-
 Professor Suk-Joong Kang Receives the Richard Brook and Helmholtz Awards
Professor Suk-Joong Kang of KAIST’s Department of Materials Sciences and Engineering received the Richard Brook Award from the European Ceramic Society at its 14th conference held on June 21, 2015, in Toledo, Spain. The award is presented to the most distinguished academic or engineer in ceramics from a non-European country. Professor Kang gave the commemorative lecture after the award ceremony.
Professor Kang is an expert in the field of sintering and microstructural evolution in ceramics and metals. He suggested a new model for grain growth and identified the principles of microstructural evolution.
He also received the 2015 Helmholtz Fellow Award in June. The Helmholtz Association, the largest scientific organization in Germany, confers the award on outstanding senior scientists based outside Germany who have made great academic and research achievements in their fields.
Professor Kang said of the Brook Award, “It is such an honor to receive an award from an eminent global institution. I take this opportunity to thank my students and colleagues for their support, and I will work harder for my research.”
2015.07.20 View 7423
Professor Suk-Joong Kang Receives the Richard Brook and Helmholtz Awards
Professor Suk-Joong Kang of KAIST’s Department of Materials Sciences and Engineering received the Richard Brook Award from the European Ceramic Society at its 14th conference held on June 21, 2015, in Toledo, Spain. The award is presented to the most distinguished academic or engineer in ceramics from a non-European country. Professor Kang gave the commemorative lecture after the award ceremony.
Professor Kang is an expert in the field of sintering and microstructural evolution in ceramics and metals. He suggested a new model for grain growth and identified the principles of microstructural evolution.
He also received the 2015 Helmholtz Fellow Award in June. The Helmholtz Association, the largest scientific organization in Germany, confers the award on outstanding senior scientists based outside Germany who have made great academic and research achievements in their fields.
Professor Kang said of the Brook Award, “It is such an honor to receive an award from an eminent global institution. I take this opportunity to thank my students and colleagues for their support, and I will work harder for my research.”
2015.07.20 View 7423 -
 KAIST Undergraduates Organize the Largest Interdisciplinary Conference in Asia
The largest interdisciplinary conference in Asia hosted by KAIST undergraduates for students around the world will be held in KAIST. The organizing committee of International Conference for the Integration of Science, Technology and Society (ICISTS) will hold the ICISTS-KAIST 2015 in KAIST and Hotel ICC from August 3-7, 2015, with around 300 Korean and international participants.
ICISTS-KAIST was established in 2005 to provide an annual platform for students to discuss the integration and the convergence of science, technology and society, regardless of their academic background.
This year’s theme is "Shaping the Future" and the topics for the conference are robotics, medicine, and science communication.
The keynote speakers are Vitalic Buterin, the winner of the World Technology Award in 2014 for the co-creation and invention of Ethereum and Alan Irwin, a well-known scholar of science, technology and society as well as the Dean of Research at the Copenhagen Business School in Denmark.
Other notable speakers include Adam Marcus, a professor of Hematology and Medical Oncology, Emory University School of Medicine; Stefan Lorenz Sorgner, the Director and co-founder of Beyond Humanism Network; Hideto Nakajima, a professor in the Department of History, Philosophy and Social Studies of Science and Technology at Tokyo Institute of Technology; Wendell Wallach, a lecturer at the Yale University Interdisciplinary Center for Bioethics; Jinil Lee, a professor in the Division of Biological Science and Technology at Yonsei University; and Sangwook Kim, an editor of APCTP web journal Crossroads and a professor in the Department of Physics Education, Pusan National University.
Last year, more than 300 students from 50 different countries attended the ICISTS-KAIST 2014 as delegates to exchange their thoughts and ideas on science, technology, and society.
To register for the event, please visit www.icists.org.
2015.07.14 View 9360
KAIST Undergraduates Organize the Largest Interdisciplinary Conference in Asia
The largest interdisciplinary conference in Asia hosted by KAIST undergraduates for students around the world will be held in KAIST. The organizing committee of International Conference for the Integration of Science, Technology and Society (ICISTS) will hold the ICISTS-KAIST 2015 in KAIST and Hotel ICC from August 3-7, 2015, with around 300 Korean and international participants.
ICISTS-KAIST was established in 2005 to provide an annual platform for students to discuss the integration and the convergence of science, technology and society, regardless of their academic background.
This year’s theme is "Shaping the Future" and the topics for the conference are robotics, medicine, and science communication.
The keynote speakers are Vitalic Buterin, the winner of the World Technology Award in 2014 for the co-creation and invention of Ethereum and Alan Irwin, a well-known scholar of science, technology and society as well as the Dean of Research at the Copenhagen Business School in Denmark.
Other notable speakers include Adam Marcus, a professor of Hematology and Medical Oncology, Emory University School of Medicine; Stefan Lorenz Sorgner, the Director and co-founder of Beyond Humanism Network; Hideto Nakajima, a professor in the Department of History, Philosophy and Social Studies of Science and Technology at Tokyo Institute of Technology; Wendell Wallach, a lecturer at the Yale University Interdisciplinary Center for Bioethics; Jinil Lee, a professor in the Division of Biological Science and Technology at Yonsei University; and Sangwook Kim, an editor of APCTP web journal Crossroads and a professor in the Department of Physics Education, Pusan National University.
Last year, more than 300 students from 50 different countries attended the ICISTS-KAIST 2014 as delegates to exchange their thoughts and ideas on science, technology, and society.
To register for the event, please visit www.icists.org.
