TR
-
 Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 8045
Professor Jae-Woong Jeong Receives Hyonwoo KAIST Academic Award
Professor Jae-Woong Jeong from the School of Electrical Engineering was selected for the Hyonwoo KAIST Academic Award, funded by the HyonWoo Cultural Foundation (Chairman Soo-il Kwak, honorary professor at Seoul National University Business School).
The Hyonwoo KAIST Academic Award, presented for the first time in 2021, is an award newly founded by the donations of Chairman Soo-il Kwak of the HyonWoo Cultural Foundation, who aims to reward excellent KAIST scholars who have made outstanding academic achievements.
Every year, through the strict evaluations of the selection committee of the HyonWoo Cultural Foundation and the faculty reward recommendation board, KAIST will choose one faculty member that may represent the school with their excellent academic achievement, and reward them with a plaque and 100 million won.
Professor Jae-Woong Jeong, the winner of this year’s award, developed the first IoT-based wireless remote brain neural network control system to overcome brain diseases, and has been leading the field. The research was published in 2021 in Nature Biomedical Engineering, one of world’s best scientific journals, and has been recognized as a novel technology that suggested a new vision for the automation of brain research and disease treatment. This study, led by Professor Jeong’s research team, was part of the KAIST College of Engineering Global Initiative Interdisciplinary Research Project, and was jointly studied by Washington University School of Medicine through an international research collaboration. The technology was introduced more than 60 times through both domestic and international media, including Medical Xpress, MBC News, and Maeil Business News.
Professor Jeong has also developed a wirelessly chargeable soft machine for brain transplants, and the results were published in Nature Communications. He thereby opened a new paradigm for implantable semi-permanent devices for transplants, and is making unprecedented research achievements.
2022.06.13 View 8045 -
 Game Design Guide Book for Middle-Aged and Older Adult Players Helps Rewrite Gaming Culture
The online book ‘Game Design Guide for Adults in Their 50s and Older’ helps to increase accessibility for adult gamers
A KAIST multi-disciplinary research team published a game guide to respond to the new demands of senior gamers and expand the gaming market. The guide will be helpful for designing interfaces fit for senior groups as a way to minimize the cognitive burdens related to aging. It also helps readers understand older users’ cognitive abilities and socioemotional characteristics.
“This guide analyzed the game experience of players in their 50s and older and converted it into a game design element that can be easily referred to by game developers and designers,” explained Professor Young Im Do from the Graduate School of Culture Technology who led the research.
The gaming industry is paying attention to the emerging trend of ‘active aging’ and senior gamers. According to the National Purchase Diary Panel Inc., game play time increased significantly in the 45-64 age group compared to other age groups during the pandemic. Despite the growing number of senior gamers, it is still difficult for older novice players to start video games because most commercial games focus on younger players. For example, older players can feel frustrated if the game requires fast reflexes and accurate timing. Font sizes and objects that are too small as well as interfaces that are too complicated can be challenging for senior gamers.
The research team presents how to handle these difficulties in game design considering the visual-motor coordination of people in age groups ranging from their 20s to 80s. It also proposes various game elements such as audio-visual elements, cognitive and motor elements, game rules, stories and characters, social aspects, in-app purchases, and advertisements for senior groups. The guide also proposes a game service model and introduces examples of game prototypes that apply supportive technology.
For this guide, the researchers operated the “International Game Living Lab”, which is an open space for creating novel and innovative solutions by converging IT technology into daily life. In the lab, ordinary citizens, research institutes, companies, and local communities formed a cooperative network and actively participated in experiments, education, and discussions for finding solutions over three years.
Researchers in multi-disciplinary fields, including computer science, psychology, game design, and gerontechnology, covered various methodologies to understand the game experience of adults in their 50s and older. In order to profile players of this age group, three different approaches were performed: visual-motor coordination experiments, an EEG (Electroencephalogram) test, and a gameplay workshop. Then, they converted the results into practical knowledge that can be used in the gaming industry.
Professor Kyung Myun Lee from the School of Digital Humanities and Computational Social Sciences at KAIST, Professor Byungjoo Shin from Yonsei University, CEO Junyoung Shin of CareU, and CEO Minseok Doh of Heartverse participated in this online book which is available to the public at https://wikidocs.net/book/7356.
2022.06.10 View 6309
Game Design Guide Book for Middle-Aged and Older Adult Players Helps Rewrite Gaming Culture
The online book ‘Game Design Guide for Adults in Their 50s and Older’ helps to increase accessibility for adult gamers
A KAIST multi-disciplinary research team published a game guide to respond to the new demands of senior gamers and expand the gaming market. The guide will be helpful for designing interfaces fit for senior groups as a way to minimize the cognitive burdens related to aging. It also helps readers understand older users’ cognitive abilities and socioemotional characteristics.
