BIO
-
 Three Professors Named KAST Fellows
(Professor Dan Keun Sung at the center)
(Professor Y.H. Cho at the center)
(Professor K.H. Cho at the center)
The Korean Academy of Science and Technology (KAST) inducted three KAIST professors as fellows at the New Year’s ceremony held at KAST on January 12. They were among the 24 newly elected fellows of the most distinguished academy in Korea. The new fellows are Professor Dan Keun Sung of the School of Electrical Engineering, Professor Kwang-Hyun Cho of the Department of Bio and Brain Engineering, and Professor Yong-Hoon Cho of the Department of Physics.
Professor Sung was recognized for his lifetime academic achievements in fields related with network protocols and energy ICT. He also played a crucial role in launching the Korean satellites KITSAT-1,2,3 and the establishment of the Satellite Technology Research Center at KAIST.
Professor Y.H.Cho has been a pioneer in the field of low-dimensional semiconductor-powered quantum photonics that enables quantum optical research in solid state. He has been recognized as a renowned scholar in this field internationally.
Professor K.H.Cho has conducted original research that combines IT and BT in systems biology and has applied novel technologies of electronic modeling and computer simulation analysis for investigating complex life sciences. Professor Cho, who is in his 40s, is the youngest fellow among the newly inducted fellows.
2018.01.16 View 16177
Three Professors Named KAST Fellows
(Professor Dan Keun Sung at the center)
(Professor Y.H. Cho at the center)
(Professor K.H. Cho at the center)
The Korean Academy of Science and Technology (KAST) inducted three KAIST professors as fellows at the New Year’s ceremony held at KAST on January 12. They were among the 24 newly elected fellows of the most distinguished academy in Korea. The new fellows are Professor Dan Keun Sung of the School of Electrical Engineering, Professor Kwang-Hyun Cho of the Department of Bio and Brain Engineering, and Professor Yong-Hoon Cho of the Department of Physics.
Professor Sung was recognized for his lifetime academic achievements in fields related with network protocols and energy ICT. He also played a crucial role in launching the Korean satellites KITSAT-1,2,3 and the establishment of the Satellite Technology Research Center at KAIST.
Professor Y.H.Cho has been a pioneer in the field of low-dimensional semiconductor-powered quantum photonics that enables quantum optical research in solid state. He has been recognized as a renowned scholar in this field internationally.
Professor K.H.Cho has conducted original research that combines IT and BT in systems biology and has applied novel technologies of electronic modeling and computer simulation analysis for investigating complex life sciences. Professor Cho, who is in his 40s, is the youngest fellow among the newly inducted fellows.
2018.01.16 View 16177 -
 Ultra-Low Power Flexible Memory Using 2D Materials
(Professor Choi and Ph.D. candidate Jang)
KAIST research team led by Professor Sung-Yool Choi at School of Electrical Engineering and Professor Sung Gap Im at the Department of Chemical and Biomolecular Engineering developed high-density, ultra-low power, non-volatile, flexible memory technology using 2D materials. The team used ultrathin molybdenum disulfide (MoS2) with atomic-scale thickness as the channel material and high-performance polymeric insulator film as the tunneling dielectric material. This research was published on the cover of Advanced Functional Materials on November 17. KAIST graduate Myung Hun Woo, a researcher at Samsung Electronics and Ph.D. candidate Byung Chul Jang are first authors.
The surge of new technologies such as Internet of Things (IoT), Artificial Intelligence (AI), and cloud server led to the paradigm shift from processor-centric computing to memory-centric computing in the industry, as well as the increase in demand of wearable devices. This led to an increased need for high-density, ultra-low power, non-volatile flexible memory. In particular, ultrathin MoS2 as semiconductor material has been recently regarded as post-silicon material. This is due to its ultrathin thickness of atomic-scale which suppresses short channel effect observed in conventional silicon material, leading to advantages in high- density and low-power consumption. Further, this thickness allows the material to be flexible, and thus the material is applicable to wearable devices.
However, due to the dangling-bond free surface of MoS2 semiconductor material, it is difficult to deposit the thin insulator film to be uniform and stable over a large area via the conventional atomic layer deposition process. Further, the currently used solution process makes it difficult to deposit uniformly low dielectric constant (k) polymeric insulator film with sub-10 nm thickness on a large area, thus indicating that the memory device utilizing the conventional solution-processed polymer insulator film cannot be operated at low-operating voltage and is not compatible with photolithography.
The research team tried to overcome the hurdles and develop high-density, ultra-low power, non-volatile flexible memory by employing a low-temperature, solvent-free, and all-dry vapor phase technique named initiated chemical vapor deposition (iCVD) process. Using iCVD process, tunneling polymeric insulator film with 10 nm thickness was deposited uniformly on MoS2 semiconductor material without being restricted by the dangling bond-free surface of MoS2. The team observed that the newly developed MoS2-based non-volatile memory can be operated at low-voltage (around 10V), in contrast to the conventional MoS2-based non-volatile memory that requires over 20V.
Professor Choi said, “As the basis for the Fourth Industrial revolution technologies including AI and IoT, semiconductor device technology needs to have characteristics of low-power and flexibility, in clear contrast to conventional memory devices.” He continued, “This new technology is significant in developing source technology in terms of materials, processes, and devices to contribute to achieve these characteristics.”
This research was supported by the Global Frontier Center for Advanced Soft Electronics and the Creative Materials Discovery Program by funded the National Research Foundation of Korea of Ministry of Science and ICT.
( Figure 1. Cover of Advanced Functional Materials)
(Figure 2. Concept map for the developed non-volatile memory material and high-resolution transmission electron microscopy image for material cross-section )
2018.01.02 View 10232
Ultra-Low Power Flexible Memory Using 2D Materials
(Professor Choi and Ph.D. candidate Jang)
KAIST research team led by Professor Sung-Yool Choi at School of Electrical Engineering and Professor Sung Gap Im at the Department of Chemical and Biomolecular Engineering developed high-density, ultra-low power, non-volatile, flexible memory technology using 2D materials. The team used ultrathin molybdenum disulfide (MoS2) with atomic-scale thickness as the channel material and high-performance polymeric insulator film as the tunneling dielectric material. This research was published on the cover of Advanced Functional Materials on November 17. KAIST graduate Myung Hun Woo, a researcher at Samsung Electronics and Ph.D. candidate Byung Chul Jang are first authors.
