that the polymerization reaction occurs independently at each site. This study also presents the structure-based mechanisms for substrate specificities of various PHA synthases from different classes.
Professor Sang Yup Lee, who has worked on this topic for more than 20 years, said,
“The results and information presented in these two papers have long been awaited not only in the PHA community, but also metabolic engineering, bacteriology/microbiology, and in general biological sciences communities. The structural information on PHA synthase together with the recently deciphered reaction mechanisms will be valuable for understanding the detailed mechanisms of biosynthesizing this important energy/redox storage material, and also for the rational engineering of PHA synthases to produce designer bioplastics from various monomers more efficiently.”
Indeed, these two papers published in Biotechnology Journal finally reveal the 30-year mystery of machinery of biological polyester synthesis, and will serve as the essential compass in creating designer and more efficient bioplastic machineries.
References:
Jieun Kim, Yeo-Jin Kim, So Young Choi, Sang Yup Lee and Kyung-Jin Kim. “Crystal structure of Ralstonia eutropha polyhydroxyalkanoate synthase C-terminal domain and reaction mechanisms” Biotechnology Journal DOI: 10.1002/biot.201600648
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600648/abstract
Yeo-Jin Kim, So Young Choi, Jieun Kim, Kyeong Sik Jin, Sang Yup Lee and Kyung-Jin Kim. “Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme” Biotechnology Journal DOI: 10.1002/biot.201600649
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600649/abstract
2016.12.02 View 11328
Mystery of Biological Plastic Synthesis Machinery Unveiled
Plastics and other polymers are used every day. These polymers are mostly made from fossil resources by refining petrochemicals. On the other hand, many microorganisms naturally synthesize polyesters known as polyhydroxyalkanoates (PHAs) as distinct granules inside cells.
PHAs are a family of microbial polyesters that have attracted much attention as biodegradable and biocompatible plastics and elastomers that can substitute petrochemical counterparts. There have been numerous papers and patents on gene cloning and metabolic engineering of PHA biosynthetic machineries, biochemical studies, and production of PHAs; simple Google search with “polyhydroxyalkanoates” yielded returns of 223,000 document pages. PHAs have always been considered amazing examples of biological polymer synthesis. It is astounding to see PHAs of 500 kDa to sometimes as high as 10,000 kDa can be synthesized in vivo by PHA synthase, the key polymerizing enzyme in PHA biosynthesis. They have attracted great interest in determining the crystal structure of PHA synthase over the last 30 years, but unfortunately without success. Thus, the characteristics and molecular mechanisms of PHA synthase were under a dark veil.
In two papers published back-to-back in Biotechnology Journal online on November 30, 2016, a Korean research team led by Professor Kyung-Jin Kim at Kyungpook National University and Distinguished Professor Sang Yup Lee at the Korea Advanced Institute of Science and Technology (KAIST) described the crystal structure of PHA synthase from Ralstonia eutropha, the best studied bacterium for PHA production, and reported the structural basis for the detailed molecular mechanisms of PHA biosynthesis. The crystal structure has been deposited to Protein Data Bank in February 2016. After deciphering the crystal structure of the catalytic domain of PHA synthase, in addition to other structural studies on whole enzyme and related proteins, the research team also performed experiments to elucidate the mechanisms of the enzyme reaction, validating detailed structures, enzyme engineering, and also N-terminal domain studies among others.
Through several biochemical studies based on crystal structure, the authors show that PHA synthase exists as a dimer and is divided into two distinct domains, the N-terminal domain (RePhaC1ND) and the C-terminal domain (RePhaC1CD). The RePhaC1CD catalyzes the polymerization reaction via a non-processive ping-pong mechanism using a Cys-His-Asp catalytic triad. The two catalytic sites of the RePhaC1CD dimer are positioned 33.4 Å apart, suggesting that the polymerization reaction occurs independently at each site. This study also presents the structure-based mechanisms for substrate specificities of various PHA synthases from different classes.
Professor Sang Yup Lee, who has worked on this topic for more than 20 years, said,
“The results and information presented in these two papers have long been awaited not only in the PHA community, but also metabolic engineering, bacteriology/microbiology, and in general biological sciences communities. The structural information on PHA synthase together with the recently deciphered reaction mechanisms will be valuable for understanding the detailed mechanisms of biosynthesizing this important energy/redox storage material, and also for the rational engineering of PHA synthases to produce designer bioplastics from various monomers more efficiently.”
Indeed, these two papers published in Biotechnology Journal finally reveal the 30-year mystery of machinery of biological polyester synthesis, and will serve as the essential compass in creating designer and more efficient bioplastic machineries.
References:
Jieun Kim, Yeo-Jin Kim, So Young Choi, Sang Yup Lee and Kyung-Jin Kim. “Crystal structure of Ralstonia eutropha polyhydroxyalkanoate synthase C-terminal domain and reaction mechanisms” Biotechnology Journal DOI: 10.1002/biot.201600648
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600648/abstract
Yeo-Jin Kim, So Young Choi, Jieun Kim, Kyeong Sik Jin, Sang Yup Lee and Kyung-Jin Kim. “Structure and function of the N-terminal domain of Ralstonia eutropha polyhydroxyalkanoate synthase, and the proposed structure and mechanisms of the whole enzyme” Biotechnology Journal DOI: 10.1002/biot.201600649
http://onlinelibrary.wiley.com/doi/10.1002/biot.201600649/abstract
2016.12.02 View 11328 -
 Professor Lee Co-chairs the Global Future Councils on Biotechnology of the WEF
The World Economic Forum (WEF) established a new global network of the world’s leading experts, “The Annual Meeting of the Global Future Councils,” to explore innovative solutions for the most pressing global challenges. The Councils’ first meeting took place on November 13-14, 2016, in Dubai, the United Arab Emirates (UAE). Some 25 nations joined as member states. The Councils have 35 committees.