“This guide analyzed the game experience of players in their 50s and older and converted it into a game design element that can be easily referred to by game developers and designers,” explained Professor Young Im Do from the Graduate School of Culture Technology who led the research.
The gaming industry is paying attention to the emerging trend of ‘active aging’ and senior gamers. According to the National Purchase Diary Panel Inc., game play time increased significantly in the 45-64 age group compared to other age groups during the pandemic. Despite the growing number of senior gamers, it is still difficult for older novice players to start video games because most commercial games focus on younger players. For example, older players can feel frustrated if the game requires fast reflexes and accurate timing. Font sizes and objects that are too small as well as interfaces that are too complicated can be challenging for senior gamers.
The research team presents how to handle these difficulties in game design considering the visual-motor coordination of people in age groups ranging from their 20s to 80s. It also proposes various game elements such as audio-visual elements, cognitive and motor elements, game rules, stories and characters, social aspects, in-app purchases, and advertisements for senior groups. The guide also proposes a game service model and introduces examples of game prototypes that apply supportive technology.
For this guide, the researchers operated the “International Game Living Lab”, which is an open space for creating novel and innovative solutions by converging IT technology into daily life. In the lab, ordinary citizens, research institutes, companies, and local communities formed a cooperative network and actively participated in experiments, education, and discussions for finding solutions over three years.
Researchers in multi-disciplinary fields, including computer science, psychology, game design, and gerontechnology, covered various methodologies to understand the game experience of adults in their 50s and older. In order to profile players of this age group, three different approaches were performed: visual-motor coordination experiments, an EEG (Electroencephalogram) test, and a gameplay workshop. Then, they converted the results into practical knowledge that can be used in the gaming industry.
Professor Kyung Myun Lee from the School of Digital Humanities and Computational Social Sciences at KAIST, Professor Byungjoo Shin from Yonsei University, CEO Junyoung Shin of CareU, and CEO Minseok Doh of Heartverse participated in this online book which is available to the public at https://wikidocs.net/book/7356.
2022.06.10 View 6309 -
 Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 9434
Now You Can See Floral Scents!
Optical interferometry visualizes how often lilies emit volatile organic compounds
Have you ever thought about when flowers emit their scents?
KAIST mechanical engineers and biological scientists directly visualized how often a lily releases a floral scent using a laser interferometry method. These measurement results can provide new insights for understanding and further exploring the biosynthesis and emission mechanisms of floral volatiles.
Why is it important to know this? It is well known that the fragrance of flowers affects their interactions with pollinators, microorganisms, and florivores. For instance, many flowering plants can tune their scent emission rates when pollinators are active for pollination. Petunias and the wild tobacco Nicotiana attenuata emit floral scents at night to attract night-active pollinators. Thus, visualizing scent emissions can help us understand the ecological evolution of plant-pollinator interactions.
Many groups have been trying to develop methods for scent analysis. Mass spectrometry has been one widely used method for investigating the fragrance of flowers. Although mass spectrometry reveals the quality and quantity of floral scents, it is impossible to directly measure the releasing frequency. A laser-based gas detection system and a smartphone-based detection system using chemo-responsive dyes have also been used to measure volatile organic compounds (VOCs) in real-time, but it is still hard to measure the time-dependent emission rate of floral scents.
However, the KAIST research team co-led by Professor Hyoungsoo Kim from the Department of Mechanical Engineering and Professor Sang-Gyu Kim from the Department of Biological Sciences measured a refractive index difference between the vapor of the VOCs of lilies and the air to measure the emission frequency. The floral scent vapor was detected and the refractive index of air was 1.0 while that of the major floral scent of a linalool lily was 1.46.
Professor Hyoungsoo Kim said, “We expect this technology to be further applicable to various industrial sectors such as developing it to detect hazardous substances in a space.” The research team also plans to identify the DNA mechanism that controls floral scent secretion.
The current work entitled “Real-time visualization of scent accumulation reveals the frequency of floral scent emissions” was published in ‘Frontiers in Plant Science’ on April 18, 2022. (https://doi.org/10.3389/fpls.2022.835305).
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2021R1A2C2007835), the Rural Development Administration (PJ016403), and the KAIST-funded Global Singularity Research PREP-Program.
-Publication:H. Kim, G. Lee, J. Song, and S.-G. Kim, "Real-time visualization of scent accumulation reveals the frequency of floral scent emissions," Frontiers in Plant Science 18, 835305 (2022) (https://doi.org/10.3389/fpls.2022.835305)
-Profile:Professor Hyoungsoo Kimhttp://fil.kaist.ac.kr
@MadeInH on TwitterDepartment of Mechanical EngineeringKAIST
Professor Sang-Gyu Kimhttps://sites.google.com/view/kimlab/home Department of Biological SciencesKAIST
2022.05.25 View 9434 -
 VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 10257
VP Sang Yup Lee Receives Honorary Doctorate from DTU
Vice President for Research, Distinguished Professor Sang Yup Lee at the Department of Chemical & Biomolecular Engineering, was awarded an honorary doctorate from the Technical University of Denmark (DTU) during the DTU Commemoration Day 2022 on April 29. The event drew distinguished guests, students, and faculty including HRH The Crown Prince Frederik Andre Henrik Christian and DTU President Anders Bjarklev.
