nanomaterials
-
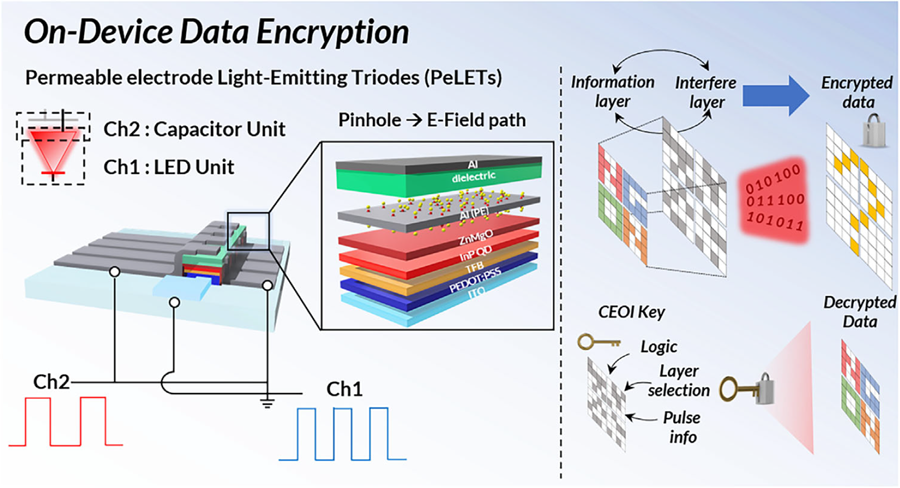 KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 646
KAIST's Li-Fi - Achieves 100 Times Faster Speed and Enhanced Security of Wi-Fi
- KAIST-KRISS Develop 'On-Device Encryption Optical Transmitter' Based on Eco-Friendly Quantum Dots
- New Li-Fi Platform Technology Achieves High Performance with 17.4% Device Efficiency and 29,000 nit Brightness, Simultaneously Improving Transmission Speed and Security
- Presents New Methodology for High-Speed and Encrypted Communication Through Single-Device-Based Dual-Channel Optical Modulation
< Photo 1. (Front row from left) Seungmin Shin, First Author; Professor Himchan Cho; (Back row from left) Hyungdoh Lee, Seungwoo Lee, Wonbeom Lee; (Top left) Dr. Kyung-geun Lim >
Li-Fi (Light Fidelity) is a wireless communication technology that utilizes the visible light spectrum (400-800 THz), similar to LED light, offering speeds up to 100 times faster than existing Wi-Fi (up to 224 Gbps). While it has fewer limitations in available frequency allocation and less radio interference, it is relatively vulnerable to security breaches as anyone can access it. Korean researchers have now proposed a new Li-Fi platform that overcomes the limitations of conventional optical communication devices and can simultaneously enhance both transmission speed and security.
KAIST (President Kwang Hyung Lee) announced on the 24th that Professor Himchan Cho's research team from the Department of Materials Science and Engineering, in collaboration with Dr. Kyung-geun Lim of the Korea Research Institute of Standards and Science (KRISS, President Ho-Seong Lee) under the National Research Council of Science & Technology (NST, Chairman Young-Sik Kim), has developed 'on-device encryption optical communication device' technology for the utilization of 'Li-Fi,' which is attracting attention as a next-generation ultra-high-speed data communication.
Professor Cho's team created high-efficiency light-emitting triode devices using eco-friendly quantum dots (low-toxicity and sustainable materials). The device developed by the research team is a mechanism that generates light using an electric field. Specifically, the electric field is concentrated in 'tiny holes (pinholes) in the permeable electrode' and transmitted beyond the electrode. This device utilizes this principle to simultaneously process two input data streams.
Using this principle, the research team developed a technology called 'on-device encryption optical transmitter.' The core of this technology is that the device itself converts information into light and simultaneously encrypts it. This means that enhanced security data transmission is possible without the need for complex, separate equipment.
External Quantum Efficiency (EQE) is an indicator of how efficiently electricity is converted into light, with a general commercialization standard of about 20%. The newly developed device recorded an EQE of 17.4%, and its luminance was 29,000 nit, significantly exceeding the maximum brightness of a smartphone OLED screen, which is 2,000 nit, demonstrating a brightness more than 10 times higher.
< Figure 1. Schematic diagram of the device structure developed by the research team and encrypted communication >
Furthermore, to more accurately understand how this device converts information into light, the research team used a method called 'transient electroluminescence analysis.' They analyzed the light-emitting characteristics generated by the device when voltage was instantaneously applied for very short durations (hundreds of nanoseconds = billionths of a second). Through this analysis, they investigated the movement of charges within the device at hundreds of nanoseconds, elucidating the operating mechanism of dual-channel optical modulation implemented within a single device.
Professor Himchan Cho of KAIST stated, "This research overcomes the limitations of existing optical communication devices and proposes a new communication platform that can both increase transmission speed and enhance security."
< Photo 2. Professor Himchan Cho, Department of Materials Science and Engineering >
He added, "This technology, which strengthens security without additional equipment and simultaneously enables encryption and transmission, can be widely applied in various fields where security is crucial in the future."
This research, with Seungmin Shin, a Ph.D. candidate at KAIST's Department of Materials Science and Engineering, participating as the first author, and Professor Himchan Cho and Dr. Kyung-geun Lim of KRISS as co-corresponding authors, was published in the international journal 'Advanced Materials' on May 30th and was selected as an inside front cover paper.※ Paper Title: High-Efficiency Quantum Dot Permeable electrode Light-Emitting Triodes for Visible-Light Communications and On-Device Data Encryption※ DOI: https://doi.org/10.1002/adma.202503189
This research was supported by the National Research Foundation of Korea, the National Research Council of Science & Technology (NST), and the Korea Institute for Advancement of Technology.
2025.06.24 View 646 -
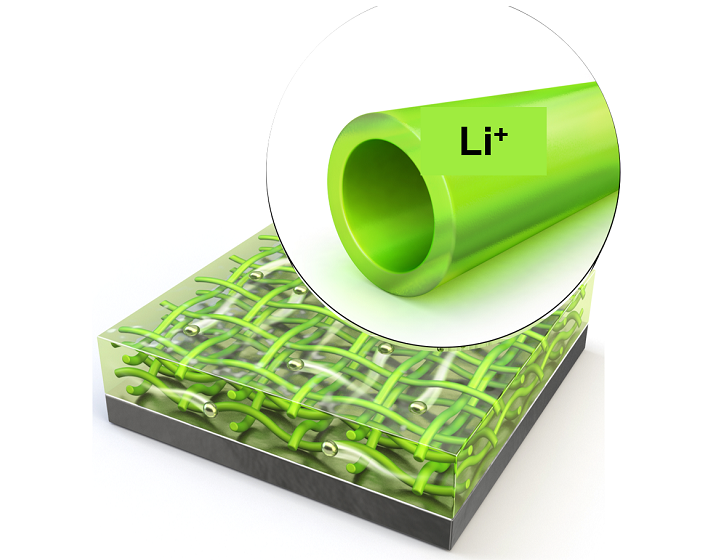 KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 6500
KAIST Extends Lithium Metal Battery Lifespan by 750% Using Water
Lithium metal, a next-generation anode material, has been highlighted for overcoming the performance limitations of commercial batteries. However, issues inherent to lithium metal have caused shortened battery lifespans and increased fire risks. KAIST researchers have achieved a world-class breakthrough by extending the lifespan of lithium metal anodes by approximately 750% only using water.