Over 700 global leaders in business, government, civil society and academia gathered at the inaugural meeting to “develop ideas and strategies to prepare the world for the Fourth Industrial Revolution, with topics including smart cities, robotics, and the future of mobility,” according to a statement issued by the WEF.
Distinguished Professor Sang Yup Lee of Chemical and Biomolecular Engineering at KAIST was appointed to co-chair one of the Councils' committees, The Annual Meeting of the Global Future Councils on Biotechnology, for two years. The other chairperson is Dr. Feng Zhang, a professor of Biomedical Engineering at the Massachusetts Institute of Technology (MIT), who played a critical role in the development of optogenetics and CRISPR technologies.
The Biotechnology Committee consists of 24 globally recognized professionals in life sciences, law, ethics and policy including Thomas Connelly, the executive director of the American Chemical Society, Tina Fano, the executive vice president of Novozymes, and Mostafa Ronaghi, the chief technology officer of Illumina.
Professor Lee also serves as a committee member of The Annual Meeting of the Global Future Councils on the Fourth Industrial Revolution.
“Life sciences and engineering will receive more attention as a key element of the Fourth Industrial Revolution that the global society as a whole has been experiencing now. Together with thought leaders gathered worldwide, I will join the international community’s concerted efforts to address issues of importance that impact greatly on the future of humanity,” Professor Lee said.
In addition, Professor Lee received the James E. Bailey Award 2016 from The Society for Biological Engineering on November 15, 2016. He is the first Asian researcher to be recognized for his contributions to the field of biotechnology.
2016.11.15 View 10594
Professor Lee Co-chairs the Global Future Councils on Biotechnology of the WEF
The World Economic Forum (WEF) established a new global network of the world’s leading experts, “The Annual Meeting of the Global Future Councils,” to explore innovative solutions for the most pressing global challenges. The Councils’ first meeting took place on November 13-14, 2016, in Dubai, the United Arab Emirates (UAE). Some 25 nations joined as member states. The Councils have 35 committees.
Over 700 global leaders in business, government, civil society and academia gathered at the inaugural meeting to “develop ideas and strategies to prepare the world for the Fourth Industrial Revolution, with topics including smart cities, robotics, and the future of mobility,” according to a statement issued by the WEF.
Distinguished Professor Sang Yup Lee of Chemical and Biomolecular Engineering at KAIST was appointed to co-chair one of the Councils' committees, The Annual Meeting of the Global Future Councils on Biotechnology, for two years. The other chairperson is Dr. Feng Zhang, a professor of Biomedical Engineering at the Massachusetts Institute of Technology (MIT), who played a critical role in the development of optogenetics and CRISPR technologies.
The Biotechnology Committee consists of 24 globally recognized professionals in life sciences, law, ethics and policy including Thomas Connelly, the executive director of the American Chemical Society, Tina Fano, the executive vice president of Novozymes, and Mostafa Ronaghi, the chief technology officer of Illumina.
Professor Lee also serves as a committee member of The Annual Meeting of the Global Future Councils on the Fourth Industrial Revolution.
“Life sciences and engineering will receive more attention as a key element of the Fourth Industrial Revolution that the global society as a whole has been experiencing now. Together with thought leaders gathered worldwide, I will join the international community’s concerted efforts to address issues of importance that impact greatly on the future of humanity,” Professor Lee said.
In addition, Professor Lee received the James E. Bailey Award 2016 from The Society for Biological Engineering on November 15, 2016. He is the first Asian researcher to be recognized for his contributions to the field of biotechnology.
2016.11.15 View 10594 -
 Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11991
Doctoral Student Receives the Best Paper Award from the International Metabolic Engineering Conference 2016
So Young Choi, a Ph.D. candidate at the Department of Chemical and Biomolecular Engineering at KAIST, received the Student and Young Investigator Poster Award at the 11th International Metabolic Engineering Conference held in Awaji, Japan on June 26-30.
Choi received the award for her research on one-step fermentative production of Poly(lactate-co-glycolate) (PLGA) from carbohydrates in Escherichia coli, which was published in the April 2016 issue of Nature Biotechnology.
In her paper, she presented a novel technology to synthesize PLGA, a non-natural copolymer, through a biological production process. Because of its biodegradability, non-toxicity, and biocompatibility, PLGA is widely used in biomedical and therapeutic applications, including surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Employing a metabolic engineering approach, Choi manipulated the metabolic pathway of an Escherichia coli bacterium to convert glucose and xylose into the biosynthesis of PLGA within the cell. Previously, PLGA could be obtained only through chemical synthesis.
Choi said, “I’m thrilled to receive an award from a flagship conference of my research field. Mindful of this recognition, I will continue my research to produce meaningful results, thereby contributing to the development of science and technology in Korea.”
The International Metabolic Engineering Conference is a leading professional gathering where state-of-the-art developments and achievements made in the field of metabolic engineering are shared. With the participation of about 400 professionals from all around the world, the conference participants discussed this year’s theme of “Design, Synthesis and System Integration for Metabolic Engineering.”