Professor Lee was recognized for his exceptional scholarship in the field of systems metabolic engineering, which led to the development of microcell factories capable of producing a wide range of fuels, chemicals, materials, and natural compounds, many for the first time.
Professor Lee said in his acceptance speech that KAIST’s continued partnership with DTU in the field of biotechnology will lead to significant contributions in the global efforts to respond to climate change and promote green growth.
DTU CPO and CSO Dina Petronovic Nielson, who heads DTU Biosustain, also lauded Professor Lee saying, “It is not only a great honor for Professor Lee to be induced at DTU but also great honor for DTU to have him.”
Professor Lee also gave commemorative lectures at DTU Biosustain in Lingby and the Bio Innovation Research Institute at the Novo Nordisk Foundation in Copenhagen while in Denmark.
DTU, one of the leading science and technology universities in Europe, has been awarding honorary doctorates since 1921, including to Nobel laureate in chemistry Professor Frances Arnold at Caltech. Professor Lee is the first Korean to receive an honorary doctorate from DTU.
2022.05.03 View 10257 -
 Quantum Technology: the Next Game Changer?
The 6th KAIST Global Strategy Institute Forum explores how quantum technology has evolved into a new growth engine for the future
The participants of the 6th KAIST Global Strategy Institute (GSI) Forum on April 20 agreed that the emerging technology of quantum computing will be a game changer of the future. As KAIST President Kwang Hyung Lee said in his opening remarks, the future is quantum and that future is rapidly approaching. Keynote speakers and panelists presented their insights on the disruptive innovations we are already experiencing.
The three keynote speakers included Dr. Jerry M. Chow, IBM fellow and director of quantum infrastructure, Professor John Preskill from Caltech, and Professor Jungsang Kim from Duke University. They discussed the academic impact and industrial applications of quantum technology, and its prospects for the future.
Dr. Chow leads IBM Quantum’s hardware system development efforts, focusing on research and system deployment. Professor Preskill is one of the leading quantum information science and quantum computation scholars. He coined the term “quantum supremacy.” Professor Kim is the co-founder and CTO of IonQ Inc., which develops general-purpose trapped ion quantum computers and software to generate, optimize, and execute quantum circuits.
Two leading quantum scholars from KAIST, Professor June-Koo Kevin Rhee and Professor Youngik Sohn, and Professor Andreas Heinrich from the IBS Center for Quantum Nanoscience also participated in the forum as panelists. Professor Rhee is the founder of the nation’s first quantum computing software company and leads the AI Quantum Computing IT Research Center at KAIST.
During the panel session, Professor Rhee said that although it is undeniable the quantum computing will be a game changer, there are several challenges. For instance, the first actual quantum computer is NISQ (Noisy intermediate-scale quantum era), which is still incomplete. However, it is expected to outperform a supercomputer. Until then, it is important to advance the accuracy of quantum computation in order to offer super computation speeds.
Professor Sohn, who worked at PsiQuantum, detailed how quantum computers will affect our society. He said that PsiQuantum is developing silicon photonics that will control photons. We can’t begin to imagine how silicon photonics will transform our society. Things will change slowly but the scale would be massive.
The keynote speakers presented how quantum cryptography communications and its sensing technology will serve as disruptive innovations. Dr. Chow stressed that to realize the potential growth and innovation, additional R&D is needed. More research groups and scholars should be nurtured. Only then will the rich R&D resources be able to create breakthroughs in quantum-related industries. Lastly, the commercialization of quantum computing must be advanced, which will enable the provision of diverse services. In addition, more technological and industrial infrastructure must be built to better accommodate quantum computing.
Professor Preskill believes that quantum computing will benefit humanity. He cited two basic reasons for his optimistic prediction: quantum complexity and quantum error corrections. He explained why quantum computing is so powerful: quantum computer can easily solve the problems classically considered difficult, such as factorization. In addition, quantum computer has the potential to efficiently simulate all of the physical processes taking place in nature.
Despite such dramatic advantages, why does it seem so difficult? Professor Preskill believes this is because we want qubits (quantum bits or ‘qubits’ are the basic unit of quantum information) to interact very strongly with each other. Because computations can fail, we don’t want qubits to interact with the environment unless we can control and predict them.