The surge of new technologies such as Internet of Things (IoT), Artificial Intelligence (AI), and cloud server led to the paradigm shift from processor-centric computing to memory-centric computing in the industry, as well as the increase in demand of wearable devices. This led to an increased need for high-density, ultra-low power, non-volatile flexible memory. In particular, ultrathin MoS2 as semiconductor material has been recently regarded as post-silicon material. This is due to its ultrathin thickness of atomic-scale which suppresses short channel effect observed in conventional silicon material, leading to advantages in high- density and low-power consumption. Further, this thickness allows the material to be flexible, and thus the material is applicable to wearable devices.
However, due to the dangling-bond free surface of MoS2 semiconductor material, it is difficult to deposit the thin insulator film to be uniform and stable over a large area via the conventional atomic layer deposition process. Further, the currently used solution process makes it difficult to deposit uniformly low dielectric constant (k) polymeric insulator film with sub-10 nm thickness on a large area, thus indicating that the memory device utilizing the conventional solution-processed polymer insulator film cannot be operated at low-operating voltage and is not compatible with photolithography.
The research team tried to overcome the hurdles and develop high-density, ultra-low power, non-volatile flexible memory by employing a low-temperature, solvent-free, and all-dry vapor phase technique named initiated chemical vapor deposition (iCVD) process. Using iCVD process, tunneling polymeric insulator film with 10 nm thickness was deposited uniformly on MoS2 semiconductor material without being restricted by the dangling bond-free surface of MoS2. The team observed that the newly developed MoS2-based non-volatile memory can be operated at low-voltage (around 10V), in contrast to the conventional MoS2-based non-volatile memory that requires over 20V.
Professor Choi said, “As the basis for the Fourth Industrial revolution technologies including AI and IoT, semiconductor device technology needs to have characteristics of low-power and flexibility, in clear contrast to conventional memory devices.” He continued, “This new technology is significant in developing source technology in terms of materials, processes, and devices to contribute to achieve these characteristics.”
This research was supported by the Global Frontier Center for Advanced Soft Electronics and the Creative Materials Discovery Program by funded the National Research Foundation of Korea of Ministry of Science and ICT.
( Figure 1. Cover of Advanced Functional Materials)
(Figure 2. Concept map for the developed non-volatile memory material and high-resolution transmission electron microscopy image for material cross-section )
2018.01.02 View 10232 -
 Professor Je-Kyun Park, Awarded by The Korean BioChip Society
On November 9, Je-Kyun Park from the Department of Bio and Brain Engineering at KAIST received an award from the 2017 Fall Meeting of The Korean BioChip Society held in Paradise Hotel Busan, Korea. This year’s meeting recognized Professor Park for developing lab-on-a-chip and microfluidic analytical technologies.
The Korean BioChip Society is a corporation of biochip professional established in 2006 for the development of biochip technology. Every year, the Society selects a recipient based on the nominees’ academic achievements and contributions to bio-fusion industry.
Professor Park served on the international editorial boards of renowned international journals in related fields, including Biosensors and Bioelectronics and Lab on a Chip. He was also the Committee Chairman of MicroTas in 2015.
2017.11.22 View 9648
Professor Je-Kyun Park, Awarded by The Korean BioChip Society
On November 9, Je-Kyun Park from the Department of Bio and Brain Engineering at KAIST received an award from the 2017 Fall Meeting of The Korean BioChip Society held in Paradise Hotel Busan, Korea. This year’s meeting recognized Professor Park for developing lab-on-a-chip and microfluidic analytical technologies.
The Korean BioChip Society is a corporation of biochip professional established in 2006 for the development of biochip technology. Every year, the Society selects a recipient based on the nominees’ academic achievements and contributions to bio-fusion industry.
Professor Park served on the international editorial boards of renowned international journals in related fields, including Biosensors and Bioelectronics and Lab on a Chip. He was also the Committee Chairman of MicroTas in 2015.
2017.11.22 View 9648 -
 In Jin Cho Earned the Best Poster Prize at ME Summit 2017
In Jin Cho, a Ph.D. student in the Department of Chemical and Biomolecular Engineering at KAIST received the best poster prize at the International Metabolic Engineering Summit 2017 held on October 24 in Beijing, China.
The International Metabolic Engineering Summit is a global conference where scientists and corporate researchers in the field of metabolic engineering present their latest research outcomes and build networks.
At this year’s summit, about 500 researchers from around the world participated in active academic exchanges, including giving keynote speeches and presenting posters.
During the poster session, the summit selects one person for the KeAi-synthetic and Systems Biotechnology Poster Award, two for Microbial Cell Factories Poster Awards, and three for Biotechnology Journal Poster Awards among the posters presented by graduate students, post-doctoral fellows and researchers. Cho received the KeAi-synthetic and Systems Biotechnology Poster Award. Her winning poster is on the biotransformation of p-xylene to terephthalic acid using engineered Escherichia coli.
Terephthalic acid is generally produced by p-xylene oxidation; however, this process requires a high temperature and pressure as well as a toxic catalyst during the reaction process.
Cho and Ziwei Luo, a Ph.D. student at KAIST, co-conducted the research and developed a successful biological conversion process. Compared to the existing chemical process, it does not require a high temperature and pressure; and it is environmentally friendly with a relatively high conversion rate of approximately 97%.
Cho’s advisor, Distinguished Professor Sang Yup Lee said, “Further research on glucose-derived terephthalic acid will enable us to produce biomass-based eco-friendly terephthalic acid through engineered Escherichia coli.”
2017.10.31 View 11460
In Jin Cho Earned the Best Poster Prize at ME Summit 2017
In Jin Cho, a Ph.D. student in the Department of Chemical and Biomolecular Engineering at KAIST received the best poster prize at the International Metabolic Engineering Summit 2017 held on October 24 in Beijing, China.
The International Metabolic Engineering Summit is a global conference where scientists and corporate researchers in the field of metabolic engineering present their latest research outcomes and build networks.
At this year’s summit, about 500 researchers from around the world participated in active academic exchanges, including giving keynote speeches and presenting posters.