2015.07.14 View 9360 -
 KAIST Startups Annually Engage 33,000 People, and Their Sales Total Nearly 10 Billion Dollars
According to a recent study, KAIST startups annually engage 33,000 people, and their sales total nearly 10 billion US dollars. Also amongst 1,245 companies, 50 were listed in stock markets including KOSDAQ and KONEX. President Kang of KAIST commissioned an evaluation of KAIST startups last year. The report consisted of six chapters: current status of entrepreneurs and companies, cross analysis based on individuals’ background and academic degree, annual performance analysis, and current status of startup assistance.
The report categorized the startups with respect to the founders’ background. Of 1,245 companies, KAIST alumni founded 929 (74.6%) of the companies under study: 191 (15.3%) were located within the KAIST campuses, 91 (7.3%) were founded by enrolled students, and 74 (2.7%) by professors. The startup founders had different levels of education: 515 (41.4%) founders had master’s degree, 443 (35.6%) Ph.D. degree, and only 213 (17.1%) had only bachelor’s degree as the highest level of education attained. The reason behind the majority of founders having a master’s degree or higher degree is that many people established a startup after obtaining specialized knowledge and skills.
Focusing on the founders’ college majors, 719 (70.6%) founders were from the engineering department, 111 (10.9%) from the business administration department, 103 (10.1%) from the natural science department, and 86 (8.4%) from other departments. Looking at the companies' locations, 462 (37.5%) were placed in Seoul, 355 (28.8%) in Daejeon, and 273 (22.2%) in Gyeonggi.
By the end of 2013, the total asset of 1,069 companies came to 12 billion and 444 million dollars. Their total sales figure was 10 billion and 13 million dollars, and annual employments summed up to 33,000 people. The companies generated a significant portion of gross regional domestic product (GRDP) in each region. They formed 0.49% of GRDP of Seoul, took up 1.67% GRDP of Gyeonggi, and 5.53% of that of Daejeon.
Along with the performance analysis, the report also took a survey of suggestions on future startup assistance and opinions on current startup assistance policies. To a question asking what constituted the most difficult part of startup, 31.7% of respondents answered “attraction of investment,” 22.8% chose “a lack of human resources,” and 16.8% said “consulting” amongst 214 respondents. The study showed that major and medium enterprises face difficulty in finding human resources whereas small businesses experience obstacles attracting investment. Some startups had help from KAIST: 44 startups were provided with the office space, 21 had educational supports, and 18 were supported in research and development.
The report demonstrates that startups established by KAIST alumni and members play a key role in the South Korean economy despite KAIST’s short startup history, which began only since the end of 1990s. Based on this report, KAIST plans to listen continuously to the needs of alumni founders, and use those responses as a guide to entrepreneurship education for current students.
The Dean of the Office of University and Industry Cooperation, Joongmyeon Bae, who oversaw the publication of this report, said, "As this report is the first in Korea to study the status of alumni startups, it will be incredibly valuable in modifying the startup assistance policies.”
To spread an entrepreneurial spirit and start-up cultures in the campus and enhance the startup supporting system, KAIST has founded various startup centers on and off the campus.
2015.07.14 View 9391
KAIST Startups Annually Engage 33,000 People, and Their Sales Total Nearly 10 Billion Dollars
According to a recent study, KAIST startups annually engage 33,000 people, and their sales total nearly 10 billion US dollars. Also amongst 1,245 companies, 50 were listed in stock markets including KOSDAQ and KONEX. President Kang of KAIST commissioned an evaluation of KAIST startups last year. The report consisted of six chapters: current status of entrepreneurs and companies, cross analysis based on individuals’ background and academic degree, annual performance analysis, and current status of startup assistance.
The report categorized the startups with respect to the founders’ background. Of 1,245 companies, KAIST alumni founded 929 (74.6%) of the companies under study: 191 (15.3%) were located within the KAIST campuses, 91 (7.3%) were founded by enrolled students, and 74 (2.7%) by professors. The startup founders had different levels of education: 515 (41.4%) founders had master’s degree, 443 (35.6%) Ph.D. degree, and only 213 (17.1%) had only bachelor’s degree as the highest level of education attained. The reason behind the majority of founders having a master’s degree or higher degree is that many people established a startup after obtaining specialized knowledge and skills.
Focusing on the founders’ college majors, 719 (70.6%) founders were from the engineering department, 111 (10.9%) from the business administration department, 103 (10.1%) from the natural science department, and 86 (8.4%) from other departments. Looking at the companies' locations, 462 (37.5%) were placed in Seoul, 355 (28.8%) in Daejeon, and 273 (22.2%) in Gyeonggi.
By the end of 2013, the total asset of 1,069 companies came to 12 billion and 444 million dollars. Their total sales figure was 10 billion and 13 million dollars, and annual employments summed up to 33,000 people. The companies generated a significant portion of gross regional domestic product (GRDP) in each region. They formed 0.49% of GRDP of Seoul, took up 1.67% GRDP of Gyeonggi, and 5.53% of that of Daejeon.
Along with the performance analysis, the report also took a survey of suggestions on future startup assistance and opinions on current startup assistance policies. To a question asking what constituted the most difficult part of startup, 31.7% of respondents answered “attraction of investment,” 22.8% chose “a lack of human resources,” and 16.8% said “consulting” amongst 214 respondents. The study showed that major and medium enterprises face difficulty in finding human resources whereas small businesses experience obstacles attracting investment. Some startups had help from KAIST: 44 startups were provided with the office space, 21 had educational supports, and 18 were supported in research and development.
The report demonstrates that startups established by KAIST alumni and members play a key role in the South Korean economy despite KAIST’s short startup history, which began only since the end of 1990s. Based on this report, KAIST plans to listen continuously to the needs of alumni founders, and use those responses as a guide to entrepreneurship education for current students.