KAIST (represented by President Kwang Hyung Lee) announced on the 2nd of December that Professor Il-Doo Kim from the Department of Materials Science and Engineering, in collaboration with Professor Jiyoung Lee from Ajou University, successfully stabilized lithium growth and significantly enhanced the lifespan of next-generation lithium metal batteries using eco-friendly hollow nanofibers as protective layers.
Conventional protective layer technologies, which involve applying a surface coating onto lithium metal in order to create an artificial interface with the electrolyte, have relied on toxic processes and expensive materials, with limited improvements in the lifespan of lithium metal anodes.
< Figure 1. Schematic illustration of the fabrication process of the newly developed protective membrane by eco-friendly electrospinning process using water >
To address these limitations, Professor Kim’s team proposed a hollow nanofiber protective layer capable of controlling lithium-ion growth through both physical and chemical means. This protective layer was manufactured through an environmentally friendly electrospinning process* using guar gum** extracted from plants as the primary material and utilizing water as the sole solvent.
*Electrospinning process: A method where polymer solutions are subjected to an electric field, producing continuous fibers with diameters ranging from tens of nanometers to several micrometers.
**Guar gum: A natural polymer extracted from guar beans, composed mainly of monosaccharides. Its oxidized functional groups regulate interactions with lithium ions.
< Figure 2. Physical and chemical control of Lithium dendrite by the newly developed protective membrane >
The nanofiber protective layer effectively controlled reversible chemical reactions between the electrolyte and lithium ions. The hollow spaces within the fibers suppressed the random accumulation of lithium ions on the metal surface, stabilizing the interface between the lithium metal surface and the electrolyte.
< Figure 3. Performance of Lithium metal battery full cells with the newly developed protective membrane >
As a result, the lithium metal anodes with this protective layer demonstrated approximately a 750% increase in lifespan compared to conventional lithium metal anodes. The battery retained 93.3% of its capacity even after 300 charge-discharge cycles, achieving world-class performance.
The researchers also verified that this natural protective layer decomposes entirely within about a month in soil, proving its eco-friendly nature throughout the manufacturing and disposal process.
< Figure 4. Excellent decomposition rate of the newly developed protective membrane >
Professor Il-Doo Kim explained, “By leveraging both physical and chemical protective functions, we were able to guide reversible reactions between lithium metal and the electrolyte more effectively and suppress dendrite growth, resulting in lithium metal anodes with unprecedented lifespan characteristics.”
He added, “As the environmental burden caused by battery production and disposal becomes a pressing issue due to surging battery demand, this water-based manufacturing method with biodegradable properties will significantly contribute to the commercialization of next-generation eco-friendly batteries.”
This study was led by Dr. Jiyoung Lee (now a professor in the Department of Chemical Engineering at Ajou University) and Dr. Hyunsub Song (currently at Samsung Electronics), both graduates of KAIST’s Department of Materials Science and Engineering. The findings were published as a front cover article in Advanced Materials, Volume 36, Issue 47, on November 21.
(Paper title: “Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer”)
The research was supported by the KAIST-LG Energy Solution Frontier Research Lab (FRL), the Alchemist Project funded by the Ministry of Trade, Industry and Energy, and the Top-Tier Research Support Program from the Ministry of Science and ICT.
2024.12.12 View 6500 -
 A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 8877
A KAIST Research Team Develops a Smart Color-Changing Flexible Battery with Ultra-high Efficiency
With the rapid growth of the smart and wearable electronic devices market, smart next-generation energy storage systems that have energy storage functions as well as additional color-changing properties are receiving a great deal of attention. However, existing electrochromic devices have low electrical conductivity, leading to low efficiency in electron and ion mobility, and low storage capacities. Such batteries have therefore been limited to use in flexible and wearable devices.
On August 21, a joint research team led by Professor Il-Doo Kim from the KAIST Department of Materials Science and Engineering (DMSE) and Professor Tae Gwang Yun from the Myongji University Department of Materials Science and Engineering announced the development of a smart electrochromic Zn-ion battery that can visually represent its charging and discharging processes using an electrochromic polymer anode incorporated with a “π-bridge spacer”, which increases electron and ion mobility efficiency.
Batteries topped with electrochromic properties are groundbreaking inventions that can visually represent their charged and discharged states using colors, and can be used as display devices that cut down energy consumption for indoor cooling by controlling solar absorbance. The research team successfully built a flexible and electrochromic smart Zn-ion battery that can maintain its excellent electrochromic and electrochemical properties, even under long-term exposure to the atmosphere and mechanical deformations.
< Figure 1. Electrochromic zinc ion battery whose anode is made of a polymer that turns dark blue when charged and transparent when discharged. >
To maximize the efficiency of electron and ion mobility, the team modelled and synthesized the first π-bridge spacer-incorporated polymer anode in the world. π-bonds can improve the mobility of electrons within a structure to speed up ion movement and maximize ion adsorption efficiency, which improves its energy storage capacity.
In anode-based batteries with a π-bridge spacer, the spacer provides room for quicker ion movement. This allows fast charging, an improved zinc-ion discharging capacity of 110 mAh/g, which is 40% greater than previously reported, and a 30% increase in electrochromic function that switches from dark blue to transparent when the device is charged/discharged. In addition, should the transparent flexible battery technology be applied to smart windows, they would display darker colors during the day while they absorb solar energy, and function as a futuristic energy storage technique that can block out UV radiation and replace curtains.
< Figure 2. A schematic diagram of the structure of the electrochromic polymer with π-π spacer and the operation of a smart flexible battery using this cathode material. >
< Figure 3. (A) Density Functional Theory (DFT) theory-based atomic and electronic structure analysis. (B) Comparison of rate characteristics for polymers with and without π-bridge spacers. (C) Electrochemical performance comparison graph with previously reported zinc ion batteries. The anode material, which has an electron donor-acceptor structure with a built-in π-bridge spacer, shows better electrochemical performance and electrochromic properties than existing zinc ion batteries and electrochromic devices. >
Professor Il-Doo Kim said, “We have developed a polymer incorporated with a π-bridge spacer and successfully built a smart Zn-ion battery with excellent electrochromic efficiency and high energy storage capacity.” He added, “This technique goes beyond the existing concept of batteries that are used simply as energy storage devices, and we expect this technology to be used as a futuristic energy storage system that accelerates innovation in smart batteries and wearable technologies.”