During the poster session, the summit selects one person for the KeAi-synthetic and Systems Biotechnology Poster Award, two for Microbial Cell Factories Poster Awards, and three for Biotechnology Journal Poster Awards among the posters presented by graduate students, post-doctoral fellows and researchers. Cho received the KeAi-synthetic and Systems Biotechnology Poster Award. Her winning poster is on the biotransformation of p-xylene to terephthalic acid using engineered Escherichia coli.
Terephthalic acid is generally produced by p-xylene oxidation; however, this process requires a high temperature and pressure as well as a toxic catalyst during the reaction process.
Cho and Ziwei Luo, a Ph.D. student at KAIST, co-conducted the research and developed a successful biological conversion process. Compared to the existing chemical process, it does not require a high temperature and pressure; and it is environmentally friendly with a relatively high conversion rate of approximately 97%.
Cho’s advisor, Distinguished Professor Sang Yup Lee said, “Further research on glucose-derived terephthalic acid will enable us to produce biomass-based eco-friendly terephthalic acid through engineered Escherichia coli.”
2017.10.31 View 11460 -
 Distinguished Professor Lee Named International Fellow of the CAS
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST was awarded the title of distinguished professor and international fellow from the Chinese Academy of Sciences (CAS), and honorary professor from its affiliated organization the Tianjin Institute of Industrial Biotechnology (TIB).
The CAS recognized Distinguished Professor Lee for his significant contributions to biotechnology. He has made significant pioneering academic achievements in the area of systems metabolic engineering, which produces useful chemicals from microorganisms. Not only did he develop the first and best source technology in that field, but also came out with processes for the production of biofuel and environmentally-friendly chemicals.”
As a global leader in systems metabolic engineering, Distinguished Professor Lee has also been appointed as an honorary professor at Jiangnan University in Wuxi, China.
Distinguished Professor Lee was listed in the ‘Top 20 Translational Researchers of 2014’ selected by the renowned international journal Nature Biotechnology. Moreover, he was the first Asian recipient of the James E. Bailey Award in 2016 and Marvin J. Johnson Award in 2012, which are given to scholars in the field of biotechnology.
He is also one of 13 global scientists who are foreign members of the renowned academic societies the National Academy of Engineering and the National Academy of Sciences in the US. Furthermore, he received the ‘2017 Korea Best Scientist Award’ from the president of Korea in July. Finally, his founding field, systems metabolic engineering, was chosen as one of the ‘Top 10 Emerging Technologies of 2016’ by the World Economic Forum.
The Chinese Academy of Sciences, established in November 1949, is an academic organization that carries out research on basic sciences and natural sciences in China. It defined its science and technology system to include the fields of basic sciences, natural sciences, and high technology. While having a base in Beijing, its branch academies are located in 12 main cities along with 117 affiliates and 100 national key labs.
2017.10.26 View 13224
Distinguished Professor Lee Named International Fellow of the CAS
Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering at KAIST was awarded the title of distinguished professor and international fellow from the Chinese Academy of Sciences (CAS), and honorary professor from its affiliated organization the Tianjin Institute of Industrial Biotechnology (TIB).
The CAS recognized Distinguished Professor Lee for his significant contributions to biotechnology. He has made significant pioneering academic achievements in the area of systems metabolic engineering, which produces useful chemicals from microorganisms. Not only did he develop the first and best source technology in that field, but also came out with processes for the production of biofuel and environmentally-friendly chemicals.”
As a global leader in systems metabolic engineering, Distinguished Professor Lee has also been appointed as an honorary professor at Jiangnan University in Wuxi, China.
Distinguished Professor Lee was listed in the ‘Top 20 Translational Researchers of 2014’ selected by the renowned international journal Nature Biotechnology. Moreover, he was the first Asian recipient of the James E. Bailey Award in 2016 and Marvin J. Johnson Award in 2012, which are given to scholars in the field of biotechnology.
He is also one of 13 global scientists who are foreign members of the renowned academic societies the National Academy of Engineering and the National Academy of Sciences in the US. Furthermore, he received the ‘2017 Korea Best Scientist Award’ from the president of Korea in July. Finally, his founding field, systems metabolic engineering, was chosen as one of the ‘Top 10 Emerging Technologies of 2016’ by the World Economic Forum.
The Chinese Academy of Sciences, established in November 1949, is an academic organization that carries out research on basic sciences and natural sciences in China. It defined its science and technology system to include the fields of basic sciences, natural sciences, and high technology. While having a base in Beijing, its branch academies are located in 12 main cities along with 117 affiliates and 100 national key labs.
2017.10.26 View 13224 -
 Professor Jin Woo Kim Wins the 14th Macrogen Scientist Award
Professor Jin Woo Kim of the Department of Biological Sciences at KAIST received the 14th Macrogen Scientist Award at the 2017 KSMCB International Conference held in COEX on September 12, 2017.
The award is given by the Korean Society for Molecular and Cellular Biology (KSMCB) and sponsored by Macrogen, a service provider of genome research. The award was established in 2004 to recognize biological scientists who have accomplished excellent performance in the field of basic life sciences.
Professor Kim has achieved outstanding research performances on nerve development, such as identifying the cause of senile retinal degenerative disease and finding retinal nerve cells that distinguish light and darkness in dark conditions.
Recently, he discovered intercellular communication, which controls the development of retinal neurons. His findings have contributed to addressing the principles of maintenance and regeneration of retinal neurons.
Since joining KAIST, he has presented approximately 20 papers and published in numerous international journals including Cell Reports, Genes and Development, and EMBO Journal. Moreover, he delivered special lectures at international conferences, universities, and institutes around the world.
2017.09.14 View 10394
Professor Jin Woo Kim Wins the 14th Macrogen Scientist Award
Professor Jin Woo Kim of the Department of Biological Sciences at KAIST received the 14th Macrogen Scientist Award at the 2017 KSMCB International Conference held in COEX on September 12, 2017.
The award is given by the Korean Society for Molecular and Cellular Biology (KSMCB) and sponsored by Macrogen, a service provider of genome research. The award was established in 2004 to recognize biological scientists who have accomplished excellent performance in the field of basic life sciences.
Professor Kim has achieved outstanding research performances on nerve development, such as identifying the cause of senile retinal degenerative disease and finding retinal nerve cells that distinguish light and darkness in dark conditions.