The Dean of the Office of University and Industry Cooperation, Joongmyeon Bae, who oversaw the publication of this report, said, "As this report is the first in Korea to study the status of alumni startups, it will be incredibly valuable in modifying the startup assistance policies.”
To spread an entrepreneurial spirit and start-up cultures in the campus and enhance the startup supporting system, KAIST has founded various startup centers on and off the campus.
2015.07.14 View 9391 -
 KAIST Undergraduate Students Volunteer in Ethiopia
World Friends (WF), one of the undergraduate student clubs at KAIST, offer students opportunities to volunteer in underdeveloped regions and countries. This year the World Friends team travels to Ethiopia from July 9 to August 17, 2015.
The aim of this trip is to help Ethiopian students fill gaps in their knowledge of information technology and encourage KAIST students build leadership skills through volunteer activities. Twenty-eight students will make the trip.
KAIST students will visit the Addis Ababa Institute of Technology and the Adama Science and Technology University, as well as some local high and elementary schools in Addis Ababa, where they will run computer classes related to the basics of information technology such as C Language, Java Programming, Photoshop, MS Office, and Windows.
The volunteers will offer Adama Science and Technology University students an advanced computer course to prepare them to participate in the ACM-ICPC, an international computer programming competition for university students.
KAIST students will also introduce Korean culture to Ethiopian students including K-pop, Korean cuisine and fashion, Korean language lessons, and traditional Korean art.
The Dean of Student Affairs and Policy at KAIST, Professor Young-Hee Kim said, “I hope the students from two very different cultures will cherish this opportunity to interact with each other and contribute to narrowing down the regional disparities in the IT field.”
2015.07.10 View 9206
KAIST Undergraduate Students Volunteer in Ethiopia
World Friends (WF), one of the undergraduate student clubs at KAIST, offer students opportunities to volunteer in underdeveloped regions and countries. This year the World Friends team travels to Ethiopia from July 9 to August 17, 2015.
The aim of this trip is to help Ethiopian students fill gaps in their knowledge of information technology and encourage KAIST students build leadership skills through volunteer activities. Twenty-eight students will make the trip.
KAIST students will visit the Addis Ababa Institute of Technology and the Adama Science and Technology University, as well as some local high and elementary schools in Addis Ababa, where they will run computer classes related to the basics of information technology such as C Language, Java Programming, Photoshop, MS Office, and Windows.
The volunteers will offer Adama Science and Technology University students an advanced computer course to prepare them to participate in the ACM-ICPC, an international computer programming competition for university students.
KAIST students will also introduce Korean culture to Ethiopian students including K-pop, Korean cuisine and fashion, Korean language lessons, and traditional Korean art.
The Dean of Student Affairs and Policy at KAIST, Professor Young-Hee Kim said, “I hope the students from two very different cultures will cherish this opportunity to interact with each other and contribute to narrowing down the regional disparities in the IT field.”
2015.07.10 View 9206 -
 Dong-Young Lee, a Doctoral Candidate, Receives the Best Paper Award
Dong-Young Lee, a Ph.D. candidate in the Mechanical Engineering Department, KAIST, received the Best Paper Award at the 18th International Conference on Composite Structures (ICCS). The event was held in Lisbon, Portugal, on June 15-18, 2015. Mr. Lee’s adviser is Professor Dai-Gil Lee of the same department.
The ICCS is held every other year, and is one of the largest and long-established conferences on composite materials and structures in the world. At this year’s conference, a total of 680 papers were presented, among which, two papers were chosen for the Best Paper Award, including Mr. Lee’s.
The paper, entitled “Gasket-integrated Carbon and Silicon Elastomer Composite Bipolar Plate for High-temperature PEMFC,” will be published in the September issue of Composite Structures which is one of the top journals in mechanical engineering as judged by the Google Scholar Metrics rankings.
Mr. Lee dropped the conventional method of PEMFC (Proton Exchange Membrane Fuel Cells) assembly and instead developed a gasket-integrated carbon and silicone elastomer composite bipolar plate. This technology significantly increased the energy efficiency of fuel cells and their productivity.
Mr. Lee said, “I would like to thank the many people who supported me, especially my Ph.D. adviser, Professor Dai-Gil Lee. Without their encouragement, I would have not won this award. I hope my research will contribute to solving energy problems in the future.”
In addition, Professor Joon-Woo Im from Chonbuk National University, Senior Researcher Il-Bum Choi from the Agency for Defense Development, and a fellow doctoral candidate Soo-Hyun Nam from KAIST participated in this research project.
2015.07.09 View 11161
Dong-Young Lee, a Doctoral Candidate, Receives the Best Paper Award
Dong-Young Lee, a Ph.D. candidate in the Mechanical Engineering Department, KAIST, received the Best Paper Award at the 18th International Conference on Composite Structures (ICCS). The event was held in Lisbon, Portugal, on June 15-18, 2015. Mr. Lee’s adviser is Professor Dai-Gil Lee of the same department.
The ICCS is held every other year, and is one of the largest and long-established conferences on composite materials and structures in the world. At this year’s conference, a total of 680 papers were presented, among which, two papers were chosen for the Best Paper Award, including Mr. Lee’s.
The paper, entitled “Gasket-integrated Carbon and Silicon Elastomer Composite Bipolar Plate for High-temperature PEMFC,” will be published in the September issue of Composite Structures which is one of the top journals in mechanical engineering as judged by the Google Scholar Metrics rankings.
Mr. Lee dropped the conventional method of PEMFC (Proton Exchange Membrane Fuel Cells) assembly and instead developed a gasket-integrated carbon and silicone elastomer composite bipolar plate. This technology significantly increased the energy efficiency of fuel cells and their productivity.