2016.07.07 View 11991 -
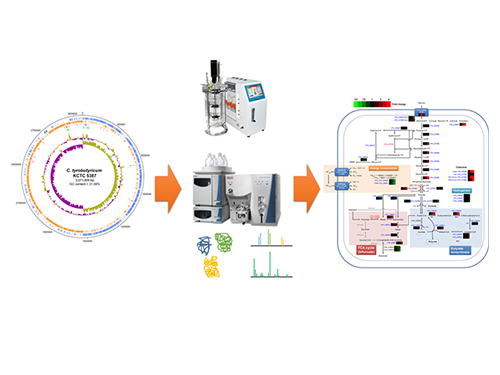 Unveiling the Distinctive Features of Industrial Microorganism
KAIST researchers have sequenced the whole genome of Clostridium tyrobutyricum, which has a higher tolerance to toxic chemicals, such as 1-butanol, compared to other clostridial bacterial strains.
Clostridium tyrobutyricum, a Gram-positive, anaerobic spore-forming bacterium, is considered a promising industrial host strain for the production of various chemicals including butyric acid which has many applications in different industries such as a precursor to biofuels. Despite such potential, C. tyrobutyricum has received little attention, mainly due to a limited understanding of its genotypic and metabolic characteristics at the genome level.
A Korean research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at the Korea Advanced Institute of Science and Technology (KAIST) deciphered the genome sequence of C. tyrobutyricum and its proteome profiles during the course of batch fermentation. As a result, the research team learned that the bacterium is not only capable of producing a large amount of butyric acid but also can tolerate toxic compounds such as 1-butanol. The research results were published in mBio on June 14, 2016.
The team adopted a genoproteomic approach, combining genomics and proteomics, to investigate the metabolic features of C. tyrobutyricum. Unlike Clostridium acetobutylicum, the most widely used organism for 1-butanol production, C. tyrobutyricum has a novel butyrate-producing pathway and various mechanisms for energy conservation under anaerobic conditions. The expression of various metabolic genes, including those involved in butyrate formation, was analyzed using the “shotgun” proteome approach.
To date, the bio-based production of 1-butanol, a next-generation biofuel, has relied on several clostridial hosts including C. acetobutylicum and C. beijerinckii. However, these organisms have a low tolerance against 1-butanol even though they are naturally capable of producing it. C. tyrobutyricum cannot produce 1-butanol itself, but has a higher 1-butanol-tolerance and rapid uptake of monosaccharides, compared to those two species.
The team identified most of the genes involved in the central metabolism of C. tyrobutyricum from the whole-genome and shotgun proteome data, and this study will accelerate the bacterium’s engineering to produce useful chemicals including butyric acid and 1-butanol, replacing traditional bacterial hosts.
Professor Lee said,
“The unique metabolic features and energy conservation mechanisms of C. tyrobutyricum can be employed in the various microbial hosts we have previously developed to further improve their productivity and yield. Moreover, findings on C. tyrobutyricum revealed by this study will be the first step to directly engineer this bacterium.”
Director Jin-Woo Kim at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said,
“Over the years, Professor Lee’s team has researched the development of a bio-refinery system to produce natural and non-natural chemicals with the systems metabolic engineering of microorganisms. They were able to design strategies for the development of diverse industrial microbial strains to produce useful chemicals from inedible biomass-based carbon dioxide fixation. We believe the efficient production of butyric acid using a metabolic engineering approach will play an important role in the establishment of a bioprocess for chemical production.”
The title of the research paper is “Deciphering Clostridium tyrobutyricum Metabolism Based on the Who-Genome Sequence and Proteome Analyses.” (DOI: 10.1128/mBio.00743-16)
The lead authors are Joungmin Lee, a post-doctoral fellow in the BioProcess Research Center at KAIST, currently working in CJ CheilJedang Research Institute; Yu-Sin Jang, a research fellow in the BioProcess Research Center at KAIST, currently working at Gyeongsang National University as an assistant professor; and Mee-Jung Han, an assistant professor in the Environmental Engineering and Energy Department at Dongyang University. Jin Young Kim, a senior researcher at the Korea Basic Science Institute, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project entitled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Schematic Diagram of C. tyrobutyricum’s Genome Sequence and Its Proteome Profiles
The picture below shows the complete genome sequence, global protein expression profiles, and the genome-based metabolic characteristics during batch fermentation of C. tyrobutyricum.
2016.06.20 View 11601
Unveiling the Distinctive Features of Industrial Microorganism
KAIST researchers have sequenced the whole genome of Clostridium tyrobutyricum, which has a higher tolerance to toxic chemicals, such as 1-butanol, compared to other clostridial bacterial strains.
Clostridium tyrobutyricum, a Gram-positive, anaerobic spore-forming bacterium, is considered a promising industrial host strain for the production of various chemicals including butyric acid which has many applications in different industries such as a precursor to biofuels. Despite such potential, C. tyrobutyricum has received little attention, mainly due to a limited understanding of its genotypic and metabolic characteristics at the genome level.
A Korean research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at the Korea Advanced Institute of Science and Technology (KAIST) deciphered the genome sequence of C. tyrobutyricum and its proteome profiles during the course of batch fermentation. As a result, the research team learned that the bacterium is not only capable of producing a large amount of butyric acid but also can tolerate toxic compounds such as 1-butanol. The research results were published in mBio on June 14, 2016.
The team adopted a genoproteomic approach, combining genomics and proteomics, to investigate the metabolic features of C. tyrobutyricum. Unlike Clostridium acetobutylicum, the most widely used organism for 1-butanol production, C. tyrobutyricum has a novel butyrate-producing pathway and various mechanisms for energy conservation under anaerobic conditions. The expression of various metabolic genes, including those involved in butyrate formation, was analyzed using the “shotgun” proteome approach.
To date, the bio-based production of 1-butanol, a next-generation biofuel, has relied on several clostridial hosts including C. acetobutylicum and C. beijerinckii. However, these organisms have a low tolerance against 1-butanol even though they are naturally capable of producing it. C. tyrobutyricum cannot produce 1-butanol itself, but has a higher 1-butanol-tolerance and rapid uptake of monosaccharides, compared to those two species.