This research, co-first authored by the alums of KAIST Departments of Material Sciences of Engineering, Professor Tae Gwang Yun of Myongji University, Dr. Jiyoung Lee, a post-doctoral associate at Northwestern University, and Professor Han Seul Kim at Chungbuk National University, was published as an inside cover article for Advanced Materials on August 3 under the title, “A π-Bridge Spacer Embedded Electron Donor-Acceptor Polymer for Flexible Electrochromic Zn-Ion Batteries”.
< Figure 4. Advanced Materials Inside Cover (August Issue) >
This research was supported by the Nanomaterial Technology Development Project under the Korean Ministry of Science and ICT, the Nano and Material Technology Development Project under the National Research Foundation of Korea, the Successive Academic Generation Development Project under the Korean Ministry of Education, and the Alchemist Project under the Korean Ministry of Trade, Industry & Energy.
2023.09.01 View 8877 -
 New Chiral Nanostructures to Extend the Material Platform
Researchers observed a wide window of chiroptical activity from nanomaterials
A research team transferred chirality from the molecular scale to a microscale to extend material platforms and applications. The optical activity from this novel chiral material encompasses to short-wave infrared region.
This platform could serve as a powerful strategy for hierarchical chirality transfer through self-assembly, generating broad optical activity and providing immense applications including bio, telecommunication, and imaging technique. This is the first observation of such a wide window of chiroptical activity from nanomaterials.
“We synthesized chiral copper sulfides using cysteine, as the stabilizer, and transferring the chirality from molecular to the microscale through self-assembly,” explained Professor Jihyeon Yeom from the Department of Materials Science and Engineering, who led the research. The result was reported in ACS Nano on September 14.
Chiral nanomaterials provide a rich platform for versatile applications. Tuning the wavelength of polarization rotation maxima in the broad range is a promising candidate for infrared neural stimulation, imaging, and nanothermometry. However, the majority of previously developed chiral nanomaterials revealed the optical activity in a relatively shorter wavelength range, not in short-wave infrared.
To achieve chiroptical activity in the short-wave infrared region, materials should be in sub-micrometer dimensions, which are compatible with the wavelength of short-wave infrared region light for strong light-matter interaction. They also should have the optical property of short-wave infrared region absorption while forming a structure with chirality.
Professor Yeom’s team induced self-assembly of the chiral nanoparticles by controlling the attraction and repulsion forces between the building block nanoparticles. During this process, molecular chirality of cysteine was transferred to the nanoscale chirality of nanoparticles, and then transferred to the micrometer scale chirality of nanoflowers with 1.5-2 2 μm dimensions formed by the self-assembly.
“We will work to expand the wavelength range of chiroptical activity to the short-wave infrared region, thus reshaping our daily lives in the form of a bio-barcode that can store vast amount of information under the skin,” said Professor Yeom.
This study was funded by the Ministry of Science and ICT, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety, the National Research Foundation of Korea,the KAIST URP Program, the KAIST Creative Challenging Research Program, Samsung and POSCO Science Fellowship.
-PublicationKi Hyun Park, Junyoung Kwon, Uichang Jeong, Ji-Young Kim, Nicholas A.Kotov, Jihyeon Yeom, “Broad Chrioptical Activity from Ultraviolet to Short-Wave Infrared by Chirality Transfer from Molecular to Micrometer Scale," September 14, 2021 ACS Nano (https://doi.org/10.1021/acsnano.1c05888)
-ProfileProfessor Jihyeon YeomNovel Nanomaterials for New Platforms LaboratoryDepartment of Materials Science and EngineeringKAIST
2021.10.22 View 11412
New Chiral Nanostructures to Extend the Material Platform
Researchers observed a wide window of chiroptical activity from nanomaterials
A research team transferred chirality from the molecular scale to a microscale to extend material platforms and applications. The optical activity from this novel chiral material encompasses to short-wave infrared region.
This platform could serve as a powerful strategy for hierarchical chirality transfer through self-assembly, generating broad optical activity and providing immense applications including bio, telecommunication, and imaging technique. This is the first observation of such a wide window of chiroptical activity from nanomaterials.
“We synthesized chiral copper sulfides using cysteine, as the stabilizer, and transferring the chirality from molecular to the microscale through self-assembly,” explained Professor Jihyeon Yeom from the Department of Materials Science and Engineering, who led the research. The result was reported in ACS Nano on September 14.
Chiral nanomaterials provide a rich platform for versatile applications. Tuning the wavelength of polarization rotation maxima in the broad range is a promising candidate for infrared neural stimulation, imaging, and nanothermometry. However, the majority of previously developed chiral nanomaterials revealed the optical activity in a relatively shorter wavelength range, not in short-wave infrared.
To achieve chiroptical activity in the short-wave infrared region, materials should be in sub-micrometer dimensions, which are compatible with the wavelength of short-wave infrared region light for strong light-matter interaction. They also should have the optical property of short-wave infrared region absorption while forming a structure with chirality.
Professor Yeom’s team induced self-assembly of the chiral nanoparticles by controlling the attraction and repulsion forces between the building block nanoparticles. During this process, molecular chirality of cysteine was transferred to the nanoscale chirality of nanoparticles, and then transferred to the micrometer scale chirality of nanoflowers with 1.5-2 2 μm dimensions formed by the self-assembly.
“We will work to expand the wavelength range of chiroptical activity to the short-wave infrared region, thus reshaping our daily lives in the form of a bio-barcode that can store vast amount of information under the skin,” said Professor Yeom.
This study was funded by the Ministry of Science and ICT, the Ministry of Health and Welfare, the Ministry of Food and Drug Safety, the National Research Foundation of Korea,the KAIST URP Program, the KAIST Creative Challenging Research Program, Samsung and POSCO Science Fellowship.
-PublicationKi Hyun Park, Junyoung Kwon, Uichang Jeong, Ji-Young Kim, Nicholas A.Kotov, Jihyeon Yeom, “Broad Chrioptical Activity from Ultraviolet to Short-Wave Infrared by Chirality Transfer from Molecular to Micrometer Scale," September 14, 2021 ACS Nano (https://doi.org/10.1021/acsnano.1c05888)
-ProfileProfessor Jihyeon YeomNovel Nanomaterials for New Platforms LaboratoryDepartment of Materials Science and EngineeringKAIST
2021.10.22 View 11412 -
 ACS Nano Special Edition Highlights Innovations at KAIST
- The collective intelligence and technological innovation of KAIST was highlighted with case studies including the Post-COVID-19 New Deal R&D Initiative Project. -
KAIST’s innovative academic achievements and R&D efforts for addressing the world’s greatest challenges such as the COVID-19 pandemic were featured in ACS Nano as part of its special virtual issue commemorating the 50th anniversary of KAIST. The issue consisted of 14 review articles contributed by KAIST faculty from five departments, including two from Professor Il-Doo Kim from the Department of Materials Science and Engineering, who serves as an associate editor of the ACS Nano.