Recently, he discovered intercellular communication, which controls the development of retinal neurons. His findings have contributed to addressing the principles of maintenance and regeneration of retinal neurons.
Since joining KAIST, he has presented approximately 20 papers and published in numerous international journals including Cell Reports, Genes and Development, and EMBO Journal. Moreover, he delivered special lectures at international conferences, universities, and institutes around the world.
2017.09.14 View 10394 -
 KAIST Researchers Receive Awards at the 13th Asian Congress on Biotechnology
(From left: Seon Young Park, Dr. So Young Choi, and Yoojin Choi)
Researchers in the laboratory of KAIST Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering swept awards at the 13th Asian Congress on Biotechnology held in Thailand last month. The conference awarded a total of eight prizes in the areas of best research and best poster presentation. This is an exceptional case in which members of one research team received almost half of the awards at an international conference.
Dr. So Young Choi received the Best Research Award, while Ph.D. candidates Yoojin Choi and Seon Young Park each received the Best Poster Presentation Award at the conference held in Khon Kaen, Thailand from July 23 to 27.
The Asian Congress on Biotechnology is an international conference in which scientists and industry experts in Asia and from around the world gather to present recent research findings in the field of biotechnology. At the conference, around 400 researchers in biotechnology from 25 countries, including Korea, gathered to present and discuss various research findings under the theme of “Bioinnovation and Bioeconomy.”
Distinguished Professor Sang Yup Lee attended the conference to give the opening plenary lecture on the topic of ‘Systems Strategies in Biotechnology.’ Professor Lee announced, “I have attended international conferences with students for the last 20 years, but this is the first in which my team received three awards at an international conference that only honors a total of eight awards, three for Best Research and five for Best Presentation.”
Dr. Choi presented research results on poly (lactate-co-glycolate) (PLGA) synthesis through a biological method using micro-organisms and received the Best Research Award. PLGA is a random copolymer of DL-lactic and glycolic acids and is a biopolymer widely used for biomedical applications. PLGA is biodegradable, biocompatible, and nontoxic, and thus has been approved by the US Food and Drug Administration (FDA) for its use in implants, drug delivery, and sutures.
Dr. Choi’s research was deemed to be innovative for synthesizing PLGA from glucose and xylose in cells through metabolic engineering of E.Coli. Dr. Choi received her Ph.D. under the supervision of Distinguished Professor Lee this February and is currently conducting post-doc research.
Ph.D. candidate Choi presented her research on the use of recombinant E.Coli for the biological synthesis of various nanoparticles and received the Best Poster Presentation award. Choi used recombinant E.Coli-expressing proteins and peptides that adsorb to heavy metals to biologically synthesize diverse metal nanoparticles such as single-nanoparticle including gold and silver, quantum dots, and magnetic nanoparticles for the first time. The synthesized nanoparticles can be used in the fields of bio-imaging, diagnosis, environment, and energy.
Ph.D. candidate Park, who also received the Best Poster Presentation award, synthesized and increased production of astanxanthin, a strong antioxidant found in nature, in E.Coli using metabolic engineering. Astanxanthin is a carotenoid pigment found in salmon and shrimp that widely used in health products and cosmetics.
2017.08.01 View 16222
KAIST Researchers Receive Awards at the 13th Asian Congress on Biotechnology
(From left: Seon Young Park, Dr. So Young Choi, and Yoojin Choi)
Researchers in the laboratory of KAIST Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering swept awards at the 13th Asian Congress on Biotechnology held in Thailand last month. The conference awarded a total of eight prizes in the areas of best research and best poster presentation. This is an exceptional case in which members of one research team received almost half of the awards at an international conference.
Dr. So Young Choi received the Best Research Award, while Ph.D. candidates Yoojin Choi and Seon Young Park each received the Best Poster Presentation Award at the conference held in Khon Kaen, Thailand from July 23 to 27.
The Asian Congress on Biotechnology is an international conference in which scientists and industry experts in Asia and from around the world gather to present recent research findings in the field of biotechnology. At the conference, around 400 researchers in biotechnology from 25 countries, including Korea, gathered to present and discuss various research findings under the theme of “Bioinnovation and Bioeconomy.”
Distinguished Professor Sang Yup Lee attended the conference to give the opening plenary lecture on the topic of ‘Systems Strategies in Biotechnology.’ Professor Lee announced, “I have attended international conferences with students for the last 20 years, but this is the first in which my team received three awards at an international conference that only honors a total of eight awards, three for Best Research and five for Best Presentation.”
Dr. Choi presented research results on poly (lactate-co-glycolate) (PLGA) synthesis through a biological method using micro-organisms and received the Best Research Award. PLGA is a random copolymer of DL-lactic and glycolic acids and is a biopolymer widely used for biomedical applications. PLGA is biodegradable, biocompatible, and nontoxic, and thus has been approved by the US Food and Drug Administration (FDA) for its use in implants, drug delivery, and sutures.
Dr. Choi’s research was deemed to be innovative for synthesizing PLGA from glucose and xylose in cells through metabolic engineering of E.Coli. Dr. Choi received her Ph.D. under the supervision of Distinguished Professor Lee this February and is currently conducting post-doc research.
Ph.D. candidate Choi presented her research on the use of recombinant E.Coli for the biological synthesis of various nanoparticles and received the Best Poster Presentation award. Choi used recombinant E.Coli-expressing proteins and peptides that adsorb to heavy metals to biologically synthesize diverse metal nanoparticles such as single-nanoparticle including gold and silver, quantum dots, and magnetic nanoparticles for the first time. The synthesized nanoparticles can be used in the fields of bio-imaging, diagnosis, environment, and energy.
Ph.D. candidate Park, who also received the Best Poster Presentation award, synthesized and increased production of astanxanthin, a strong antioxidant found in nature, in E.Coli using metabolic engineering. Astanxanthin is a carotenoid pigment found in salmon and shrimp that widely used in health products and cosmetics.
2017.08.01 View 16222 -
 Top 10 Emerging Technologies of 2017
The World Economic Forum’s Expert Network and Global Future Councils in collaboration with Scientific American and its Board of Advisors announced the top 10 emerging technologies of 2017 on June 26 in Dalian, China where the 2017 Summer Davos Forum is being held. Each technology was chosen for its potential to improve lives, transform industries, and safeguard the planet.