The team identified most of the genes involved in the central metabolism of C. tyrobutyricum from the whole-genome and shotgun proteome data, and this study will accelerate the bacterium’s engineering to produce useful chemicals including butyric acid and 1-butanol, replacing traditional bacterial hosts.
Professor Lee said,
“The unique metabolic features and energy conservation mechanisms of C. tyrobutyricum can be employed in the various microbial hosts we have previously developed to further improve their productivity and yield. Moreover, findings on C. tyrobutyricum revealed by this study will be the first step to directly engineer this bacterium.”
Director Jin-Woo Kim at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said,
“Over the years, Professor Lee’s team has researched the development of a bio-refinery system to produce natural and non-natural chemicals with the systems metabolic engineering of microorganisms. They were able to design strategies for the development of diverse industrial microbial strains to produce useful chemicals from inedible biomass-based carbon dioxide fixation. We believe the efficient production of butyric acid using a metabolic engineering approach will play an important role in the establishment of a bioprocess for chemical production.”
The title of the research paper is “Deciphering Clostridium tyrobutyricum Metabolism Based on the Who-Genome Sequence and Proteome Analyses.” (DOI: 10.1128/mBio.00743-16)
The lead authors are Joungmin Lee, a post-doctoral fellow in the BioProcess Research Center at KAIST, currently working in CJ CheilJedang Research Institute; Yu-Sin Jang, a research fellow in the BioProcess Research Center at KAIST, currently working at Gyeongsang National University as an assistant professor; and Mee-Jung Han, an assistant professor in the Environmental Engineering and Energy Department at Dongyang University. Jin Young Kim, a senior researcher at the Korea Basic Science Institute, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project entitled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Schematic Diagram of C. tyrobutyricum’s Genome Sequence and Its Proteome Profiles
The picture below shows the complete genome sequence, global protein expression profiles, and the genome-based metabolic characteristics during batch fermentation of C. tyrobutyricum.
2016.06.20 View 11601 -
 Special Lecture by Professor Sung-Hou Kim of UC Berkeley
As part of its special lecture series, the Department of Biological Sciences at KAIST has invited Professor Sung-Hou Kim of the Department of Chemistry at the University of California, Berkeley, to lecture on his research in structural biology. He will speak twice on May 23 and 30, respectively, on the topics “Origin of Universe and Earth—A Narrative” and “Origin of Life and Human Species—A Narrative.”
Professor Kim's research addresses the structural basis of molecules to reveal how they communicate with each other to activate or inhibit particular processes in cell growth, cell differentiation, and cancer. Using the single-crystal X-ray diffraction technology, he discovered, for the first time in the world, the three-dimensional (3-D) structure of a transfer RNA (t-RNA) and received much praise for this work from the scientific community. Since then, he has been cited as a candidate for a Nobel Prize in Chemistry for many years.
He also examined the 3-D structures of a RAS protein in normal and cancer cells and identified the mutations of the RAS protein as a cause for cancer. His work has assisted in the development of target drugs for cancer treatment. In recent years, he has adopted a computational biology approach to study the structure and function of biological genomics, with which he has tried to predict disease-sensitive genes.
Professor Kim graduated from Seoul National University in 1962 and received his Ph.D. degree in chemistry from the University of Pittsburgh in the United States in 1966. He worked at the Massachusetts Institute of Technology (MIT) as a senior research scientist, and has taught at UC Berkeley since 1978.
2016.05.23 View 7740
Special Lecture by Professor Sung-Hou Kim of UC Berkeley
As part of its special lecture series, the Department of Biological Sciences at KAIST has invited Professor Sung-Hou Kim of the Department of Chemistry at the University of California, Berkeley, to lecture on his research in structural biology. He will speak twice on May 23 and 30, respectively, on the topics “Origin of Universe and Earth—A Narrative” and “Origin of Life and Human Species—A Narrative.”
Professor Kim's research addresses the structural basis of molecules to reveal how they communicate with each other to activate or inhibit particular processes in cell growth, cell differentiation, and cancer. Using the single-crystal X-ray diffraction technology, he discovered, for the first time in the world, the three-dimensional (3-D) structure of a transfer RNA (t-RNA) and received much praise for this work from the scientific community. Since then, he has been cited as a candidate for a Nobel Prize in Chemistry for many years.
He also examined the 3-D structures of a RAS protein in normal and cancer cells and identified the mutations of the RAS protein as a cause for cancer. His work has assisted in the development of target drugs for cancer treatment. In recent years, he has adopted a computational biology approach to study the structure and function of biological genomics, with which he has tried to predict disease-sensitive genes.
Professor Kim graduated from Seoul National University in 1962 and received his Ph.D. degree in chemistry from the University of Pittsburgh in the United States in 1966. He worked at the Massachusetts Institute of Technology (MIT) as a senior research scientist, and has taught at UC Berkeley since 1978.
2016.05.23 View 7740 -
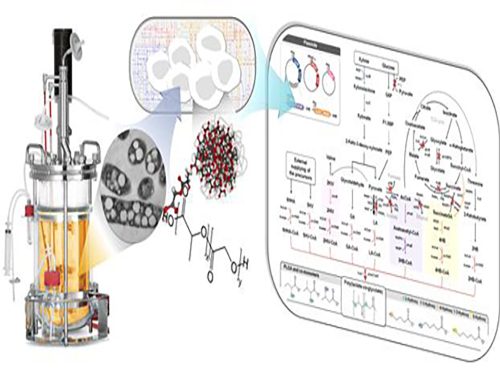 Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12806
Non-Natural Biomedical Polymers Produced from Microorganisms
KAIST researchers have developed metabolically engineered Escherichia coli strains to synthesize non-natural, biomedically important polymers including poly(lactate-co-glycolate) (PLGA), previously considered impossible to obtain from biobased materials.