As for quantum computing in the NISQ era, he said that NISQ will be an exciting tool for exploring physics. Professor Preskill does not believe that NISQ will change the world alone, rather it is a step forward toward more powerful quantum technologies in the future. He added that a potentially transformable, scalable quantum computer could still be decades away.
Professor Preskill said that a large number of qubits, higher accuracy, and better quality will require a significant investment. He said if we expand with better ideas, we can make a better system. In the longer term, quantum technology will bring significant benefits to the technological sectors and society in the fields of materials, physics, chemistry, and energy production.
Professor Kim from Duke University presented on the practical applications of quantum computing, especially in the startup environment. He said that although there is no right answer for the early applications of quantum computing, developing new approaches to solve difficult problems and raising the accessibility of the technology will be important for ensuring the growth of technology-based startups.
2022.04.21 View 11473
Quantum Technology: the Next Game Changer?
The 6th KAIST Global Strategy Institute Forum explores how quantum technology has evolved into a new growth engine for the future
The participants of the 6th KAIST Global Strategy Institute (GSI) Forum on April 20 agreed that the emerging technology of quantum computing will be a game changer of the future. As KAIST President Kwang Hyung Lee said in his opening remarks, the future is quantum and that future is rapidly approaching. Keynote speakers and panelists presented their insights on the disruptive innovations we are already experiencing.
The three keynote speakers included Dr. Jerry M. Chow, IBM fellow and director of quantum infrastructure, Professor John Preskill from Caltech, and Professor Jungsang Kim from Duke University. They discussed the academic impact and industrial applications of quantum technology, and its prospects for the future.
Dr. Chow leads IBM Quantum’s hardware system development efforts, focusing on research and system deployment. Professor Preskill is one of the leading quantum information science and quantum computation scholars. He coined the term “quantum supremacy.” Professor Kim is the co-founder and CTO of IonQ Inc., which develops general-purpose trapped ion quantum computers and software to generate, optimize, and execute quantum circuits.
Two leading quantum scholars from KAIST, Professor June-Koo Kevin Rhee and Professor Youngik Sohn, and Professor Andreas Heinrich from the IBS Center for Quantum Nanoscience also participated in the forum as panelists. Professor Rhee is the founder of the nation’s first quantum computing software company and leads the AI Quantum Computing IT Research Center at KAIST.
During the panel session, Professor Rhee said that although it is undeniable the quantum computing will be a game changer, there are several challenges. For instance, the first actual quantum computer is NISQ (Noisy intermediate-scale quantum era), which is still incomplete. However, it is expected to outperform a supercomputer. Until then, it is important to advance the accuracy of quantum computation in order to offer super computation speeds.
Professor Sohn, who worked at PsiQuantum, detailed how quantum computers will affect our society. He said that PsiQuantum is developing silicon photonics that will control photons. We can’t begin to imagine how silicon photonics will transform our society. Things will change slowly but the scale would be massive.
The keynote speakers presented how quantum cryptography communications and its sensing technology will serve as disruptive innovations. Dr. Chow stressed that to realize the potential growth and innovation, additional R&D is needed. More research groups and scholars should be nurtured. Only then will the rich R&D resources be able to create breakthroughs in quantum-related industries. Lastly, the commercialization of quantum computing must be advanced, which will enable the provision of diverse services. In addition, more technological and industrial infrastructure must be built to better accommodate quantum computing.
Professor Preskill believes that quantum computing will benefit humanity. He cited two basic reasons for his optimistic prediction: quantum complexity and quantum error corrections. He explained why quantum computing is so powerful: quantum computer can easily solve the problems classically considered difficult, such as factorization. In addition, quantum computer has the potential to efficiently simulate all of the physical processes taking place in nature.
Despite such dramatic advantages, why does it seem so difficult? Professor Preskill believes this is because we want qubits (quantum bits or ‘qubits’ are the basic unit of quantum information) to interact very strongly with each other. Because computations can fail, we don’t want qubits to interact with the environment unless we can control and predict them.
As for quantum computing in the NISQ era, he said that NISQ will be an exciting tool for exploring physics. Professor Preskill does not believe that NISQ will change the world alone, rather it is a step forward toward more powerful quantum technologies in the future. He added that a potentially transformable, scalable quantum computer could still be decades away.
Professor Preskill said that a large number of qubits, higher accuracy, and better quality will require a significant investment. He said if we expand with better ideas, we can make a better system. In the longer term, quantum technology will bring significant benefits to the technological sectors and society in the fields of materials, physics, chemistry, and energy production.
Professor Kim from Duke University presented on the practical applications of quantum computing, especially in the startup environment. He said that although there is no right answer for the early applications of quantum computing, developing new approaches to solve difficult problems and raising the accessibility of the technology will be important for ensuring the growth of technology-based startups.