The KAIST delegation, headed by President Sung-Chul Shin, is participating in the forum’s diverse activities including IdeasLab and GULF (Global University Leaders Forum). KAIST is the only Korean representative participating in the IdeasLab. KAIST Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, director of KAIST Institute, has served as a committee member of the Global Agenda Council on Emerging Technologies since 2012 and Global Future Council on the Fourth Industrial Revolution. He also chairs the Global Future Council on Biotechnologies.
Professor Lee said, “Very diverse technological breakthroughs were proposed for the final list of candidates. We made the final selections through very in-depth discussion with experts in each field.
We focused on the technologies which have a level of maturity that will enable them to be adopted widely within three to five years."
The top 10 emerging technologies are
(courtesy from https://
www.weforum.org/agenda/2017/06/these-are-the-top-10-emerging-technologies-of-2017):
2017 10대 기술.
1. Liquid biopsies
Liquid biopsies mark a step forward in the fight against cancer. First, they are an alternative where traditional tissue-based biopsies are not possible. Second, they provide a full spectrum of information compared to tissue samples, which only reflect the information available in the sample. Lastly, by homing in on circulating-tumor DNA (ctDNA), genetic material that routinely finds its way from cancer cells into the bloodstream, disease progression or resistance to treatment can be spotted much faster than otherwise relying on symptoms or imaging.
2. Harvesting clean water from air
The ability to extract clean water from air is not new, however existing techniques require high moisture levels and a lot of electricity. This is changing. A team from MIT and University of California, Berkeley has successfully tested a process using porous crystals that convert the water using no energy at all.
3. Deep learning for visual tasks
Computers are beginning to recognize images better than humans. Thanks to deep learning, an emerging field of artificial intelligence, computer-vision technologies are increasingly being used in applications as diverse as driving autonomous vehicles, medical diagnostics, damage assessment for insurance claims, and monitoring water levels and crop yield.
4. Liquid fuels from sunshine
Can we mimic the humble leaf to create artificial photosynthesis to generate and store energy? The prospects are looking increasingly positive. The answer lies in using sunlight-activated catalysts to split water molecules into water and hydrogen, and then using the same hydrogen to convert CO2 into hydrocarbons.
5. The Human Cell Atlas
An international collaboration aimed at deciphering the human body, called the Human Cell Atlas, was launched in October 2016. The project aims to identify every cell type in every tissue; learn exactly which genes, proteins, and other molecules are active in each type, and the processes which control that activity.
6. Precision farming
The Fourth Industrial Revolution is providing farmers with a new set of tools to boost crop yield and quality while reducing water and chemical use. Sensors, robots, GPS, mapping tools, and data-analytics software are all being used to customize the care that plants need.
7. Affordable catalysts for green vehicles
Progress is being made on a promising zero-emission technology, the hydrogen-fed fuel cell. Progress to date has been stymied by the high price of catalysts which contain platinum. However, much progress has been made in reducing reliance on this rare and expensive metal, and the latest developments involve catalysts that include no platinum, or in some cases no metal at all.
8. Genomic vaccines
Vaccines based on genes are superior to more conventional ones in a number of ways. They are faster to manufacture, which is crucial during violent outbreaks. Compared to manufacturing proteins in cell cultures or eggs, producing genetic material should also be simpler and less expensive.
9. Sustainable design of communities
Applying green construction to multiple buildings at once has the potential to revolutionize the amount of energy and water we consume. Sending locally-generated solar power to a smart microgrid could reduce electricity consumption by half and reduce carbon emissions to zero if a project currently under development at the University of California at Berkeley goes according to plan.
10. Quantum computing
Quantum computers’ almost limitless potential has only ever been matched by the difficulty and cost of their construction. This explains why today the small ones that have been built have not yet managed to exceed the power of supercomputers. But progress is being made and in 2016 the technology firm IBM provided public access to the first quantum computer in the cloud.
2017.06.28 View 13968
Top 10 Emerging Technologies of 2017
The World Economic Forum’s Expert Network and Global Future Councils in collaboration with Scientific American and its Board of Advisors announced the top 10 emerging technologies of 2017 on June 26 in Dalian, China where the 2017 Summer Davos Forum is being held. Each technology was chosen for its potential to improve lives, transform industries, and safeguard the planet.
The KAIST delegation, headed by President Sung-Chul Shin, is participating in the forum’s diverse activities including IdeasLab and GULF (Global University Leaders Forum). KAIST is the only Korean representative participating in the IdeasLab. KAIST Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering, director of KAIST Institute, has served as a committee member of the Global Agenda Council on Emerging Technologies since 2012 and Global Future Council on the Fourth Industrial Revolution. He also chairs the Global Future Council on Biotechnologies.
Professor Lee said, “Very diverse technological breakthroughs were proposed for the final list of candidates. We made the final selections through very in-depth discussion with experts in each field.
We focused on the technologies which have a level of maturity that will enable them to be adopted widely within three to five years."
The top 10 emerging technologies are
(courtesy from https://
www.weforum.org/agenda/2017/06/these-are-the-top-10-emerging-technologies-of-2017):
2017 10대 기술.
1. Liquid biopsies
Liquid biopsies mark a step forward in the fight against cancer. First, they are an alternative where traditional tissue-based biopsies are not possible. Second, they provide a full spectrum of information compared to tissue samples, which only reflect the information available in the sample. Lastly, by homing in on circulating-tumor DNA (ctDNA), genetic material that routinely finds its way from cancer cells into the bloodstream, disease progression or resistance to treatment can be spotted much faster than otherwise relying on symptoms or imaging.
2. Harvesting clean water from air
The ability to extract clean water from air is not new, however existing techniques require high moisture levels and a lot of electricity. This is changing. A team from MIT and University of California, Berkeley has successfully tested a process using porous crystals that convert the water using no energy at all.
3. Deep learning for visual tasks
Computers are beginning to recognize images better than humans. Thanks to deep learning, an emerging field of artificial intelligence, computer-vision technologies are increasingly being used in applications as diverse as driving autonomous vehicles, medical diagnostics, damage assessment for insurance claims, and monitoring water levels and crop yield.
4. Liquid fuels from sunshine
Can we mimic the humble leaf to create artificial photosynthesis to generate and store energy? The prospects are looking increasingly positive. The answer lies in using sunlight-activated catalysts to split water molecules into water and hydrogen, and then using the same hydrogen to convert CO2 into hydrocarbons.