ACS Nano, the leading international journal in nanoscience and nanotechnology, published a special virtual issue last month, titled ‘Celebrating 50 Years of KAIST: Collective Intelligence and Innovation for Confronting Contemporary Issues.’
This special virtual issue introduced KAIST’s vision of becoming a ‘global value-creative leading university’ and its progress toward this vision over the last 50 years. The issue explained how KAIST has served as the main hub for advanced scientific research and technological innovation in South Korea since its establishment in 1971, and how its faculty and over 69,000 graduates played a key role in propelling the nation’s rapid industrialization and economic development.
The issue also emphasized the need for KAIST to enhance global cooperation and the exchange of ideas in the years to come, especially during the post-COVID era intertwined with the Fourth Industrial Revolution (4IR). In this regard, the issue cited the first ‘KAIST Emerging Materials e-Symposium (EMS)’, which was held online for five days in September of last year with a global audience of over 10,000 participating live via Zoom and YouTube, as a successful example of what academic collaboration could look like in the post-COVID and 4IR eras.
In addition, the “Science & Technology New Deal Project for COVID-19 Response,” a project conducted by KAIST with support from the Ministry of Science and ICT (MSIT) of South Korea, was also introduced as another excellent case of KAIST’s collective intelligence and technological innovation. The issue highlighted some key achievements from this project for overcoming the pandemic-driven crisis, such as: reusable anti-virus filters, negative-pressure ambulances for integrated patient transport and hospitalization, and movable and expandable negative-pressure ward modules.
“We hold our expectations high for the outstanding achievements and progress KAIST will have made by its centennial,” said Professor Kim on the background of curating the 14 review articles contributed by KAIST faculty from the fields of Materials Science and Engineering (MSE), Chemical and Biomolecular Engineering (CBE), Nuclear and Quantum Engineering (NQE), Electrical Engineering (EE), and Chemistry (Chem).
Review articles discussing emerging materials and their properties covered photonic carbon dots (Professor Chan Beum Park, MSE), single-atom and ensemble catalysts (Professor Hyunjoo Lee, CBE), and metal/metal oxide electrocatalysts (Professor Sung-Yoon Chung, MSE).
Review articles discussing materials processing covered 2D layered materials synthesis based on interlayer engineering (Professor Kibum Kang, MSE), eco-friendly methods for solar cell production (Professor Bumjoon J. Kim, CBE), an ex-solution process for the synthesis of highly stable catalysts (Professor WooChul Jung, MSE), and 3D light-patterning synthesis of ordered nanostructures (Professor Seokwoo Jeon, MSE, and Professor Dongchan Jang, NQE).
Review articles discussing advanced analysis techniques covered operando materials analyses (Professor Jeong Yeong Park, Chem), graphene liquid cell transmission electron microscopy (Professor Jong Min Yuk, MSE), and multiscale modeling and visualization of materials systems (Professor Seungbum Hong, MSE).
Review articles discussing practical state-of-the-art devices covered chemiresistive hydrogen sensors (Professor Il-Doo Kim, MSE), patient-friendly diagnostics and implantable treatment devices (Professor Steve Park, MSE), triboelectric nanogenerators (Professor Yang-Kyu Choi, EE), and next-generation lithium-air batteries (Professor Hye Ryung Byon, Chem, and Professor Il-Doo Kim, MSE).
In addition to Professor Il-Doo Kim, post-doctoral researcher Dr. Jaewan Ahn from the KAIST Applied Science Research Institute, Dean of the College of Engineering at KAIST Professor Choongsik Bae, and ACS Nano Editor-in-Chief Professor Paul S. Weiss from the University of California, Los Angeles also contributed to the publication of this ACS Nano special virtual issue.
The issue can be viewed and downloaded from the ACS Nano website at https://doi.org/10.1021/acsnano.1c01101.
Image credit: KAIST
Image usage restrictions: News organizations may use or redistribute this image,with proper attribution, as part of news coverage of this paper only.
Publication:
Ahn, J., et al. (2021) Celebrating 50 Years of KAIST: Collective Intelligence and Innovation for Confronting Contemporary Issues. ACS Nano 15(3): 1895-1907. Available online at https://doi.org/10.1021/acsnano.1c01101
Profile:
Il-Doo Kim, Ph.D
Chair Professor
idkim@kaist.ac.kr
http://advnano.kaist.ac.kr
Advanced Nanomaterials and Energy Lab.
Department of Materials Science and Engineering
Membrane Innovation Center for Anti-Virus and Air-Quality Control
https://kaist.ac.kr/
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
(END)
2021.03.05 View 34084
ACS Nano Special Edition Highlights Innovations at KAIST
- The collective intelligence and technological innovation of KAIST was highlighted with case studies including the Post-COVID-19 New Deal R&D Initiative Project. -
KAIST’s innovative academic achievements and R&D efforts for addressing the world’s greatest challenges such as the COVID-19 pandemic were featured in ACS Nano as part of its special virtual issue commemorating the 50th anniversary of KAIST. The issue consisted of 14 review articles contributed by KAIST faculty from five departments, including two from Professor Il-Doo Kim from the Department of Materials Science and Engineering, who serves as an associate editor of the ACS Nano.
ACS Nano, the leading international journal in nanoscience and nanotechnology, published a special virtual issue last month, titled ‘Celebrating 50 Years of KAIST: Collective Intelligence and Innovation for Confronting Contemporary Issues.’
This special virtual issue introduced KAIST’s vision of becoming a ‘global value-creative leading university’ and its progress toward this vision over the last 50 years. The issue explained how KAIST has served as the main hub for advanced scientific research and technological innovation in South Korea since its establishment in 1971, and how its faculty and over 69,000 graduates played a key role in propelling the nation’s rapid industrialization and economic development.
The issue also emphasized the need for KAIST to enhance global cooperation and the exchange of ideas in the years to come, especially during the post-COVID era intertwined with the Fourth Industrial Revolution (4IR). In this regard, the issue cited the first ‘KAIST Emerging Materials e-Symposium (EMS)’, which was held online for five days in September of last year with a global audience of over 10,000 participating live via Zoom and YouTube, as a successful example of what academic collaboration could look like in the post-COVID and 4IR eras.