2022.04.21 View 11473 -
 Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 7055
Professor Hyunjoo Jenny Lee to Co-Chair IEEE MEMS 2025
Professor Hyunjoo Jenny Lee from the School of Electrical Engineering has been appointed General Chair of the 38th IEEE MEMS 2025 (International Conference on Micro Electro Mechanical Systems). Professor Lee, who is 40, is the conference’s youngest General Chair to date and will work jointly with Professor Sheng-Shian Li of Taiwan’s National Tsing Hua University as co-chairs in 2025.
IEEE MEMS is a top-tier international conference on microelectromechanical systems and it serves as a core academic showcase for MEMS research and technology in areas such as microsensors and actuators.
With over 800 MEMS paper submissions each year, the conference only accepts and publishes about 250 of them after a rigorous review process recognized for its world-class prestige. Of all the submissions, fewer than 10% are chosen for oral presentations.
2022.04.18 View 7055 -
 Distinguished Professor Sukbok Chang Named the 2022 Ho-Am Laureate
Distinguished Professor Sukbok Chang from the Department of Chemistry was named the awardee of the Ho-Am Prize in the fields of chemistry and life sciences. The award has recognized the most distinguished scholars, individuals, and organizations in physics and mathematics, chemistry and life sciences, engineering, medicine, arts, and community service in honor of the late founder of Samsung Group Byong-Chul Lee, whose penname is Ho-Am. The awards ceremony will be held on May 31 and awardees will receive 300 million KRW in prize money.
Professor Chang became the fourth KAIST Ho-Am laureate following Distinguished Professor Sang Yup Lee in engineering in 2014, Distinguished Professor Jun Ho Oh in engineering in 2016, and Distinguished Professor Gou Young Koh in medicine in 2018.
Professor Chang is a renowned chemist who has made pioneering research in the area of transition metal catalysis for organic transformations. Professor Chang is also one of the Highly Cited Researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. He has made the list seven years in a row from 2016.
Professor Chang has developed a range of new and impactful C-H bond functionalization reactions. By using his approaches, value-added molecules can be readily produced from chemical feedstocks, representatively hydrocarbons and (hetero)arenes. His research team elucidated fundamental key mechanistic aspects in the course of the essential C-H bond activation process of unreactive starting materials. He was able to utilize the obtained mechanistic understanding for the subsequent catalyst design to develop more efficient and highly (stereo)selective catalytic reactions.
Among the numerous contributions he made, the design of new mechanistic approaches toward metal nitrenoid transfers are of especially high impact to the chemical community. Indeed, a series of important transition metal catalyst systems were developed by Professor Chang to enable the direct and selective C-H amidation of unreactive organic compounds, thereby producing aminated compounds that have important applicability in synthetic, medicinal, and materials science. He has also pioneered in the area of asymmetric C-H amination chemistry by creatively devising various types of chiral transition metal catalyst systems, and his team proved for the first time that chiral lactam compounds can be obtained at an excellent level of stereoselectivity.
Another significant contribution of Professor. Chang was the introduction of dioxazolones as a robust but highly reactive source of acyl nitrenoids for the catalytic C-H amidation reactions, and this reagent is now broadly utilized in synthetic chemistry worldwide.
Professor Chang also leads a research group in the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science.
2022.04.06 View 7890
Distinguished Professor Sukbok Chang Named the 2022 Ho-Am Laureate
Distinguished Professor Sukbok Chang from the Department of Chemistry was named the awardee of the Ho-Am Prize in the fields of chemistry and life sciences. The award has recognized the most distinguished scholars, individuals, and organizations in physics and mathematics, chemistry and life sciences, engineering, medicine, arts, and community service in honor of the late founder of Samsung Group Byong-Chul Lee, whose penname is Ho-Am. The awards ceremony will be held on May 31 and awardees will receive 300 million KRW in prize money.
Professor Chang became the fourth KAIST Ho-Am laureate following Distinguished Professor Sang Yup Lee in engineering in 2014, Distinguished Professor Jun Ho Oh in engineering in 2016, and Distinguished Professor Gou Young Koh in medicine in 2018.
Professor Chang is a renowned chemist who has made pioneering research in the area of transition metal catalysis for organic transformations. Professor Chang is also one of the Highly Cited Researchers who rank in the top 1% of citations by field and publication year in the Web of Science citation index. He has made the list seven years in a row from 2016.
Professor Chang has developed a range of new and impactful C-H bond functionalization reactions. By using his approaches, value-added molecules can be readily produced from chemical feedstocks, representatively hydrocarbons and (hetero)arenes. His research team elucidated fundamental key mechanistic aspects in the course of the essential C-H bond activation process of unreactive starting materials. He was able to utilize the obtained mechanistic understanding for the subsequent catalyst design to develop more efficient and highly (stereo)selective catalytic reactions.