Mr. Lee said, “I would like to thank the many people who supported me, especially my Ph.D. adviser, Professor Dai-Gil Lee. Without their encouragement, I would have not won this award. I hope my research will contribute to solving energy problems in the future.”
In addition, Professor Joon-Woo Im from Chonbuk National University, Senior Researcher Il-Bum Choi from the Agency for Defense Development, and a fellow doctoral candidate Soo-Hyun Nam from KAIST participated in this research project.
2015.07.09 View 11161 -
 Omnidirectional Free Space Wireless Charging Developed
The simultaneous charging of multiple mobile devices at 0.5 meter away from the power source is now possible under the international electromagnetic field guidelines.
Mobile devices, such as smartphones and laptops, have become indispensable portable items in modern life, but one big challenge remains to fully enjoying these devices: keeping their batteries charged.
A group of researchers at KAIST has developed a wireless-power transfer (WPT) technology that allows mobile devices to be charged at any location and in any direction, even if the devices are away from the power source, just as Wi-Fi works for Internet connections. With this technology, so long as mobile users stay in a designated area where the charging is available, e.g., the Wi-Power zone, the device, without being tethered to a charger, will pick up power automatically, as needed.
The research team led by Professor Chun T. Rim of the Nuclear and Quantum Engineering Department at KAIST has made great strides in WPT development. Their WPT system is capable of charging multiple mobile devices concurrently and with unprecedented freedom in any direction, even while holding the devices in midair or a half meter away from the power source, which is a transmitter. The research result was published in the June 2015 on-line issue of IEEE Transactions on Power Electronics, which is entitled “Six Degrees of Freedom Mobile Inductive Power Transfer by Crossed Dipole Tx (Transmitter) and Rx (Receiver) Coils.”
Professor Rim’s team has successfully showcased the technology on July 7, 2015 at a lab on KAIST’s campus. They used high-frequency magnetic materials in a dipole coil structure to build a thin, flat transmitter (Tx) system shaped in a rectangle with a size of 1m2. Either 30 smartphones with a power capacity of one watt each or 5 laptops with 2.4 watts each can be simultaneously and wirelessly charged at a 50 cm distance from the transmitter with six degrees of freedom, regardless of the devices’ three-axes positions and directions. This means that the device can receive power all around the transmitter in three-dimensional space. The maximum power transfer efficiency for the laptops was 34%. The researchers said that to fabricate plane Tx and Rx coils with the six-degree-of-freedom characteristic was a bottleneck of WPT for mobile applications.
Dipole Coil Resonance System (DCRS)
The research team used the Dipole Coil Resonance System (DCRS) to induce magnetic fields, which was developed by the team in 2014 for inductive power transfer over an extended distance. The DCRS is composed of two (transmitting and receiving) magnetic dipole coils, placed in parallel, with each coil having a ferrite core and connected with a resonant capacitor. Comparing to a conventional loop coil, the dipole coil is very compact and has a less dimension. Therefore, a crossed dipole structure has 2-dimension rather than 3-dimension of a crossed loop coil structure. The DCRS has a great advantage to transfer power even when the resonance frequency changes in the range of 1% (Q factor is below 100). The ferrite cores are optimally designed to reduce the core volume by half, and their ability to transfer power is nearly unaffected by human bodies or surrounding metal objects, making DCRS ideal to transmit wireless power in emergency situations. In a test conducted in 2014, Professor Rim succeeded in transferring 209 watts of power wirelessly to the distance of five meters. (See KAIST’s press release on DCRS for details: http://www.eurekalert.org/pub_releases/2014-04/tkai-wpt041714.php.)
Greater Flexibility and Safer Charging
The research team rearranged the two dipole coils from a parallel position to cross them in order to generate rotating magnetic fields, which was embedded in the Tx’s flat platform. This has made it possible for mobile devices to receive power from any direction.
Although wireless-power technology has been applied to smartphones, it could not offer any substantial advantages over traditional wired charging because the devices still require close contact with the transmitter, a charging pad. To use the devices freely and safely, including in public spaces, the WPT technology should provide mobile users with six degrees of freedom at a distance. Until now, all wireless-charging technologies have had difficulties with the problem of short charging distance, mostly less than 10 cm, as well as charging conditions that the devices should be placed in a fixed position. For example, the Galaxy S6 could only be charged wirelessly in a fixed position, having one degree of freedom. The degree of freedom represents mobile devices’ freedom of movement in three-dimensional space.
In addition, the DCRS works at a low magnetic field environment. Based on the magnetic flux shielding technology developed by the research team, the level of magnetic flux is below the safety level of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) guideline (27µT) for general public exposure to electromagnetic field (EMF).
Professor Rim said, “Our transmitter system is safe for humans and compatible with other electronic devices. We have solved three major issues of short charging distance, the dependence on charging directions, and plane coil structures of both Tx and Rx, which have blocked the commercialization of WPT.”
Currently, the research team and KAIST’s spin-off company, TESLAS, Inc., have been conducting pilot projects to apply DCRS in various places such as cafes and offices.
YouTube Link: https://www.youtube.com/watch?v=JU64pMyJioc
Demonstration of 30 Watts Range Omnidirectional Wireless-charging at a Laboratory on KAIST’s Campus
Figure 1: Wide-range omnidirectional wireless-charging system based on DCRS can charge multiple numbers of mobile devices simultaneously in a 1m3 range. The above is a transmitter, and the below is a Samsung Galaxy Note with a receiver embedded inside.