Renewable non-food biomass could potentially replace petrochemical raw materials to produce energy sources, useful chemicals, or a vast array of petroleum-based end products such as plastics, lubricants, paints, fertilizers, and vitamin capsules. In recent years, biorefineries which transform non-edible biomass into fuel, heat, power, chemicals, and materials have received a great deal of attention as a sustainable alternative to decreasing the reliance on fossil fuels.
A research team headed by Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department at KAIST has established a biorefinery system to create non-natural polymers from natural sources, allowing various plastics to be made in an environmentally-friendly and sustainable manner. The research results were published online in Nature Biotechnology on March 7, 2016. The print version will be issued in April 2016.
The research team adopted a systems metabolic engineering approach to develop a microorganism that can produce diverse non-natural, biomedically important polymers and succeeded in synthesizing poly(lactate-co-glycolate) (PLGA), a copolymer of two different polymer monomers, lactic and glycolic acid. PLGA is biodegradable, biocompatible, and non-toxic, and has been widely used in biomedical and therapeutic applications such as surgical sutures, prosthetic devices, drug delivery, and tissue engineering.
Inspired by the biosynthesis process for polyhydroxyalkanoates (PHAs), biologically-derived polyesters produced in nature by the bacterial fermentation of sugar or lipids, the research team designed a metabolic pathway for the biosynthesis of PLGA through microbial fermentation directly from carbohydrates in Escherichia coli (E. coli) strains.
The team had previously reported a recombinant E. coli producing PLGA by using the glyoxylate shunt pathway for the generation of glycolate from glucose, which was disclosed in their patents KR10-1575585-0000 (filing date of March 11, 2011), US08883463 and JP5820363. However, they discovered that the polymer content and glycolate fraction of PLGA could not be significantly enhanced via further engineering techniques. Thus, in this research, the team introduced a heterologous pathway to produce glycolate from xylose and succeeded in developing the recombinant E. coli producing PLGA and various novel copolymers much more efficiently.
In order to produce PLGA by microbial fermentation directly from carbohydrates, the team incorporated external and engineered enzymes as catalysts to co-polymerize PLGA while establishing a few additional metabolic pathways for the biosynthesis to produce a range of different non-natural polymers, some for the first time. This bio-based synthetic process for PLGA and other polymers could substitute for the existing complicated chemical production that involves the preparation and purification of precursors, chemical polymerization processes, and the elimination of metal catalysts.
Professor Lee and his team performed in silico genome-scale metabolic simulations of the E. coli cell to predict and analyze changes in the metabolic fluxes of cells which were caused by the introduction of external metabolic pathways. Based on these results, genes are manipulated to optimize metabolic fluxes by eliminating the genes responsible for byproducts formation and enhancing the expression levels of certain genes, thereby achieving the effective production of target polymers as well as stimulating cell growth.
The team utilized the structural basis of broad substrate specificity of the key synthesizing enzyme, PHA synthase, to incorporate various co-monomers with main and side chains of different lengths. These monomers were produced inside the cell by metabolic engineering, and then copolymerized to improve the material properties of PLGA. As a result, a variety of PLGA copolymers with different monomer compositions such as the US Food and Drug Administration (FDA)-approved monomers, 3-hydroxyburate, 4-hydroxyburate, and 6-hydroxyhexanoate, were produced. Newly applied bioplastics such as 5-hydroxyvalerate and 2-hydroxyisovalerate were also made.
The team employed a systems metabolic engineering application which, according to the researchers, is the first successful example of biological production of PGLA and several novel copolymers from renewable biomass by one-step direct fermentation of metabolically engineered E.coli.
Professor Lee said, “We presented important findings that non-natural polymers, such as PLGA which is commonly used for drug delivery or biomedical devices, were produced by a metabolically engineered gut bacterium. Our research is meaningful in that it proposes a platform strategy in metabolic engineering, which can be further utilized in the development of numerous non-natural, useful polymers.”
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning of Korea, who oversees the Technology Development Program to Solve Climate Change, said, “Professor Lee has led one of our research projects, the Systems Metabolic Engineering for Biorefineries, which began as part of the Ministry’s Technology Development Program to Solve Climate Change. He and his team have continuously achieved promising results and been attracting greater interest from the global scientific community. As climate change technology grows more important, this research on the biological production of non-natural, high value polymers will have a great impact on science and industry.”
The title of the research paper is “One-step Fermentative Production of Poly(lactate-co-glycolate) from Carbohydrates in Escherichia coli (DOI: 10.1038/nbt.3485).” The lead authors are So Young Choi, a Ph.D. candidate in the Department of Chemical and Biomolecular Engineering at KAIST, and Si Jae Park, Assistant Professor of the Environmental Engineering and Energy Department at Myongji University. Won Jun Kim and Jung Eun Yang, both doctoral students in the Department of Chemical and Biomolecular Engineering at KAIST, also participated in the research.
This research was supported by the Technology Development Program to Solve Climate Change’s research project titled “Systems Metabolic Engineering for Biorefineries” from the Ministry of Science, ICT and Future Planning through the National Research Foundation of Korea (NRF-2012M1A2A2026556).
Figure: Production of PLGA and Other Non-Natural Copolymers
This schematic diagram shows the overall conceptualization of how metabolically engineered E. coli produced a variety of PLGAs with different monomer compositions, proposing the chemosynthetic process of non-natural polymers from biomass. The non-natural polymer PLGA and its other copolymers, which were produced by engineered bacteria developed by taking a systems metabolic engineering approach, accumulate in granule forms within a cell.