Among the numerous contributions he made, the design of new mechanistic approaches toward metal nitrenoid transfers are of especially high impact to the chemical community. Indeed, a series of important transition metal catalyst systems were developed by Professor Chang to enable the direct and selective C-H amidation of unreactive organic compounds, thereby producing aminated compounds that have important applicability in synthetic, medicinal, and materials science. He has also pioneered in the area of asymmetric C-H amination chemistry by creatively devising various types of chiral transition metal catalyst systems, and his team proved for the first time that chiral lactam compounds can be obtained at an excellent level of stereoselectivity.
Another significant contribution of Professor. Chang was the introduction of dioxazolones as a robust but highly reactive source of acyl nitrenoids for the catalytic C-H amidation reactions, and this reagent is now broadly utilized in synthetic chemistry worldwide.
Professor Chang also leads a research group in the Center for Catalytic Hydrocarbon Functionalizations at the Institute for Basic Science.
2022.04.06 View 7890 -
 Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 10055
Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 10055 -
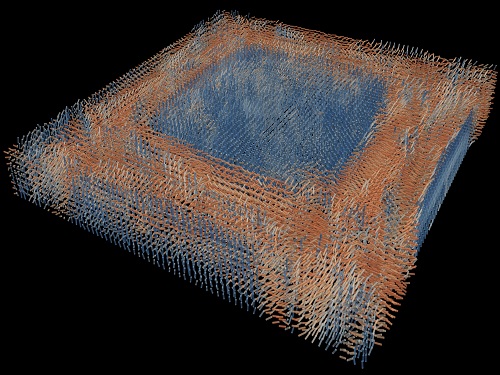 Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 8192
Tomographic Measurement of Dielectric Tensors
Dielectric tensor tomography allows the direct measurement of the 3D dielectric tensors of optically anisotropic structures
A research team reported the direct measurement of dielectric tensors of anisotropic structures including the spatial variations of principal refractive indices and directors. The group also demonstrated quantitative tomographic measurements of various nematic liquid-crystal structures and their fast 3D nonequilibrium dynamics using a 3D label-free tomographic method. The method was described in Nature Materials.
Light-matter interactions are described by the dielectric tensor. Despite their importance in basic science and applications, it has not been possible to measure 3D dielectric tensors directly. The main challenge was due to the vectorial nature of light scattering from a 3D anisotropic structure. Previous approaches only addressed 3D anisotropic information indirectly and were limited to two-dimensional, qualitative, strict sample conditions or assumptions.
The research team developed a method enabling the tomographic reconstruction of 3D dielectric tensors without any preparation or assumptions. A sample is illuminated with a laser beam with various angles and circularly polarization states. Then, the light fields scattered from a sample are holographically measured and converted into vectorial diffraction components. Finally, by inversely solving a vectorial wave equation, the 3D dielectric tensor is reconstructed.
Professor YongKeun Park said, “There were a greater number of unknowns in direct measuring than with the conventional approach. We applied our approach to measure additional holographic images by slightly tilting the incident angle.”
He said that the slightly tilted illumination provides an additional orthogonal polarization, which makes the underdetermined problem become the determined problem. “Although scattered fields are dependent on the illumination angle, the Fourier differentiation theorem enables the extraction of the same dielectric tensor for the slightly tilted illumination,” Professor Park added.
His team’s method was validated by reconstructing well-known liquid crystal (LC) structures, including the twisted nematic, hybrid aligned nematic, radial, and bipolar configurations. Furthermore, the research team demonstrated the experimental measurements of the non-equilibrium dynamics of annihilating, nucleating, and merging LC droplets, and the LC polymer network with repeating 3D topological defects.
“This is the first experimental measurement of non-equilibrium dynamics and 3D topological defects in LC structures in a label-free manner. Our method enables the exploration of inaccessible nematic structures and interactions in non-equilibrium dynamics,” first author Dr. Seungwoo Shin explained.
-PublicationSeungwoo Shin, Jonghee Eun, Sang Seok Lee, Changjae Lee, Herve Hugonnet, Dong Ki Yoon, Shin-Hyun Kim, Jongwoo Jeong, YongKeun Park, “Tomographic Measurement ofDielectric Tensors at Optical Frequency,” Nature Materials March 02, 2022 (https://doi.org/10/1038/s41563-022-01202-8)
-ProfileProfessor YongKeun ParkBiomedical Optics Laboratory (http://bmol.kaist.ac.kr)Department of PhysicsCollege of Natural SciencesKAIST
2022.03.22 View 8192 -
 Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 12071
Decoding Brain Signals to Control a Robotic Arm
Advanced brain-machine interface system successfully interprets arm movement directions from neural signals in the brain
Researchers have developed a mind-reading system for decoding neural signals from the brain during arm movement. The method, described in the journal Applied Soft Computing, can be used by a person to control a robotic arm through a brain-machine interface (BMI).