In addition, the “Science & Technology New Deal Project for COVID-19 Response,” a project conducted by KAIST with support from the Ministry of Science and ICT (MSIT) of South Korea, was also introduced as another excellent case of KAIST’s collective intelligence and technological innovation. The issue highlighted some key achievements from this project for overcoming the pandemic-driven crisis, such as: reusable anti-virus filters, negative-pressure ambulances for integrated patient transport and hospitalization, and movable and expandable negative-pressure ward modules.
“We hold our expectations high for the outstanding achievements and progress KAIST will have made by its centennial,” said Professor Kim on the background of curating the 14 review articles contributed by KAIST faculty from the fields of Materials Science and Engineering (MSE), Chemical and Biomolecular Engineering (CBE), Nuclear and Quantum Engineering (NQE), Electrical Engineering (EE), and Chemistry (Chem).
Review articles discussing emerging materials and their properties covered photonic carbon dots (Professor Chan Beum Park, MSE), single-atom and ensemble catalysts (Professor Hyunjoo Lee, CBE), and metal/metal oxide electrocatalysts (Professor Sung-Yoon Chung, MSE).
Review articles discussing materials processing covered 2D layered materials synthesis based on interlayer engineering (Professor Kibum Kang, MSE), eco-friendly methods for solar cell production (Professor Bumjoon J. Kim, CBE), an ex-solution process for the synthesis of highly stable catalysts (Professor WooChul Jung, MSE), and 3D light-patterning synthesis of ordered nanostructures (Professor Seokwoo Jeon, MSE, and Professor Dongchan Jang, NQE).
Review articles discussing advanced analysis techniques covered operando materials analyses (Professor Jeong Yeong Park, Chem), graphene liquid cell transmission electron microscopy (Professor Jong Min Yuk, MSE), and multiscale modeling and visualization of materials systems (Professor Seungbum Hong, MSE).
Review articles discussing practical state-of-the-art devices covered chemiresistive hydrogen sensors (Professor Il-Doo Kim, MSE), patient-friendly diagnostics and implantable treatment devices (Professor Steve Park, MSE), triboelectric nanogenerators (Professor Yang-Kyu Choi, EE), and next-generation lithium-air batteries (Professor Hye Ryung Byon, Chem, and Professor Il-Doo Kim, MSE).
In addition to Professor Il-Doo Kim, post-doctoral researcher Dr. Jaewan Ahn from the KAIST Applied Science Research Institute, Dean of the College of Engineering at KAIST Professor Choongsik Bae, and ACS Nano Editor-in-Chief Professor Paul S. Weiss from the University of California, Los Angeles also contributed to the publication of this ACS Nano special virtual issue.
The issue can be viewed and downloaded from the ACS Nano website at https://doi.org/10.1021/acsnano.1c01101.
Image credit: KAIST
Image usage restrictions: News organizations may use or redistribute this image,with proper attribution, as part of news coverage of this paper only.
Publication:
Ahn, J., et al. (2021) Celebrating 50 Years of KAIST: Collective Intelligence and Innovation for Confronting Contemporary Issues. ACS Nano 15(3): 1895-1907. Available online at https://doi.org/10.1021/acsnano.1c01101
Profile:
Il-Doo Kim, Ph.D
Chair Professor
idkim@kaist.ac.kr
http://advnano.kaist.ac.kr
Advanced Nanomaterials and Energy Lab.
Department of Materials Science and Engineering
Membrane Innovation Center for Anti-Virus and Air-Quality Control
https://kaist.ac.kr/
Korea Advanced Institute of Science and Technology (KAIST) Daejeon, Republic of Korea
(END)
2021.03.05 View 34084 -
 Chemical Scissors Snip 2D Transition Metal Dichalcogenides into Nanoribbon
New ‘nanoribbon’ catalyst should slash cost of hydrogen production for clean fuels
Researchers have identified a potential catalyst alternative – and an innovative way to produce them using chemical ‘scissors’ – that could make hydrogen production more economical.
The research team led by Professor Sang Ouk Kim at the Department of Materials Science and Engineering published their work in Nature Communications.
Hydrogen is likely to play a key role in the clean transition away from fossil fuels and other processes that produce greenhouse gas emissions. There is a raft of transportation sectors such as long-haul shipping and aviation that are difficult to electrify and so will require cleanly produced hydrogen as a fuel or as a feedstock for other carbon-neutral synthetic fuels. Likewise, fertilizer production and the steel sector are unlikely to be “de-carbonized” without cheap and clean hydrogen.
The problem is that the cheapest methods by far of producing hydrogen gas is currently from natural gas, a process that itself produces the greenhouse gas carbon dioxide–which defeats the purpose.
Alternative techniques of hydrogen production, such as electrolysis using an electric current between two electrodes plunged into water to overcome the chemical bonds holding water together, thereby splitting it into its constituent elements, oxygen and hydrogen are very well established. But one of the factors contributing to the high cost, beyond being extremely energy-intensive, is the need for the very expensive precious and relatively rare metal platinum. The platinum is used as a catalyst–a substance that kicks off or speeds up a chemical reaction–in the hydrogen production process.
As a result, researchers have long been on the hunt for a substitution for platinum -- another catalyst that is abundant in the earth and thus much cheaper.
Transition metal dichalcogenides, or TMDs, in a nanomaterial form, have for some time been considered a good candidate as a catalyst replacement for platinum. These are substances composed of one atom of a transition metal (the elements in the middle part of the periodic table) and two atoms of a chalcogen element (the elements in the third-to-last column in the periodic table, specifically sulfur, selenium and tellurium).
What makes TMDs a good bet as a platinum replacement is not just that they are much more abundant, but also their electrons are structured in a way that gives the electrodes a boost.
In addition, a TMD that is a nanomaterial is essentially a two-dimensional super-thin sheet only a few atoms thick, just like graphene. The ultrathin nature of a 2-D TMD nanosheet allows for a great many more TMD molecules to be exposed during the catalysis process than would be the case in a block of the stuff, thus kicking off and speeding up the hydrogen-making chemical reaction that much more.
However, even here the TMD molecules are only reactive at the four edges of a nanosheet. In the flat interior, not much is going on. In order to increase the chemical reaction rate in the production of hydrogen, the nanosheet would need to be cut into very thin – almost one-dimensional strips, thereby creating many edges.
In response, the research team developed what are in essence a pair of chemical scissors that can snip TMD into tiny strips.
“Up to now, the only substances that anyone has been able to turn into these ‘nano-ribbons’ are graphene and phosphorene,” said Sang Professor Kim, one of the researchers involved in devising the process.
“But they’re both made up of just one element, so it’s pretty straightforward. Figuring out how to do it for TMD, which is made of two elements was going to be much harder.”