2016.03.08 View 12806 -
 Meditox Donates 600 Million KRW Scholarship
On February 17, a Korean biopharmaceutical company Meditox, headed by Chief Executive Officer (CEO) Hyun-Ho Jeong, signed a memorandum of understanding (MOU) with KAIST to establish the “Meditox Fellowship” and donated a total of 600 million Korean won (KRW) to the university to assist in promoting more scientists in the field of biology.
Meditox CEO Hyun-Ho Jeong, KAIST President Steve Kang, Dean of Life Science and Bioengineering College Jung-Hoe Kim, and Dean of the Department of Biological Sciences Byung-Ha Oh participated in the agreement ceremony.
According to the MOU, Meditox will donate 60,000,000 KRW over a ten year period, from which KAIST can draw on to grant scholarships for master’s and doctoral students.
The “Meditox Fellowship” will support promising and enthusiastic students whose finances limit their studies. The first scholarship students for 2016 were: Kwang-Uk Min, In-suk Yeo, Sung-ryung- Lee, Si-on Lee, and Jung-hyun Kim.
Meditox CEO Jeong, who graduated from KAIST’s Department of Biological Sciences, said, "I felt it was important to start the Meditox Fellowship at my alma mater to contribute to the cultivation of outstanding scientists in the field of biological sciences."
He also said that he would plan to launch projects that aim to support not only those who receive the scholarship but also the development of Korea’s biological sciences in general.
President Steve Kang (right) and Chief Executive Officer Hyun-Ho Jeong (left) of Meditox hold the signed memorandum of understanding together.
2016.02.18 View 11617
Meditox Donates 600 Million KRW Scholarship
On February 17, a Korean biopharmaceutical company Meditox, headed by Chief Executive Officer (CEO) Hyun-Ho Jeong, signed a memorandum of understanding (MOU) with KAIST to establish the “Meditox Fellowship” and donated a total of 600 million Korean won (KRW) to the university to assist in promoting more scientists in the field of biology.
Meditox CEO Hyun-Ho Jeong, KAIST President Steve Kang, Dean of Life Science and Bioengineering College Jung-Hoe Kim, and Dean of the Department of Biological Sciences Byung-Ha Oh participated in the agreement ceremony.
According to the MOU, Meditox will donate 60,000,000 KRW over a ten year period, from which KAIST can draw on to grant scholarships for master’s and doctoral students.
The “Meditox Fellowship” will support promising and enthusiastic students whose finances limit their studies. The first scholarship students for 2016 were: Kwang-Uk Min, In-suk Yeo, Sung-ryung- Lee, Si-on Lee, and Jung-hyun Kim.
Meditox CEO Jeong, who graduated from KAIST’s Department of Biological Sciences, said, "I felt it was important to start the Meditox Fellowship at my alma mater to contribute to the cultivation of outstanding scientists in the field of biological sciences."
He also said that he would plan to launch projects that aim to support not only those who receive the scholarship but also the development of Korea’s biological sciences in general.
President Steve Kang (right) and Chief Executive Officer Hyun-Ho Jeong (left) of Meditox hold the signed memorandum of understanding together.
2016.02.18 View 11617 -
 Asia Pacific Biotech News' Special Coverage of Korean Biotechnology
The Asia Pacific Biotech News covered five major biotechnology research projects sponsored by the Korean government in the areas of biofuels, biomedicine, bio-nano healthcare, and biorefinery.
The Asia Pacific Biotech News (APBN), a monthly magazine based in Singapore, which offers comprehensive reports on the fields of pharmaceuticals, healthcare, and biotechnology, recently published a special feature on Korea’s biotechnology research and development (R&D) programs.
The magazine feature selected five research programs sponsored by the Korean government, which are either part of the Global Frontier or the Climate Change Technology Development Projects.
The programs are:
Systems Metabolic Engineering Research: Distinguished Professor Sang Yup Lee
of the Chemical and Biomolecular Engineering Department at the Korea Advanced
Institute of Science and Technology (KAIST) has been leading a research group to
develop biorefining technology using renewable non-food biomass to produce
chemicals, fuels, and materials that were largely drawn from fossil resources
through petrochemical refinery processes. Applying a systems metabolic
engineering approach, the group succeeded in modifying the metabolic pathways of
microorganisms. As a result, they produced, for the first time in the world,
engineered plastic raw materials and gasoline. The team also developed a technique
to produce butanol and succinic acid with a higher titer and yield using metabolically
engineered microorganisms.
Next-generation Biomass Research: Under the leadership of Professor Yong-
Keun Chang of the Chemical and Biomolecular Engineering Department at KAIST,
the research project, which belongs to the Global Frontier Project, develops biofuels
and bioproducts utilizing microalgae typically found in water and other marine
systems.
Convergence Research for Biomedicine: Professor Sung-Hoon Kim of Seoul
National University leads this project that develops targeted new drugs based on
convergence research strategies.
Bionano Healthcare Chip Research: Director Bong-Hyun Chung of the Korea
Research Institute of Bioscience and Biotechnology has integrated information and
communications technology, nanotechnology, and biotechnology to develop a
diagnostic kit that can screen toxic germs, virus, and toxic materials in a prompt
and accurate manner.
Biosynergy Research: Led by Professor Do-Hun Lee of the Bio and Brain
Engineering Department at KAIST, this research project develops new treatments
with a multi-target, multi-component approach in the context of systems biology
through an analysis of synergistic reactions between multi-compounds in traditional
East Asian medicine and human metabolites. In East Asian medicine, treatment and
caring of the human body are considered analogous to the politics of governing a
nation. Based on such system, the research focuses on designing a foundation for
the integration of traditional medicine with modern drug discovery and development.