A BMI is a device that translates nerve signals into commands to control a machine, such as a computer or a robotic limb. There are two main techniques for monitoring neural signals in BMIs: electroencephalography (EEG) and electrocorticography (ECoG).
The EEG exhibits signals from electrodes on the surface of the scalp and is widely employed because it is non-invasive, relatively cheap, safe and easy to use. However, the EEG has low spatial resolution and detects irrelevant neural signals, which makes it difficult to interpret the intentions of individuals from the EEG.
On the other hand, the ECoG is an invasive method that involves placing electrodes directly on the surface of the cerebral cortex below the scalp. Compared with the EEG, the ECoG can monitor neural signals with much higher spatial resolution and less background noise. However, this technique has several drawbacks.
“The ECoG is primarily used to find potential sources of epileptic seizures, meaning the electrodes are placed in different locations for different patients and may not be in the optimal regions of the brain for detecting sensory and movement signals,” explained Professor Jaeseung Jeong, a brain scientist at KAIST. “This inconsistency makes it difficult to decode brain signals to predict movements.”
To overcome these problems, Professor Jeong’s team developed a new method for decoding ECoG neural signals during arm movement. The system is based on a machine-learning system for analysing and predicting neural signals called an ‘echo-state network’ and a mathematical probability model called the Gaussian distribution.
In the study, the researchers recorded ECoG signals from four individuals with epilepsy while they were performing a reach-and-grasp task. Because the ECoG electrodes were placed according to the potential sources of each patient’s epileptic seizures, only 22% to 44% of the electrodes were located in the regions of the brain responsible for controlling movement.
During the movement task, the participants were given visual cues, either by placing a real tennis ball in front of them, or via a virtual reality headset showing a clip of a human arm reaching forward in first-person view. They were asked to reach forward, grasp an object, then return their hand and release the object, while wearing motion sensors on their wrists and fingers. In a second task, they were instructed to imagine reaching forward without moving their arms.
The researchers monitored the signals from the ECoG electrodes during real and imaginary arm movements, and tested whether the new system could predict the direction of this movement from the neural signals. They found that the novel decoder successfully classified arm movements in 24 directions in three-dimensional space, both in the real and virtual tasks, and that the results were at least five times more accurate than chance. They also used a computer simulation to show that the novel ECoG decoder could control the movements of a robotic arm.
Overall, the results suggest that the new machine learning-based BCI system successfully used ECoG signals to interpret the direction of the intended movements. The next steps will be to improve the accuracy and efficiency of the decoder. In the future, it could be used in a real-time BMI device to help people with movement or sensory impairments.
This research was supported by the KAIST Global Singularity Research Program of 2021, Brain Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning, and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education.
-PublicationHoon-Hee Kim, Jaeseung Jeong, “An electrocorticographic decoder for arm movement for brain-machine interface using an echo state network and Gaussian readout,” Applied SoftComputing online December 31, 2021 (doi.org/10.1016/j.asoc.2021.108393)
-ProfileProfessor Jaeseung JeongDepartment of Bio and Brain EngineeringCollege of EngineeringKAIST
2022.03.18 View 12071 -
 'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 21848
'Fingerprint' Machine Learning Technique Identifies Different Bacteria in Seconds
A synergistic combination of surface-enhanced Raman spectroscopy and deep learning serves as an effective platform for separation-free detection of bacteria in arbitrary media
Bacterial identification can take hours and often longer, precious time when diagnosing infections and selecting appropriate treatments. There may be a quicker, more accurate process according to researchers at KAIST. By teaching a deep learning algorithm to identify the “fingerprint” spectra of the molecular components of various bacteria, the researchers could classify various bacteria in different media with accuracies of up to 98%.
Their results were made available online on Jan. 18 in Biosensors and Bioelectronics, ahead of publication in the journal’s April issue.
Bacteria-induced illnesses, those caused by direct bacterial infection or by exposure to bacterial toxins, can induce painful symptoms and even lead to death, so the rapid detection of bacteria is crucial to prevent the intake of contaminated foods and to diagnose infections from clinical samples, such as urine. “By using surface-enhanced Raman spectroscopy (SERS) analysis boosted with a newly proposed deep learning model, we demonstrated a markedly simple, fast, and effective route to classify the signals of two common bacteria and their resident media without any separation procedures,” said Professor Sungho Jo from the School of Computing.
Raman spectroscopy sends light through a sample to see how it scatters. The results reveal structural information about the sample — the spectral fingerprint — allowing researchers to identify its molecules. The surface-enhanced version places sample cells on noble metal nanostructures that help amplify the sample’s signals.
However, it is challenging to obtain consistent and clear spectra of bacteria due to numerous overlapping peak sources, such as proteins in cell walls. “Moreover, strong signals of surrounding media are also enhanced to overwhelm target signals, requiring time-consuming and tedious bacterial separation steps,” said Professor Yeon Sik Jung from the Department of Materials Science and Engineering.