The ‘scissors’ involve a two-step process involving first inserting lithium ions into the layered structure of the TMD sheets, and then using ultrasound to cause a spontaneous ‘unzipping’ in straight lines.
“It works sort of like how when you split a plank of plywood: it breaks easily in one direction along the grain,” Professor Kim continued. “It’s actually really simple.”
The researchers then tried it with various types of TMDs, including those made of molybdenum, selenium, sulfur, tellurium and tungsten. All worked just as well, with a catalytic efficiency as effective as platinum’s.
Because of the simplicity of the procedure, this method should be able to be used not just in the large-scale production of TMD nanoribbons, but also to make similar nanoribbons from other multi-elemental 2D materials for purposes beyond just hydrogen production.
-ProfileProfessor Sang Ouk KimSoft Nanomaterials Laboratory (http://snml.kaist.ac.kr)Department of Materials Science and EngineeringKAIST
2020.10.29 View 9331
Chemical Scissors Snip 2D Transition Metal Dichalcogenides into Nanoribbon
New ‘nanoribbon’ catalyst should slash cost of hydrogen production for clean fuels
Researchers have identified a potential catalyst alternative – and an innovative way to produce them using chemical ‘scissors’ – that could make hydrogen production more economical.
The research team led by Professor Sang Ouk Kim at the Department of Materials Science and Engineering published their work in Nature Communications.
Hydrogen is likely to play a key role in the clean transition away from fossil fuels and other processes that produce greenhouse gas emissions. There is a raft of transportation sectors such as long-haul shipping and aviation that are difficult to electrify and so will require cleanly produced hydrogen as a fuel or as a feedstock for other carbon-neutral synthetic fuels. Likewise, fertilizer production and the steel sector are unlikely to be “de-carbonized” without cheap and clean hydrogen.
The problem is that the cheapest methods by far of producing hydrogen gas is currently from natural gas, a process that itself produces the greenhouse gas carbon dioxide–which defeats the purpose.
Alternative techniques of hydrogen production, such as electrolysis using an electric current between two electrodes plunged into water to overcome the chemical bonds holding water together, thereby splitting it into its constituent elements, oxygen and hydrogen are very well established. But one of the factors contributing to the high cost, beyond being extremely energy-intensive, is the need for the very expensive precious and relatively rare metal platinum. The platinum is used as a catalyst–a substance that kicks off or speeds up a chemical reaction–in the hydrogen production process.
As a result, researchers have long been on the hunt for a substitution for platinum -- another catalyst that is abundant in the earth and thus much cheaper.
Transition metal dichalcogenides, or TMDs, in a nanomaterial form, have for some time been considered a good candidate as a catalyst replacement for platinum. These are substances composed of one atom of a transition metal (the elements in the middle part of the periodic table) and two atoms of a chalcogen element (the elements in the third-to-last column in the periodic table, specifically sulfur, selenium and tellurium).
What makes TMDs a good bet as a platinum replacement is not just that they are much more abundant, but also their electrons are structured in a way that gives the electrodes a boost.
In addition, a TMD that is a nanomaterial is essentially a two-dimensional super-thin sheet only a few atoms thick, just like graphene. The ultrathin nature of a 2-D TMD nanosheet allows for a great many more TMD molecules to be exposed during the catalysis process than would be the case in a block of the stuff, thus kicking off and speeding up the hydrogen-making chemical reaction that much more.
However, even here the TMD molecules are only reactive at the four edges of a nanosheet. In the flat interior, not much is going on. In order to increase the chemical reaction rate in the production of hydrogen, the nanosheet would need to be cut into very thin – almost one-dimensional strips, thereby creating many edges.
In response, the research team developed what are in essence a pair of chemical scissors that can snip TMD into tiny strips.
“Up to now, the only substances that anyone has been able to turn into these ‘nano-ribbons’ are graphene and phosphorene,” said Sang Professor Kim, one of the researchers involved in devising the process.
“But they’re both made up of just one element, so it’s pretty straightforward. Figuring out how to do it for TMD, which is made of two elements was going to be much harder.”
The ‘scissors’ involve a two-step process involving first inserting lithium ions into the layered structure of the TMD sheets, and then using ultrasound to cause a spontaneous ‘unzipping’ in straight lines.
“It works sort of like how when you split a plank of plywood: it breaks easily in one direction along the grain,” Professor Kim continued. “It’s actually really simple.”
The researchers then tried it with various types of TMDs, including those made of molybdenum, selenium, sulfur, tellurium and tungsten. All worked just as well, with a catalytic efficiency as effective as platinum’s.
Because of the simplicity of the procedure, this method should be able to be used not just in the large-scale production of TMD nanoribbons, but also to make similar nanoribbons from other multi-elemental 2D materials for purposes beyond just hydrogen production.
-ProfileProfessor Sang Ouk KimSoft Nanomaterials Laboratory (http://snml.kaist.ac.kr)Department of Materials Science and EngineeringKAIST
2020.10.29 View 9331 -
 The 10th KINC Fusion Research Awardees
The KAIST Institute for NanoCentury (KINC) recognized three distinguished researchers whose convergence studies made significant impacts. The KINC presented the 10th KINC Fusion Research Awards during a ceremony that took place at KAIST’s main campus in Daejeon on May 19.
This year’s ‘best’ convergence research award went to a joint research group led by Professor Hee Tak Kim from the Department of Chemical and Biomolecular Engineering and Professor Sang Ouk Kim from the Department of Materials Science and Engineering. Their research, featured in the December 27 issue of Advanced Materials as a front cover article last year, introduced the world’s first high-energy efficiency, membraneless, flowless, zinc-bromine battery. This study, in which research professor Gyoung Hwa Jeong, postdoctoral researcher Yearin Byun, and PhD candidate Ju-Hyuck Lee took part as co-lead authors, is deemed as an example of a best practice in convergence research in which two groups’ respective expertise in the fields of carbon materials and electrochemical analysis created a synergistic effect.
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering was also recognized for having published the most interdisciplinary research papers on polymer electronics and nanomaterials at home and abroad.
Professor Hee-Tae Jung, the Director of KINC and the host of the KINC Fusion Research Awards, said, “The KINC is happy to announce the 10th awardees in nano-fusion research this year. Since convergence is crucial for making revolutionary changes, the importance of convergence studies should be recognized. Our institute will spare no effort to create a research environment suitable for convergence studies, which will be crucial for making a significant difference.”
The KINC was established in June 2006 under the KAIST Institute with the mission of facilitating convergence studies by tearing down boarders among departments and carrying out interdisciplinary joint research. Currently, the institute is comprised of approximately 90 professors from 13 departments. It aims to become a hub of university institutes for nano-fusion research.