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning, Republic of Korea, who is responsible for the Global Frontier Program and the Technology to Solve Climate Change, said, “It is great to see that Asia Pacific Biotech News published an extensive coverage of Korea’s several key research programs on biotechnology as its first issue of this year. I am sure that these programs will lead to great outcomes to solve many worldwide pending issues including climate change and healthcare in the aging society.”
Professor Sang Yup Lee, who served as an editor of the feature, said, “At the request of the magazine, we have already published lead articles on our biotechnology research three times in the past in 2002, 2006, and 2011. I am pleased to see continued coverage of Korean biotechnology by the magazine because it recognizes the excellence of our research. Biotechnology has emerged as one of the strong fields that addresses important global issues such as climate change and sustainability.”
2016.02.04 View 14241
Asia Pacific Biotech News' Special Coverage of Korean Biotechnology
The Asia Pacific Biotech News covered five major biotechnology research projects sponsored by the Korean government in the areas of biofuels, biomedicine, bio-nano healthcare, and biorefinery.
The Asia Pacific Biotech News (APBN), a monthly magazine based in Singapore, which offers comprehensive reports on the fields of pharmaceuticals, healthcare, and biotechnology, recently published a special feature on Korea’s biotechnology research and development (R&D) programs.
The magazine feature selected five research programs sponsored by the Korean government, which are either part of the Global Frontier or the Climate Change Technology Development Projects.
The programs are:
Systems Metabolic Engineering Research: Distinguished Professor Sang Yup Lee
of the Chemical and Biomolecular Engineering Department at the Korea Advanced
Institute of Science and Technology (KAIST) has been leading a research group to
develop biorefining technology using renewable non-food biomass to produce
chemicals, fuels, and materials that were largely drawn from fossil resources
through petrochemical refinery processes. Applying a systems metabolic
engineering approach, the group succeeded in modifying the metabolic pathways of
microorganisms. As a result, they produced, for the first time in the world,
engineered plastic raw materials and gasoline. The team also developed a technique
to produce butanol and succinic acid with a higher titer and yield using metabolically
engineered microorganisms.
Next-generation Biomass Research: Under the leadership of Professor Yong-
Keun Chang of the Chemical and Biomolecular Engineering Department at KAIST,
the research project, which belongs to the Global Frontier Project, develops biofuels
and bioproducts utilizing microalgae typically found in water and other marine
systems.
Convergence Research for Biomedicine: Professor Sung-Hoon Kim of Seoul
National University leads this project that develops targeted new drugs based on
convergence research strategies.
Bionano Healthcare Chip Research: Director Bong-Hyun Chung of the Korea
Research Institute of Bioscience and Biotechnology has integrated information and
communications technology, nanotechnology, and biotechnology to develop a
diagnostic kit that can screen toxic germs, virus, and toxic materials in a prompt
and accurate manner.
Biosynergy Research: Led by Professor Do-Hun Lee of the Bio and Brain
Engineering Department at KAIST, this research project develops new treatments
with a multi-target, multi-component approach in the context of systems biology
through an analysis of synergistic reactions between multi-compounds in traditional
East Asian medicine and human metabolites. In East Asian medicine, treatment and
caring of the human body are considered analogous to the politics of governing a
nation. Based on such system, the research focuses on designing a foundation for
the integration of traditional medicine with modern drug discovery and development.
Director Ilsub Baek at the Platform Technology Division of the Ministry of Science, ICT and Future Planning, Republic of Korea, who is responsible for the Global Frontier Program and the Technology to Solve Climate Change, said, “It is great to see that Asia Pacific Biotech News published an extensive coverage of Korea’s several key research programs on biotechnology as its first issue of this year. I am sure that these programs will lead to great outcomes to solve many worldwide pending issues including climate change and healthcare in the aging society.”
Professor Sang Yup Lee, who served as an editor of the feature, said, “At the request of the magazine, we have already published lead articles on our biotechnology research three times in the past in 2002, 2006, and 2011. I am pleased to see continued coverage of Korean biotechnology by the magazine because it recognizes the excellence of our research. Biotechnology has emerged as one of the strong fields that addresses important global issues such as climate change and sustainability.”
2016.02.04 View 14241 -
 IdeasLab Presents Biotechnology Solutions for Aging Populations at 2016 Davos Forum
KAIST researchers will discuss how biological sciences and health technologies can address challenges and opportunities posed by aging populations in an era of increasing longevity.
Many countries around the world today are experiencing the rapid growth of aging populations, with a decline in fertility rate and longer life expectancy.
At this year's Annual Meeting of the World Economic Forum (a.k.a. Davos Forum) on January 20-23, 2016 in Davos-Klosters, Switzerland, four researchers in the field of biological sciences and biotechnology at the Korea Advanced Institute of Science and Technology (KAIST) will discuss the implications of an aging population and explore possible solutions to provide better health care services to the elderly.
KAIST will host an IdeasLab twice on the theme "Biotechnology Solutions for Ageing Populations" on January 21st and 23rd, respectively.
Professor Byung-Kwan Cho of the Biological Sciences Department will give a presentation on "Rejuvenation via the Microbiome," explaining how microorganisms in the human gut play an important role in preventing aging, or even rejuvenating it.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will talk about "Traditional Medicine Reimagined through Modern Systems Biology." Professor Lee will introduce his research results published in Nature Biotechnology (March 6, 2015) and some more new results. He discovered the mechanisms of traditional oriental medicine's (TOM) efficacy by applying systems biology to study structural similarities between natural and nontoxic multi-compounds in the medicine and human metabolites. He will discuss TOM's multi-target approach, which is based on the synergistic combinations of multi-compounds to treat symptoms of a disease, can contribute to the development of new drugs, cosmetics, and nutrients.