5. The Human Cell Atlas
An international collaboration aimed at deciphering the human body, called the Human Cell Atlas, was launched in October 2016. The project aims to identify every cell type in every tissue; learn exactly which genes, proteins, and other molecules are active in each type, and the processes which control that activity.
6. Precision farming
The Fourth Industrial Revolution is providing farmers with a new set of tools to boost crop yield and quality while reducing water and chemical use. Sensors, robots, GPS, mapping tools, and data-analytics software are all being used to customize the care that plants need.
7. Affordable catalysts for green vehicles
Progress is being made on a promising zero-emission technology, the hydrogen-fed fuel cell. Progress to date has been stymied by the high price of catalysts which contain platinum. However, much progress has been made in reducing reliance on this rare and expensive metal, and the latest developments involve catalysts that include no platinum, or in some cases no metal at all.
8. Genomic vaccines
Vaccines based on genes are superior to more conventional ones in a number of ways. They are faster to manufacture, which is crucial during violent outbreaks. Compared to manufacturing proteins in cell cultures or eggs, producing genetic material should also be simpler and less expensive.
9. Sustainable design of communities
Applying green construction to multiple buildings at once has the potential to revolutionize the amount of energy and water we consume. Sending locally-generated solar power to a smart microgrid could reduce electricity consumption by half and reduce carbon emissions to zero if a project currently under development at the University of California at Berkeley goes according to plan.
10. Quantum computing
Quantum computers’ almost limitless potential has only ever been matched by the difficulty and cost of their construction. This explains why today the small ones that have been built have not yet managed to exceed the power of supercomputers. But progress is being made and in 2016 the technology firm IBM provided public access to the first quantum computer in the cloud.
2017.06.28 View 13968 -
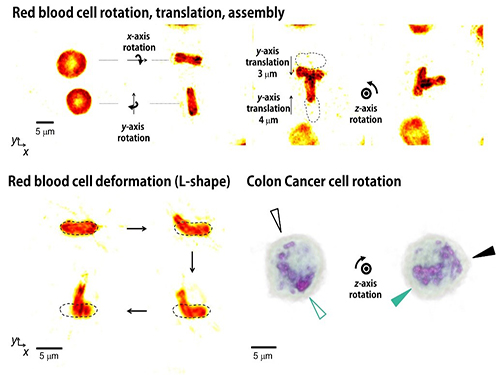 Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9457
Controlling 3D Behavior of Biological Cells Using Laser Holographic Techniques
A research team led by Professor YongKeun Park of the Physics Department at KAIST has developed an optical manipulation technique that can freely control the position, orientation, and shape of microscopic samples having complex shapes. The study has been published online in Nature Communications on May 22.
Conventional optical manipulation techniques called “optical tweezers,” have been used as an invaluable tool for exerting micro-scale force on microscopic particles and manipulating three-dimensional (3-D) positions of particles. Optical tweezers employ a tightly-focused laser whose beam diameter is smaller than one micrometer (1/100 of hair thickness), which can generate attractive force on neighboring microscopic particles moving toward the beam focus. Controlling the positions of the beam focus enabled researchers to hold the particles and move them freely to other locations so they coined the name “optical tweezers,” and have been widely used in various fields of physical and biological studies.
So far, most experiments using optical tweezers have been conducted for trapping spherical particles because physical principles can easily predict optical forces and the responding motion of microspheres. For trapping objects having complicated shapes, however, conventional optical tweezers induce unstable motion of such particles, and controllable orientation of such objects is limited, which hinder controlling the 3-D motion of microscopic objects having complex shapes such as living cells.
The research team has developed a new optical manipulation technique that can trap complex objects of arbitrary shapes. This technique first measures 3-D structures of an object in real time using a 3-D holographic microscope, which shares the same physical principle of X-Ray CT imaging. Based on the measured 3-D shape of the object, the researchers precisely calculates the shape of light that can stably control the object. When the shape of light is the same as the shape of the object, the energy of the object is minimized, which provides the stable trapping of the object having the complicated shape.
Moreover, by controlling the shape of light to have various positions, directions, and shapes of objects, it is possible to freely control the 3-D motion of the object and make the object have a desired shape. This process resembles the generation of a mold for casting a statue having desired shape so the researchers coined the name of the present technique “tomographic mold for optical trapping (TOMOTRAP).” The team succeeded in trapping individual human red blood cells stably, rotating them with desired orientations, folding them in an L-shape, and assembling two red blood cells together to form a new structure. In addition, colon cancer cells having a complex structure could be stably trapped and rotated at desired orientations. All of which have been difficult to be realized by the conventional optical techniques.
Professor Park said, “Our technique has the advantage of controlling the 3-D motion of complex shaped objects without knowing prior information about their shape and optical characteristics, and can be applied in various fields including physics, optics, nanotechnology, and medical science.”
Dr. Kyoohyun Kim, the lead author of this paper, noted that this technique can induce controlled deformation of biological cells with desired shapes. “This approach can be also applied to real-time monitoring of surgical prognosis of cellular-level surgeries for capturing and deforming cells as well as subcellular organelles,” added Kim.
Figure 1. Concept of optical manipulation techniques
Figure 2. Experimental setup
Figure 3. Research results
2017.05.25 View 9457 -
 Distinguished Professor Lee Elected to the NAS
Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering was elected as a foreign associate to the US National Academy of Sciences (NAS) on May 2. The National Academy of Sciences elected 84 new members and 21 foreign associates in recognition of their distinguished and continuing achievements in their original research. Election to the Academy is widely regarded as one of the highest honors that a scientist can receive.
Professor Lee was also elected in 2010 as a member of the US National Academy of Engineering (NAE) for his leadership in microbial biotechnology and metabolic engineering, including the development of fermentation processes for biodegradable polymers and organic acids. Until 2016, there are only 12 people worldwide who are foreign associates of both NAS and NAE.
He is the first Korean elected to both prestigious academies, the NAS and the NAE in the US. Professor Lee is currently the dean of KAIST Institutes, the world leading institute for multi-and interdisciplinary research. He is also serving as co-chair of the Global Council on Biotechnology and member of the Global Future Council on the Fourth Industrial Revolution, the World Economic Forum.