Figure 2: Demonstration of the omnidirectional wireless-charging system (clockwise from top of the left, robust charging despite the presence of metal obstacles, omnidirectional charging, long distance charging, and multiple devices charging)
2015.07.08 View 17516
Omnidirectional Free Space Wireless Charging Developed
The simultaneous charging of multiple mobile devices at 0.5 meter away from the power source is now possible under the international electromagnetic field guidelines.
Mobile devices, such as smartphones and laptops, have become indispensable portable items in modern life, but one big challenge remains to fully enjoying these devices: keeping their batteries charged.
A group of researchers at KAIST has developed a wireless-power transfer (WPT) technology that allows mobile devices to be charged at any location and in any direction, even if the devices are away from the power source, just as Wi-Fi works for Internet connections. With this technology, so long as mobile users stay in a designated area where the charging is available, e.g., the Wi-Power zone, the device, without being tethered to a charger, will pick up power automatically, as needed.
The research team led by Professor Chun T. Rim of the Nuclear and Quantum Engineering Department at KAIST has made great strides in WPT development. Their WPT system is capable of charging multiple mobile devices concurrently and with unprecedented freedom in any direction, even while holding the devices in midair or a half meter away from the power source, which is a transmitter. The research result was published in the June 2015 on-line issue of IEEE Transactions on Power Electronics, which is entitled “Six Degrees of Freedom Mobile Inductive Power Transfer by Crossed Dipole Tx (Transmitter) and Rx (Receiver) Coils.”
Professor Rim’s team has successfully showcased the technology on July 7, 2015 at a lab on KAIST’s campus. They used high-frequency magnetic materials in a dipole coil structure to build a thin, flat transmitter (Tx) system shaped in a rectangle with a size of 1m2. Either 30 smartphones with a power capacity of one watt each or 5 laptops with 2.4 watts each can be simultaneously and wirelessly charged at a 50 cm distance from the transmitter with six degrees of freedom, regardless of the devices’ three-axes positions and directions. This means that the device can receive power all around the transmitter in three-dimensional space. The maximum power transfer efficiency for the laptops was 34%. The researchers said that to fabricate plane Tx and Rx coils with the six-degree-of-freedom characteristic was a bottleneck of WPT for mobile applications.
Dipole Coil Resonance System (DCRS)
The research team used the Dipole Coil Resonance System (DCRS) to induce magnetic fields, which was developed by the team in 2014 for inductive power transfer over an extended distance. The DCRS is composed of two (transmitting and receiving) magnetic dipole coils, placed in parallel, with each coil having a ferrite core and connected with a resonant capacitor. Comparing to a conventional loop coil, the dipole coil is very compact and has a less dimension. Therefore, a crossed dipole structure has 2-dimension rather than 3-dimension of a crossed loop coil structure. The DCRS has a great advantage to transfer power even when the resonance frequency changes in the range of 1% (Q factor is below 100). The ferrite cores are optimally designed to reduce the core volume by half, and their ability to transfer power is nearly unaffected by human bodies or surrounding metal objects, making DCRS ideal to transmit wireless power in emergency situations. In a test conducted in 2014, Professor Rim succeeded in transferring 209 watts of power wirelessly to the distance of five meters. (See KAIST’s press release on DCRS for details: http://www.eurekalert.org/pub_releases/2014-04/tkai-wpt041714.php.)
Greater Flexibility and Safer Charging
The research team rearranged the two dipole coils from a parallel position to cross them in order to generate rotating magnetic fields, which was embedded in the Tx’s flat platform. This has made it possible for mobile devices to receive power from any direction.
Although wireless-power technology has been applied to smartphones, it could not offer any substantial advantages over traditional wired charging because the devices still require close contact with the transmitter, a charging pad. To use the devices freely and safely, including in public spaces, the WPT technology should provide mobile users with six degrees of freedom at a distance. Until now, all wireless-charging technologies have had difficulties with the problem of short charging distance, mostly less than 10 cm, as well as charging conditions that the devices should be placed in a fixed position. For example, the Galaxy S6 could only be charged wirelessly in a fixed position, having one degree of freedom. The degree of freedom represents mobile devices’ freedom of movement in three-dimensional space.
In addition, the DCRS works at a low magnetic field environment. Based on the magnetic flux shielding technology developed by the research team, the level of magnetic flux is below the safety level of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) guideline (27µT) for general public exposure to electromagnetic field (EMF).
Professor Rim said, “Our transmitter system is safe for humans and compatible with other electronic devices. We have solved three major issues of short charging distance, the dependence on charging directions, and plane coil structures of both Tx and Rx, which have blocked the commercialization of WPT.”
Currently, the research team and KAIST’s spin-off company, TESLAS, Inc., have been conducting pilot projects to apply DCRS in various places such as cafes and offices.
YouTube Link: https://www.youtube.com/watch?v=JU64pMyJioc
Demonstration of 30 Watts Range Omnidirectional Wireless-charging at a Laboratory on KAIST’s Campus
Figure 1: Wide-range omnidirectional wireless-charging system based on DCRS can charge multiple numbers of mobile devices simultaneously in a 1m3 range. The above is a transmitter, and the below is a Samsung Galaxy Note with a receiver embedded inside.
Figure 2: Demonstration of the omnidirectional wireless-charging system (clockwise from top of the left, robust charging despite the presence of metal obstacles, omnidirectional charging, long distance charging, and multiple devices charging)
2015.07.08 View 17516 -
 Experts Gather to Develop a Korean Supercomputer on KAIST Campus
KAIST hosted an inauguration ceremony for the Super-Capacity Computing Advancement Forum on July 2, 2015, to increase Korea's national computing capacity. It represents a gathering consisting of experts drawn across industry, university, and institutes in super-capacity computing.