To parse through the noisy signals, the researchers implemented an artificial intelligence method called deep learning that can hierarchically extract certain features of the spectral information to classify data. They specifically designed their model, named the dual-branch wide-kernel network (DualWKNet), to efficiently learn the correlation between spectral features. Such an ability is critical for analyzing one-dimensional spectral data, according to Professor Jo.
“Despite having interfering signals or noise from the media, which make the general shapes of different bacterial spectra and their residing media signals look similar, high classification accuracies of bacterial types and their media were achieved,” Professor Jo said, explaining that DualWKNet allowed the team to identify key peaks in each class that were almost indiscernible in individual spectra, enhancing the classification accuracies. “Ultimately, with the use of DualWKNet replacing the bacteria and media separation steps, our method dramatically reduces analysis time.”
The researchers plan to use their platform to study more bacteria and media types, using the information to build a training data library of various bacterial types in additional media to reduce the collection and detection times for new samples.
“We developed a meaningful universal platform for rapid bacterial detection with the collaboration between SERS and deep learning,” Professor Jo said. “We hope to extend the use of our deep learning-based SERS analysis platform to detect numerous types of bacteria in additional media that are important for food or clinical analysis, such as blood.”
The National R&D Program, through a National Research Foundation of Korea grant funded by the Ministry of Science and ICT, supported this research.
-PublicationEojin Rho, Minjoon Kim, Seunghee H. Cho, Bongjae Choi, Hyungjoon Park, Hanhwi Jang, Yeon Sik Jung, Sungho Jo, “Separation-free bacterial identification in arbitrary media via deepneural network-based SERS analysis,” Biosensors and Bioelectronics online January 18, 2022 (doi.org/10.1016/j.bios.2022.113991)
-ProfileProfessor Yeon Sik JungDepartment of Materials Science and EngineeringKAIST
Professor Sungho JoSchool of ComputingKAIST
2022.03.04 View 21848 -
 KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 7853
KAA Recognizes 4 Distinguished Alumni of the Year
The KAIST Alumni Association (KAA) recognized four distinguished alumni of the year during a ceremony on February 25 in Seoul. The four Distinguished Alumni Awardees are Distinguished Professor Sukbok Chang from the KAIST Department of Chemistry, Hyunshil Ahn, head of the AI Economy Institute and an editorial writer at The Korea Economic Daily, CEO Hwan-ho Sung of PSTech, and President Hark Kyu Park of Samsung Electronics.
Distinguished Professor Sukbok Chang who received his MS from the Department of Chemistry in 1985 has been a pioneer in the novel field of ‘carbon-hydrogen bond activation reactions’. He has significantly contributed to raising Korea’s international reputation in natural sciences and received the Kyungam Academic Award in 2013, the 14th Korea Science Award in 2015, the 1st Science and Technology Prize of Korea Toray in 2018, and the Best Scientist/Engineer Award Korea in 2019. Furthermore, he was named as a Highly Cited Researcher who ranked in the top 1% of citations by field and publication year in the Web of Science citation index for seven consecutive years from 2015 to 2021, demonstrating his leadership as a global scholar.
Hyunshil Ahn, a graduate of the School of Business and Technology Management with an MS in 1985 and a PhD in 1987, was appointed as the first head of the AI Economy Institute when The Korea Economic Daily was the first Korean media outlet to establish an AI economy lab. He has contributed to creating new roles for the press and media in the 4th industrial revolution, and added to the popularization of AI technology through regulation reform and consulting on industrial policies.
PSTech CEO Hwan-ho Sung is a graduate of the School of Electrical Engineering where he received an MS in 1988 and a PhD in EMBA in 2008. He has run the electronics company PSTech for over 20 years and successfully localized the production of power equipment, which previously depended on foreign technology. His development of the world’s first power equipment that can be applied to new industries including semiconductors and displays was recognized through this award.
Samsung Electronics President Hark Kyu Park graduated from the School of Business and Technology Management with an MS in 1986. He not only enhanced Korea’s national competitiveness by expanding the semiconductor industry, but also established contract-based semiconductor departments at Korean universities including KAIST, Sungkyunkwan University, Yonsei University, and Postech, and semiconductor track courses at KAIST, Sogang University, Seoul National University, and Postech to nurture professional talents. He also led the national semiconductor coexistence system by leading private sector-government-academia collaborations to strengthen competence in semiconductors, and continues to make unconditional investments in strong small businesses.
KAA President Chilhee Chung said, “Thanks to our alumni contributing at the highest levels of our society, the name of our alma mater shines brighter. As role models for our younger alumni, I hope greater honours will follow our awardees in the future.”
2022.03.03 View 7853