2017.05.16 View 11135
Distinguished Professor Lee Elected to the NAS
Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering was elected as a foreign associate to the US National Academy of Sciences (NAS) on May 2. The National Academy of Sciences elected 84 new members and 21 foreign associates in recognition of their distinguished and continuing achievements in their original research. Election to the Academy is widely regarded as one of the highest honors that a scientist can receive.
Professor Lee was also elected in 2010 as a member of the US National Academy of Engineering (NAE) for his leadership in microbial biotechnology and metabolic engineering, including the development of fermentation processes for biodegradable polymers and organic acids. Until 2016, there are only 12 people worldwide who are foreign associates of both NAS and NAE.
He is the first Korean elected to both prestigious academies, the NAS and the NAE in the US. Professor Lee is currently the dean of KAIST Institutes, the world leading institute for multi-and interdisciplinary research. He is also serving as co-chair of the Global Council on Biotechnology and member of the Global Future Council on the Fourth Industrial Revolution, the World Economic Forum.
2017.05.16 View 11135 -
 ANSYS Korea Donates Engineering Simulation Software
ANSYS Korea made an in-kind donation of engineering simulation software, Multiphysics Campus Solution, to KAIST on March 24. ANSYS Korea donated 10,000 copies for education and 1,000 copies for research valued at about 4 billion KRW (about 200 billion KRW commercially).
The ANSYS software will benefit the engineering simulation work in nine departments and 60 labs for three years, including the departments of mechanical engineering, aerospace engineering, electrical engineering, civil and environmental engineering, nuclear and quantum engineering, chemical and bimolecular engineering, bio and brain engineering, materials science and engineering, and the Cho Chun Shik Graduate School of Green Transportation.
ANSYS is a global engineering simulation company. It provides ANSYS CAE (Computer Aided Engineering) software products in various industries in the world as well as various support, training, and consulting services. Deemed an exemplary model of university-industry R&D collaboration especially in the Industry 4.0 era, their donation will help create the best engineering education environment possible at KAIST.
ANSYS's multi-physics campus solution is a comprehensive software suite that spans the entire range of physics, providing access to virtually any field of engineering simulation that a design process requires. It expands the fields of fluids, structures, electromagnetics, and semiconductors. Undergraduates use it to learn physics principles and gain hands-on, real-world experience that can lead to a deeper understanding of engineering concepts. Postgraduate researchers apply simulation tools to solve complex engineering problems and produce data for their theses.
"Engineering simulations are playing a stronger role in science and engineering. ANSYS software will help our undergraduates and our researchers learn the principles of physics and deepen their understanding of engineering concepts. We hope this will serve as an instrumental tool for multidisciplinary studies, critical to fostering our students," said President Sung-Chul Shin.
ANSYS Korea CEO Yong-Won Cho added, "We sincerely hope our software will help KAIST students and researchers experience the best engineering education and achieve significant research results."
(Photo caption: President Shin (left) poses with ANSYS Korea CEO Yong-Won Cho at the donation ceremony on March 24 at KAIST)
2017.03.24 View 10058
ANSYS Korea Donates Engineering Simulation Software
ANSYS Korea made an in-kind donation of engineering simulation software, Multiphysics Campus Solution, to KAIST on March 24. ANSYS Korea donated 10,000 copies for education and 1,000 copies for research valued at about 4 billion KRW (about 200 billion KRW commercially).
The ANSYS software will benefit the engineering simulation work in nine departments and 60 labs for three years, including the departments of mechanical engineering, aerospace engineering, electrical engineering, civil and environmental engineering, nuclear and quantum engineering, chemical and bimolecular engineering, bio and brain engineering, materials science and engineering, and the Cho Chun Shik Graduate School of Green Transportation.
ANSYS is a global engineering simulation company. It provides ANSYS CAE (Computer Aided Engineering) software products in various industries in the world as well as various support, training, and consulting services. Deemed an exemplary model of university-industry R&D collaboration especially in the Industry 4.0 era, their donation will help create the best engineering education environment possible at KAIST.
ANSYS's multi-physics campus solution is a comprehensive software suite that spans the entire range of physics, providing access to virtually any field of engineering simulation that a design process requires. It expands the fields of fluids, structures, electromagnetics, and semiconductors. Undergraduates use it to learn physics principles and gain hands-on, real-world experience that can lead to a deeper understanding of engineering concepts. Postgraduate researchers apply simulation tools to solve complex engineering problems and produce data for their theses.
"Engineering simulations are playing a stronger role in science and engineering. ANSYS software will help our undergraduates and our researchers learn the principles of physics and deepen their understanding of engineering concepts. We hope this will serve as an instrumental tool for multidisciplinary studies, critical to fostering our students," said President Sung-Chul Shin.
ANSYS Korea CEO Yong-Won Cho added, "We sincerely hope our software will help KAIST students and researchers experience the best engineering education and achieve significant research results."
(Photo caption: President Shin (left) poses with ANSYS Korea CEO Yong-Won Cho at the donation ceremony on March 24 at KAIST)
2017.03.24 View 10058 -
 Professor Jae Kyoung Kim Receives the 2017 HSFP Award
The Human Frontier Science Program (HSFP), one of the most competitive research grants in life sciences, has funded researchers worldwide across and beyond the field since 1990. Each year, the program selects a handful of recipients who push the envelope of basic research in biology to bring breakthroughs from novel approaches. Among its 7,000 recipients thus far, 26 scientists have received the Nobel Prize. For that reason, HSFP grants are often referred to as “Nobel Prize Grants.”
Professor Jae Kyoung Kim of the Mathematical Sciences Department at KAIST and his international collaborators, Professor Robert Havekes from the University of Groningen, the Netherlands, Professor Sara Aton from the University of Michigan in Ann Arbor, the United States, and Professor Matias Zurbriggen from the University of Düsseldorf, Germany, won the Young Investigator Grants of the 2017 HSFP.
The 30 winning teams of the 2017 competition (in 9 Young Investigator Grants and 21 Program Grants) went through a rigorous year-long review process from a total of 1,073 applications submitted from more than 60 countries around the world. Each winning team will receive financial support averaging 110,000-125,000 USD per year for three years.