More than ten experts from the university, including President Steve Kang and Professor Oh-Joon Kwon of the Department of Aerospace Engineering, attended the ceremony. This forum was created to secure a competitive edge in the global market by establishing a long-term strategy for the development of super computers.
The recent rise of new service industries, such as voice recognition, artificial intelligence, and the Internet of Things, has increased the need for super-capacity computing to deal more rapidly with big data. The need is made more urgent by increased investment by leading countries in this field.
The forum will organize and operate working-level subcommittees to promote in-depth discussions on issues related to super-capacity computing systems. Open forums and public hearings will be held until October, to gather information and insights needed to advance the field.
President Steve Kang, the Chairman of the Forum, said, “The forum will have a great impact on Korea’s effort to become a world leader in super-capacity computing. We plan to debate the pros and cons of potential solutions to the Korean government, to assist them in building the nation’s competitiveness in super-capacity computing capability.”
2015.07.07 View 7328
Experts Gather to Develop a Korean Supercomputer on KAIST Campus
KAIST hosted an inauguration ceremony for the Super-Capacity Computing Advancement Forum on July 2, 2015, to increase Korea's national computing capacity. It represents a gathering consisting of experts drawn across industry, university, and institutes in super-capacity computing.
More than ten experts from the university, including President Steve Kang and Professor Oh-Joon Kwon of the Department of Aerospace Engineering, attended the ceremony. This forum was created to secure a competitive edge in the global market by establishing a long-term strategy for the development of super computers.
The recent rise of new service industries, such as voice recognition, artificial intelligence, and the Internet of Things, has increased the need for super-capacity computing to deal more rapidly with big data. The need is made more urgent by increased investment by leading countries in this field.
The forum will organize and operate working-level subcommittees to promote in-depth discussions on issues related to super-capacity computing systems. Open forums and public hearings will be held until October, to gather information and insights needed to advance the field.
President Steve Kang, the Chairman of the Forum, said, “The forum will have a great impact on Korea’s effort to become a world leader in super-capacity computing. We plan to debate the pros and cons of potential solutions to the Korean government, to assist them in building the nation’s competitiveness in super-capacity computing capability.”
2015.07.07 View 7328 -
 The Minister of Education of Kazakhstan Visits KAIST
The Minister of Education of the Republic of Kazakhstan, Aslan Sarinzhipov, and his delegation visited KAIST on June 30, 2015.
Dr. Young-Suk Ji, the Chairman of Elsevier, an academic publishing company that publishes medical and scientific literature, arranged the visit.
The Kazakh delegation showed great interest in KAIST’s educational system and research programs during their meeting with President Steve Kang of KAIST. In particular, the delegation was most impressed by the startups and entrepreneurship programs established at the KAIST Pangyo Innovation Center.
President Kang said, “I hope the Minister’s visit will help inspire more Kazakh students to come to Korea and study at KAIST.”
Kazakhstan, located in the northern part of Central Asia, gained its independence in 1991 after the collapse of the Soviet Union. Currently, there are 22 Kazakh students studying at KAIST.
2015.07.03 View 5635
The Minister of Education of Kazakhstan Visits KAIST
The Minister of Education of the Republic of Kazakhstan, Aslan Sarinzhipov, and his delegation visited KAIST on June 30, 2015.
Dr. Young-Suk Ji, the Chairman of Elsevier, an academic publishing company that publishes medical and scientific literature, arranged the visit.
The Kazakh delegation showed great interest in KAIST’s educational system and research programs during their meeting with President Steve Kang of KAIST. In particular, the delegation was most impressed by the startups and entrepreneurship programs established at the KAIST Pangyo Innovation Center.
President Kang said, “I hope the Minister’s visit will help inspire more Kazakh students to come to Korea and study at KAIST.”
Kazakhstan, located in the northern part of Central Asia, gained its independence in 1991 after the collapse of the Soviet Union. Currently, there are 22 Kazakh students studying at KAIST.
2015.07.03 View 5635 -
 Professor Jae Kyu Lee Appointed the President of Association for Information Systems
Chair Professor Jae Kyu Lee of KAIST’s College of Business was appointed the President of Association for Information Systems (AIS) on July 1, 2015. Professor Lee will serve a one-year term, which will end in June 2016. With four thousand members researching information systems from 90 different nations, AIS is the largest academic society in the fields of information system and business process engineering.
Professor Lee has proposed his idea of “the Bright Internet” as the official vision of AIS. Employing this vision, AIS will create technology and systems, as well as sponsor international cooperation to solve fundamental issues of the Internet including concerns over hacking and cyber-related crimes.
The extent of damage from cyber-related crimes grows each year. Every day, 56 billion junk emails are sent to computers which are hacked and become “zombie” computers. The social cost of such crimes is estimated to be 400 billion US dollars annually.
Based on "the Bright Internet," AIS will build a preventative Internet security system by adopting ground rules that make attackers responsible for the damages from such crimes. The system will also modify technology and other systems to minimize privacy infringement while maintaining security. Finally, the Bright Internet proposes to adopt an international standard for this security system through collaboration with the International Telecommunications Union (ITU).
Professor Lee said, “The vision of the Bright Internet started from an awareness that we needed to resolve issues such as Internet addiction, indiscriminate media exposure, and verbal violence. This vision developed by the experts from all around the world will not only bring a revolution of a reliable Internet platform to a global scale but also reshape the Korean Internet platform.”
2015.07.02 View 8873
Professor Jae Kyu Lee Appointed the President of Association for Information Systems
Chair Professor Jae Kyu Lee of KAIST’s College of Business was appointed the President of Association for Information Systems (AIS) on July 1, 2015. Professor Lee will serve a one-year term, which will end in June 2016. With four thousand members researching information systems from 90 different nations, AIS is the largest academic society in the fields of information system and business process engineering.