(END)
2020.05.19 View 15736
The 10th KINC Fusion Research Awardees
The KAIST Institute for NanoCentury (KINC) recognized three distinguished researchers whose convergence studies made significant impacts. The KINC presented the 10th KINC Fusion Research Awards during a ceremony that took place at KAIST’s main campus in Daejeon on May 19.
This year’s ‘best’ convergence research award went to a joint research group led by Professor Hee Tak Kim from the Department of Chemical and Biomolecular Engineering and Professor Sang Ouk Kim from the Department of Materials Science and Engineering. Their research, featured in the December 27 issue of Advanced Materials as a front cover article last year, introduced the world’s first high-energy efficiency, membraneless, flowless, zinc-bromine battery. This study, in which research professor Gyoung Hwa Jeong, postdoctoral researcher Yearin Byun, and PhD candidate Ju-Hyuck Lee took part as co-lead authors, is deemed as an example of a best practice in convergence research in which two groups’ respective expertise in the fields of carbon materials and electrochemical analysis created a synergistic effect.
Professor Bumjoon Kim from the Department of Chemical and Biomolecular Engineering was also recognized for having published the most interdisciplinary research papers on polymer electronics and nanomaterials at home and abroad.
Professor Hee-Tae Jung, the Director of KINC and the host of the KINC Fusion Research Awards, said, “The KINC is happy to announce the 10th awardees in nano-fusion research this year. Since convergence is crucial for making revolutionary changes, the importance of convergence studies should be recognized. Our institute will spare no effort to create a research environment suitable for convergence studies, which will be crucial for making a significant difference.”
The KINC was established in June 2006 under the KAIST Institute with the mission of facilitating convergence studies by tearing down boarders among departments and carrying out interdisciplinary joint research. Currently, the institute is comprised of approximately 90 professors from 13 departments. It aims to become a hub of university institutes for nano-fusion research.
(END)
2020.05.19 View 15736 -
 Recyclable Nano-Fiber Filtered Face Masks a Boon for Supply Fiasco
Wearing a face mask is a common sight in Korea during the COVID-19 outbreak. Due to the overwhelming demand, last week the government started to ration two masks per person per week, as a drastic measure to address the supply fiasco.
The face masks most commonly used are disposable ones, originally made for filtering out up to 94 or 95 percent of fine dust, referred to as N94 or N95 masks.
A KAIST research team announced that they have developed a nano-filter that maintains excellent filtering efficiency even after hand washing through the development of proprietary technology that aligns nanofibers with a diameter of 100~500 nm in orthogonal or unidirectional directions. This reusable nano-filtered face mask could help to relieve the challenges arising from the supply shortage of face masks.
Professor Il-Doo Kim’s nano-fiber filtered mask will maintain its sturdy frame and filtering function even after being washed more than 20 times. Professor Kim, who has continued to study the filtering of fine dust using nano-filters, is now awaiting final approval from the Ministry of Food and Drug Safety to bring his product into the market.
Professor Kim used an insulation block electrospinning process to manufacture orthogonal nanofibers by controlling the alignment of nanofibers. This structure can minimize delivering of the pressure toward the air filter and maximize the filtration efficiency, which is different from existing disposable masks without nano-fibers.
Existing masks also fail to maintain their air filtering function because their electrostatic function disappears when exposed to water. Thus, their filtering efficiency is reduced significantly, making it almost impossible to reuse them. However, this nano-fiber design was proven to be water resistant with more than 94% filtering efficiency in 20 repeated bactericidal tests with ethanol. The nano-fiber mask also showed no deformation in its nano-membrane structure despite the 20 hand washes. In particular, it was confirmed that there were no deformations in the membrane, even after soaking in ethanol more than three hours.
Professor Kim said, “We believe that this mask can be reusable for about a month even after washing in ethanol. The inner filter can also be replaced.” He added, “We found that the mask filters out up to 80 percent of 600-nanometer particles even after undergoing a bending test more than 4,000 times.”
Professor Kim established his startup company, the “Kim Il-Doo Research Institute,” last February. It can currently produce 1,500 nano-fiber filters per day.
2020.03.17 View 23842
Recyclable Nano-Fiber Filtered Face Masks a Boon for Supply Fiasco
Wearing a face mask is a common sight in Korea during the COVID-19 outbreak. Due to the overwhelming demand, last week the government started to ration two masks per person per week, as a drastic measure to address the supply fiasco.
The face masks most commonly used are disposable ones, originally made for filtering out up to 94 or 95 percent of fine dust, referred to as N94 or N95 masks.
A KAIST research team announced that they have developed a nano-filter that maintains excellent filtering efficiency even after hand washing through the development of proprietary technology that aligns nanofibers with a diameter of 100~500 nm in orthogonal or unidirectional directions. This reusable nano-filtered face mask could help to relieve the challenges arising from the supply shortage of face masks.
Professor Il-Doo Kim’s nano-fiber filtered mask will maintain its sturdy frame and filtering function even after being washed more than 20 times. Professor Kim, who has continued to study the filtering of fine dust using nano-filters, is now awaiting final approval from the Ministry of Food and Drug Safety to bring his product into the market.
Professor Kim used an insulation block electrospinning process to manufacture orthogonal nanofibers by controlling the alignment of nanofibers. This structure can minimize delivering of the pressure toward the air filter and maximize the filtration efficiency, which is different from existing disposable masks without nano-fibers.
Existing masks also fail to maintain their air filtering function because their electrostatic function disappears when exposed to water. Thus, their filtering efficiency is reduced significantly, making it almost impossible to reuse them. However, this nano-fiber design was proven to be water resistant with more than 94% filtering efficiency in 20 repeated bactericidal tests with ethanol. The nano-fiber mask also showed no deformation in its nano-membrane structure despite the 20 hand washes. In particular, it was confirmed that there were no deformations in the membrane, even after soaking in ethanol more than three hours.
Professor Kim said, “We believe that this mask can be reusable for about a month even after washing in ethanol. The inner filter can also be replaced.” He added, “We found that the mask filters out up to 80 percent of 600-nanometer particles even after undergoing a bending test more than 4,000 times.”
Professor Kim established his startup company, the “Kim Il-Doo Research Institute,” last February. It can currently produce 1,500 nano-fiber filters per day.
2020.03.17 View 23842 -
 New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19065
New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19065 -
 Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 28175
Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 28175 -
 Synthesizing Single-Crystalline Hexagonal Graphene Quantum Dots
(Figure: Uniformly ordered single-crystalline graphene quantum dots of various sizes synthesized through solution chemistry.)
A KAIST team has designed a novel strategy for synthesizing single-crystalline graphene quantum dots, which emit stable blue light. The research team confirmed that a display made of their synthesized graphene quantum dots successfully emitted blue light with stable electric pressure, reportedly resolving the long-standing challenges of blue light emission in manufactured displays. The study, led by Professor O Ok Park in the Department of Chemical and Biological Engineering, was featured online in Nano Letters on July 5.