Although Professor Kim was trained as a mathematician, he has extended his research focus into biological sciences and attempted to solve some of the most difficult problems in biology by employing mathematical theories and applications including nonlinear dynamics, stochastic process, singular perturbation, and parameter estimation.
The project that won the Young Investigator Grants was a study on how a molecular circadian clock may affect sleep-regulated neurophysiology in mammals. Physiological and metabolic processes such as sleep, blood pressure, and hormone secretion exhibit circadian rhythms in mammals. Professor Kim used mathematical modeling and analysis to explain that the mammalian circadian clock is a hierarchical system, in which the master clock in the superchiasmatic nucleus, a tiny region in the brain that controls circadian rhythms, functions as a pacemaker and synchronizer of peripheral clocks to generate coherent systematic rhythms throughout the body.
Professor Kim said, “The mechanisms of our neuronal and hormonal activities regulating many of our bodily functions over a 24-hour cycle are not yet fully known. We go to sleep every night, but do not really know how it affects our brain functions. I hope my experience in mathematics, along with insights from biologists, can find meaningful answers to some of today’s puzzling problems in biological sciences, for example, revealing the complexities of our brains and showing how they work.”
“In the meantime, I hope collaborations between the fields of mathematics and biology, as yet a rare phenomenon in the Korean scientific community, will become more popular in the near future.”
Professor Kim received his doctoral degree in Applied and Interdisciplinary Mathematics in 2013 from the University of Michigan and joined KAIST in 2015. He has published numerous articles in reputable science journals such as Science, Molecular Cell, Proceedings of the National Academy of Sciences, and Nature Communications.
Both the Program Grants and Young Investigator Grants support international teams with members from at least two countries for innovative and creative research. This year, the Program Grants were awarded to research topics ranging from the evolution of counting and the role of extracellular vesicles in breast cancer bone metastasis to the examination of obesity from a mechanobiological point of view.
The Young Investigator Grants are limited to teams that established their independent research within the last five years and received their doctoral degrees within the last decade. Besides Professor Kim’s study, such topics as the use of infrasound for navigation by seabirds and protein formation in photochemistry and photophysics were awarded in 2017.
Full lists of the 2017 HFSP winners are available at: http://www.hfsp.org/awardees/newly-awarded.
About the Human Frontier Science Program (HFSP):
The HFSP is a research funding program implemented by the International Human Frontier Science Program (HFSPO) based in Strasbourg, France. It promotes intercontinental collaboration and training in cutting-edge, interdisciplinary research specializing in life sciences. Founded in 1989, the HFSPO consists of the European Union and 14 other countries including the G7 nations and South Korea.
2017.03.21 View 11932
Professor Jae Kyoung Kim Receives the 2017 HSFP Award
The Human Frontier Science Program (HSFP), one of the most competitive research grants in life sciences, has funded researchers worldwide across and beyond the field since 1990. Each year, the program selects a handful of recipients who push the envelope of basic research in biology to bring breakthroughs from novel approaches. Among its 7,000 recipients thus far, 26 scientists have received the Nobel Prize. For that reason, HSFP grants are often referred to as “Nobel Prize Grants.”
Professor Jae Kyoung Kim of the Mathematical Sciences Department at KAIST and his international collaborators, Professor Robert Havekes from the University of Groningen, the Netherlands, Professor Sara Aton from the University of Michigan in Ann Arbor, the United States, and Professor Matias Zurbriggen from the University of Düsseldorf, Germany, won the Young Investigator Grants of the 2017 HSFP.
The 30 winning teams of the 2017 competition (in 9 Young Investigator Grants and 21 Program Grants) went through a rigorous year-long review process from a total of 1,073 applications submitted from more than 60 countries around the world. Each winning team will receive financial support averaging 110,000-125,000 USD per year for three years.
Although Professor Kim was trained as a mathematician, he has extended his research focus into biological sciences and attempted to solve some of the most difficult problems in biology by employing mathematical theories and applications including nonlinear dynamics, stochastic process, singular perturbation, and parameter estimation.
The project that won the Young Investigator Grants was a study on how a molecular circadian clock may affect sleep-regulated neurophysiology in mammals. Physiological and metabolic processes such as sleep, blood pressure, and hormone secretion exhibit circadian rhythms in mammals. Professor Kim used mathematical modeling and analysis to explain that the mammalian circadian clock is a hierarchical system, in which the master clock in the superchiasmatic nucleus, a tiny region in the brain that controls circadian rhythms, functions as a pacemaker and synchronizer of peripheral clocks to generate coherent systematic rhythms throughout the body.
Professor Kim said, “The mechanisms of our neuronal and hormonal activities regulating many of our bodily functions over a 24-hour cycle are not yet fully known. We go to sleep every night, but do not really know how it affects our brain functions. I hope my experience in mathematics, along with insights from biologists, can find meaningful answers to some of today’s puzzling problems in biological sciences, for example, revealing the complexities of our brains and showing how they work.”
“In the meantime, I hope collaborations between the fields of mathematics and biology, as yet a rare phenomenon in the Korean scientific community, will become more popular in the near future.”
Professor Kim received his doctoral degree in Applied and Interdisciplinary Mathematics in 2013 from the University of Michigan and joined KAIST in 2015. He has published numerous articles in reputable science journals such as Science, Molecular Cell, Proceedings of the National Academy of Sciences, and Nature Communications.
Both the Program Grants and Young Investigator Grants support international teams with members from at least two countries for innovative and creative research. This year, the Program Grants were awarded to research topics ranging from the evolution of counting and the role of extracellular vesicles in breast cancer bone metastasis to the examination of obesity from a mechanobiological point of view.
The Young Investigator Grants are limited to teams that established their independent research within the last five years and received their doctoral degrees within the last decade. Besides Professor Kim’s study, such topics as the use of infrasound for navigation by seabirds and protein formation in photochemistry and photophysics were awarded in 2017.
Full lists of the 2017 HFSP winners are available at: http://www.hfsp.org/awardees/newly-awarded.
About the Human Frontier Science Program (HFSP):
The HFSP is a research funding program implemented by the International Human Frontier Science Program (HFSPO) based in Strasbourg, France. It promotes intercontinental collaboration and training in cutting-edge, interdisciplinary research specializing in life sciences. Founded in 1989, the HFSPO consists of the European Union and 14 other countries including the G7 nations and South Korea.
2017.03.21 View 11932