Professor Lee has proposed his idea of “the Bright Internet” as the official vision of AIS. Employing this vision, AIS will create technology and systems, as well as sponsor international cooperation to solve fundamental issues of the Internet including concerns over hacking and cyber-related crimes.
The extent of damage from cyber-related crimes grows each year. Every day, 56 billion junk emails are sent to computers which are hacked and become “zombie” computers. The social cost of such crimes is estimated to be 400 billion US dollars annually.
Based on "the Bright Internet," AIS will build a preventative Internet security system by adopting ground rules that make attackers responsible for the damages from such crimes. The system will also modify technology and other systems to minimize privacy infringement while maintaining security. Finally, the Bright Internet proposes to adopt an international standard for this security system through collaboration with the International Telecommunications Union (ITU).
Professor Lee said, “The vision of the Bright Internet started from an awareness that we needed to resolve issues such as Internet addiction, indiscriminate media exposure, and verbal violence. This vision developed by the experts from all around the world will not only bring a revolution of a reliable Internet platform to a global scale but also reshape the Korean Internet platform.”
2015.07.02 View 8873 -
 KAIST Professor Sung-Ju Lee Appointed a Technical Program Chair of INFOCOM
Professor Sung-Ju Lee of the Department of Computer Science at KAIST has been appointed to serve as a technical program chair of IEEE INFOCOME. The computer communication conference, started in 1982, is influential in the research fields of the Internet, wireless, and data centers.
Professor Lee is the first Korean to serve as a program chair. He has been acknowledged for his work in network communications. In the 34th conference, which will be held next year, he will take part in selecting 650 experts in the field to become members and supervise the evaluation of around 1,600 papers.
Professor Lee is the leading researcher in the field of wireless mobile network systems. He is a fellow of the Institute of Electrical and Electronics Engineers (IEEE) and served as the general chair of the 20th Association for Computing Machinery (ACM) SIGMOBILE Annual International Conference on Mobile Computing & Networking (MobiCom 2014). He is on the editorial boards of IEEE Transactions on Mobile Computing (TMC) and IEEE Internet of Things Journals.
Professor Lee said, “I hope to continue the traditions of the conference, as well as integrating research from various areas of network communication. I will strive to create a program with high technology transfer probability.”
The 34th IEEE INFOCOM will take place in San Francisco in April 2016.
2015.07.02 View 9810
KAIST Professor Sung-Ju Lee Appointed a Technical Program Chair of INFOCOM
Professor Sung-Ju Lee of the Department of Computer Science at KAIST has been appointed to serve as a technical program chair of IEEE INFOCOME. The computer communication conference, started in 1982, is influential in the research fields of the Internet, wireless, and data centers.
Professor Lee is the first Korean to serve as a program chair. He has been acknowledged for his work in network communications. In the 34th conference, which will be held next year, he will take part in selecting 650 experts in the field to become members and supervise the evaluation of around 1,600 papers.
Professor Lee is the leading researcher in the field of wireless mobile network systems. He is a fellow of the Institute of Electrical and Electronics Engineers (IEEE) and served as the general chair of the 20th Association for Computing Machinery (ACM) SIGMOBILE Annual International Conference on Mobile Computing & Networking (MobiCom 2014). He is on the editorial boards of IEEE Transactions on Mobile Computing (TMC) and IEEE Internet of Things Journals.
Professor Lee said, “I hope to continue the traditions of the conference, as well as integrating research from various areas of network communication. I will strive to create a program with high technology transfer probability.”
The 34th IEEE INFOCOM will take place in San Francisco in April 2016.
2015.07.02 View 9810 -
 Professor Naehyuck Jang was Appointed Technical Program Chair of the Design Automation Conference
Professor Naehyuck Jang of the Electrical Engineering Department at KAIST was appointed as the technical program chair of the Design Automation Conference (DAC). He is the first Asian to serve the conference as the chair. At next year’s conference, he will select 150 program committee members and supervise the selection process of 1,000 papers.
Founded in 1964, DAC encompasses research related to automation of semiconductor processes, which usually involve billions of transistors. More than seven thousand people and 150 companies from all around the world participate, of which only the top 20% of the submitted papers are selected. It is the most prestigious conference in the field of semiconductor automation.
The Design Automation Conference also introduces optimization and automation of design processes of systems, hardware security, automobiles, and the Internet of things. Professor Jang specializes in low power system designs. As an ACM Distinguished Scientist, Professor Jang was elected as the chairman after contributing to this year’s program committee by reforming the process of selection of papers.
Professor Jang said, “This year’s conference represents a departure, where we move from the field of traditional semiconductors to the optimization of embedded system, the Internet of things, and security. He added that “we want to create a paper selection process that can propose the future of design automation.”
The 53rd annual DAC will take place at the Austin Convention Center in Texas in June 2016.
2015.07.02 View 7969
Professor Naehyuck Jang was Appointed Technical Program Chair of the Design Automation Conference
Professor Naehyuck Jang of the Electrical Engineering Department at KAIST was appointed as the technical program chair of the Design Automation Conference (DAC). He is the first Asian to serve the conference as the chair. At next year’s conference, he will select 150 program committee members and supervise the selection process of 1,000 papers.
Founded in 1964, DAC encompasses research related to automation of semiconductor processes, which usually involve billions of transistors. More than seven thousand people and 150 companies from all around the world participate, of which only the top 20% of the submitted papers are selected. It is the most prestigious conference in the field of semiconductor automation.
The Design Automation Conference also introduces optimization and automation of design processes of systems, hardware security, automobiles, and the Internet of things. Professor Jang specializes in low power system designs. As an ACM Distinguished Scientist, Professor Jang was elected as the chairman after contributing to this year’s program committee by reforming the process of selection of papers.