Professor Youn-Kyung Lim of the Industrial Design Department will speak about a mobile and the Internet of Things-based health care service called "Dr. M" in her presentation on "Advanced Mobile Healthcare Systems."
Professor Daesoo Kim of the Biological Sciences Department will share his research on human's happiness and greed in the context of nueroscience and behavioral and biological sciences in a talk entitled "A Neural Switch for Being Happy with Less on a Crowded Planet."
KAIST has hosted IdeasLabs several times at the Summer Davos Forum in China, but this is the first time it will participate in the Davos Forum in January.
Professor Lee said, "Just like climate change, the issue of how to address aging populations has become a major global issue. We will share some exciting research results and hope to have in depth discussion on this issue with the leaders attending the Davos Forum. KAIST will engage actively in finding solutions that benefit not only Korea but also the international community."
2016.01.19 View 12429
IdeasLab Presents Biotechnology Solutions for Aging Populations at 2016 Davos Forum
KAIST researchers will discuss how biological sciences and health technologies can address challenges and opportunities posed by aging populations in an era of increasing longevity.
Many countries around the world today are experiencing the rapid growth of aging populations, with a decline in fertility rate and longer life expectancy.
At this year's Annual Meeting of the World Economic Forum (a.k.a. Davos Forum) on January 20-23, 2016 in Davos-Klosters, Switzerland, four researchers in the field of biological sciences and biotechnology at the Korea Advanced Institute of Science and Technology (KAIST) will discuss the implications of an aging population and explore possible solutions to provide better health care services to the elderly.
KAIST will host an IdeasLab twice on the theme "Biotechnology Solutions for Ageing Populations" on January 21st and 23rd, respectively.
Professor Byung-Kwan Cho of the Biological Sciences Department will give a presentation on "Rejuvenation via the Microbiome," explaining how microorganisms in the human gut play an important role in preventing aging, or even rejuvenating it.
Distinguished Professor Sang Yup Lee of the Chemical and Biomolecular Engineering Department will talk about "Traditional Medicine Reimagined through Modern Systems Biology." Professor Lee will introduce his research results published in Nature Biotechnology (March 6, 2015) and some more new results. He discovered the mechanisms of traditional oriental medicine's (TOM) efficacy by applying systems biology to study structural similarities between natural and nontoxic multi-compounds in the medicine and human metabolites. He will discuss TOM's multi-target approach, which is based on the synergistic combinations of multi-compounds to treat symptoms of a disease, can contribute to the development of new drugs, cosmetics, and nutrients.
Professor Youn-Kyung Lim of the Industrial Design Department will speak about a mobile and the Internet of Things-based health care service called "Dr. M" in her presentation on "Advanced Mobile Healthcare Systems."
Professor Daesoo Kim of the Biological Sciences Department will share his research on human's happiness and greed in the context of nueroscience and behavioral and biological sciences in a talk entitled "A Neural Switch for Being Happy with Less on a Crowded Planet."
KAIST has hosted IdeasLabs several times at the Summer Davos Forum in China, but this is the first time it will participate in the Davos Forum in January.
Professor Lee said, "Just like climate change, the issue of how to address aging populations has become a major global issue. We will share some exciting research results and hope to have in depth discussion on this issue with the leaders attending the Davos Forum. KAIST will engage actively in finding solutions that benefit not only Korea but also the international community."
2016.01.19 View 12429 -
 Professor Joonho Choe Appointed as the President of the KSMCB
Professor Joonho Choe of the Biological Sciences Department at KAIST has been elected the 25th president of Korean Society for Molecular and Cellular Biology (KSMCB).
His presidency will last one year, beginning on January 1, 2016.
Established in 1989, the Society has served as the largest academic gathering in the field of life sciences, holding an international conference every fall. It has more than 12,400 fellows.
Professor Choe served as the vice president of KSMC as well as the editor of its journal, Molecules and Cells.
He said, “The 2016 International Conference of the KSMCB will take place on October 12-14, 2016 at the COEX Convention and Exhibition Hall in Seoul. This year, we are preparing 20 symposiums and will invite four international renowned keynote speakers in the field including a Nobel Laureate. We hope many people, students and young researchers in particular, from academia and industry will join the conference.”
Professor Choe received his doctoral degree from the University of California, Los Angeles (UCLA) after graduating from Seoul National University with his bachelor and master’s degrees.
2016.01.05 View 9817
Professor Joonho Choe Appointed as the President of the KSMCB
Professor Joonho Choe of the Biological Sciences Department at KAIST has been elected the 25th president of Korean Society for Molecular and Cellular Biology (KSMCB).
His presidency will last one year, beginning on January 1, 2016.
Established in 1989, the Society has served as the largest academic gathering in the field of life sciences, holding an international conference every fall. It has more than 12,400 fellows.
Professor Choe served as the vice president of KSMC as well as the editor of its journal, Molecules and Cells.
He said, “The 2016 International Conference of the KSMCB will take place on October 12-14, 2016 at the COEX Convention and Exhibition Hall in Seoul. This year, we are preparing 20 symposiums and will invite four international renowned keynote speakers in the field including a Nobel Laureate. We hope many people, students and young researchers in particular, from academia and industry will join the conference.”
Professor Choe received his doctoral degree from the University of California, Los Angeles (UCLA) after graduating from Seoul National University with his bachelor and master’s degrees.
2016.01.05 View 9817