Professor Jang said, “This year’s conference represents a departure, where we move from the field of traditional semiconductors to the optimization of embedded system, the Internet of things, and security. He added that “we want to create a paper selection process that can propose the future of design automation.”
The 53rd annual DAC will take place at the Austin Convention Center in Texas in June 2016.
2015.07.02 View 7969 -
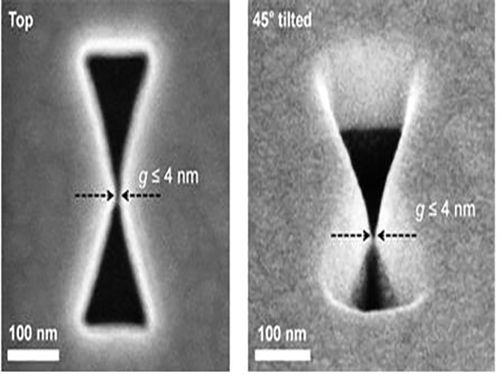 3D Plasmon Antenna Capable of Focusing Light into Few Nanometers
Professors Myung-Ki Kim and Yong-Hee Lee, both of the Physics Department at KAIST, and their research teams have developed a three dimensional (3D) gap-plasmon antenna which can focus light into a space a few nanometers wide. Their research findings were published in the June 10th issue of Nano Letters.
Focusing light into a point-like space is an active research field with many applications. However, concentrating light into a smaller space than its wavelength is often hindered by diffraction. To tackle this problem, many researchers have utilized the plasmonic phenomenon of a metal where light can be confined to a greater extent by overcoming the diffraction limit.
Many researchers have focused on developing a two dimensional (2D) plasmon antenna and were able to focus a light under 5 nanometers wide. However, this 2D antenna revealed a challenge: the light disperses to the opposite end regardless of how small its beam was focused. To solve this difficulty, a 3D structure had to be employed to maximize the light's intensity.
Adopting the proximal focused-ion-beam milling technology, the KAIST research team developed a 3D four nanometer wide gap-plasmon antenna. By squeezing the photons into a 3D nano space of 4 x 10 x 10 nm3 size, the researchers were able to increase the intensity of light by 400,000 times stronger than that of the incident light. Capitalizing on the enhanced intensity of light within the antenna, they intensified the second-harmonic signal and verified that the light was focused in the nano gap by scanning cathodoluminescent images.
The researchers anticipate that this technology will improve the speed of data transfer and processing up to the level of a terahertz (one trillion times per second) and to enlarge the storage volume per unit area on hard disks by 100 times. In addition, high definition images of submolecule size can be taken with actual light, instead of with an electron microscope, while improving the semiconductor process to a smaller size of few nanometers.
Professor Kim said, “A simple yet ingenious idea has shifted the research paradigm from 2D gap-plasmon antennas to 3D antennas. This technology will see numerous applications including in the field of information technology, data storage, imaging medical science, and semiconductor processes.”
The research was sponsored by the National Research Foundation of Korea.
Figure 1: 3D Gap-Plasmon Antenna Structure and Simulation Results
Figure 2 – Constructed 3D Gap-Plasmon Antenna Structure
Figure 3 – Amplified Second Harmonic Signal Generation and Light Focused in the Nano Gap
2015.06.24 View 10976
3D Plasmon Antenna Capable of Focusing Light into Few Nanometers
Professors Myung-Ki Kim and Yong-Hee Lee, both of the Physics Department at KAIST, and their research teams have developed a three dimensional (3D) gap-plasmon antenna which can focus light into a space a few nanometers wide. Their research findings were published in the June 10th issue of Nano Letters.
Focusing light into a point-like space is an active research field with many applications. However, concentrating light into a smaller space than its wavelength is often hindered by diffraction. To tackle this problem, many researchers have utilized the plasmonic phenomenon of a metal where light can be confined to a greater extent by overcoming the diffraction limit.
Many researchers have focused on developing a two dimensional (2D) plasmon antenna and were able to focus a light under 5 nanometers wide. However, this 2D antenna revealed a challenge: the light disperses to the opposite end regardless of how small its beam was focused. To solve this difficulty, a 3D structure had to be employed to maximize the light's intensity.
Adopting the proximal focused-ion-beam milling technology, the KAIST research team developed a 3D four nanometer wide gap-plasmon antenna. By squeezing the photons into a 3D nano space of 4 x 10 x 10 nm3 size, the researchers were able to increase the intensity of light by 400,000 times stronger than that of the incident light. Capitalizing on the enhanced intensity of light within the antenna, they intensified the second-harmonic signal and verified that the light was focused in the nano gap by scanning cathodoluminescent images.
The researchers anticipate that this technology will improve the speed of data transfer and processing up to the level of a terahertz (one trillion times per second) and to enlarge the storage volume per unit area on hard disks by 100 times. In addition, high definition images of submolecule size can be taken with actual light, instead of with an electron microscope, while improving the semiconductor process to a smaller size of few nanometers.
Professor Kim said, “A simple yet ingenious idea has shifted the research paradigm from 2D gap-plasmon antennas to 3D antennas. This technology will see numerous applications including in the field of information technology, data storage, imaging medical science, and semiconductor processes.”
The research was sponsored by the National Research Foundation of Korea.
Figure 1: 3D Gap-Plasmon Antenna Structure and Simulation Results
Figure 2 – Constructed 3D Gap-Plasmon Antenna Structure
Figure 3 – Amplified Second Harmonic Signal Generation and Light Focused in the Nano Gap
2015.06.24 View 10976