Graphene has gained increased attention as a next-generation material for its heat and electrical conductivity as well as its transparency. However, single and multi-layered graphene have characteristics of a conductor so that it is difficult to apply into semiconductor. Only when downsized to the nanoscale, semiconductor’s distinct feature of bandgap will be exhibited to emit the light in the graphene. This illuminating featuring of dot is referred to as a graphene quantum dot.
Conventionally, single-crystalline graphene has been fabricated by chemical vapor deposition (CVD) on copper or nickel thin films, or by peeling graphite physically and chemically. However, graphene made via chemical vapor deposition is mainly used for large-surface transparent electrodes. Meanwhile, graphene made by chemical and physical peeling carries uneven size defects.
The research team explained that their graphene quantum dots exhibited a very stable single-phase reaction when they mixed amine and acetic acid with an aqueous solution of glucose. Then, they synthesized single-crystalline graphene quantum dots from the self-assembly of the reaction intermediate. In the course of fabrication, the team developed a new separation method at a low-temperature precipitation, which led to successfully creating a homogeneous nucleation of graphene quantum dots via a single-phase reaction.
Professor Park and his colleagues have developed solution phase synthesis technology that allows for the creation of the desired crystal size for single nanocrystals down to 100 nano meters. It is reportedly the first synthesis of the homogeneous nucleation of graphene through a single-phase reaction.
Professor Park said, "This solution method will significantly contribute to the grafting of graphene in various fields. The application of this new graphene will expand the scope of its applications such as for flexible displays and varistors.”
This research was a joint project with a team from Korea University under Professor Sang Hyuk Im from the Department of Chemical and Biological Engineering, and was supported by the National Research Foundation of Korea, the Nano-Material Technology Development Program from the Electronics and Telecommunications Research Institute (ETRI), KAIST EEWS, and the BK21+ project from the Korean government.
2019.08.02 View 35335
Synthesizing Single-Crystalline Hexagonal Graphene Quantum Dots
(Figure: Uniformly ordered single-crystalline graphene quantum dots of various sizes synthesized through solution chemistry.)
A KAIST team has designed a novel strategy for synthesizing single-crystalline graphene quantum dots, which emit stable blue light. The research team confirmed that a display made of their synthesized graphene quantum dots successfully emitted blue light with stable electric pressure, reportedly resolving the long-standing challenges of blue light emission in manufactured displays. The study, led by Professor O Ok Park in the Department of Chemical and Biological Engineering, was featured online in Nano Letters on July 5.
Graphene has gained increased attention as a next-generation material for its heat and electrical conductivity as well as its transparency. However, single and multi-layered graphene have characteristics of a conductor so that it is difficult to apply into semiconductor. Only when downsized to the nanoscale, semiconductor’s distinct feature of bandgap will be exhibited to emit the light in the graphene. This illuminating featuring of dot is referred to as a graphene quantum dot.
Conventionally, single-crystalline graphene has been fabricated by chemical vapor deposition (CVD) on copper or nickel thin films, or by peeling graphite physically and chemically. However, graphene made via chemical vapor deposition is mainly used for large-surface transparent electrodes. Meanwhile, graphene made by chemical and physical peeling carries uneven size defects.
The research team explained that their graphene quantum dots exhibited a very stable single-phase reaction when they mixed amine and acetic acid with an aqueous solution of glucose. Then, they synthesized single-crystalline graphene quantum dots from the self-assembly of the reaction intermediate. In the course of fabrication, the team developed a new separation method at a low-temperature precipitation, which led to successfully creating a homogeneous nucleation of graphene quantum dots via a single-phase reaction.
Professor Park and his colleagues have developed solution phase synthesis technology that allows for the creation of the desired crystal size for single nanocrystals down to 100 nano meters. It is reportedly the first synthesis of the homogeneous nucleation of graphene through a single-phase reaction.
Professor Park said, "This solution method will significantly contribute to the grafting of graphene in various fields. The application of this new graphene will expand the scope of its applications such as for flexible displays and varistors.”
This research was a joint project with a team from Korea University under Professor Sang Hyuk Im from the Department of Chemical and Biological Engineering, and was supported by the National Research Foundation of Korea, the Nano-Material Technology Development Program from the Electronics and Telecommunications Research Institute (ETRI), KAIST EEWS, and the BK21+ project from the Korean government.
2019.08.02 View 35335 -
 Professor Il-Doo Kim Recevies the Song-gok Award
Professor Il-Doo Kim from the Department of Materials Science and Engineering at KAIST received the 20th Song-gok Science and Technology Award from Korea Institute of Science and Technology (KSIT).
The Song-gok Science and Technology Award was established to praise the accomplishments of the first president, Hyung-seop Choi, whose penname is Song-gok. The award selects a recipient in the field of materials and technology every other year.
Professor Kim, in recognition of his outstanding research and contributions to materials science in Korea, received the award during the 52nd anniversary ceremony of KIST on February 9.
Professor Kim focuses on developing nanofiber gas sensors for diagnosing disease in advance by analyzing exhaled biomarkers with electrospinning technology.
He has published more than 211 papers and has recorded more than 9,650 citations and 50 h-index.
Professor Kim has registered 107 patents and applied 38 patents in Korea while registering 29 patents and applying 16 patents overseas. Also, he transferred four technologies in 2017.
Professor Kim is recognized as one of the researchers who is leading nanofiber technology. On January 17, he made a keynote speech at the 5th International Conference on Electrospinning, which was his fourth keynote speech at that conference.
Moreover, he received the Technology Innovation Award at the College of Engineering, KAIST on December 19, 2017.
Professor Kim said, “It is my great honor to receive the Song-gok Science and Technology Award. I would like to bring distinction to KAIST by taking the lead in the commercializing a nanofiber-based highly sensitive nanosensors, diversifying and commercializing technology using nanofiber.”
2018.02.13 View 9666
Professor Il-Doo Kim Recevies the Song-gok Award
Professor Il-Doo Kim from the Department of Materials Science and Engineering at KAIST received the 20th Song-gok Science and Technology Award from Korea Institute of Science and Technology (KSIT).
The Song-gok Science and Technology Award was established to praise the accomplishments of the first president, Hyung-seop Choi, whose penname is Song-gok. The award selects a recipient in the field of materials and technology every other year.
Professor Kim, in recognition of his outstanding research and contributions to materials science in Korea, received the award during the 52nd anniversary ceremony of KIST on February 9.
Professor Kim focuses on developing nanofiber gas sensors for diagnosing disease in advance by analyzing exhaled biomarkers with electrospinning technology.
He has published more than 211 papers and has recorded more than 9,650 citations and 50 h-index.
Professor Kim has registered 107 patents and applied 38 patents in Korea while registering 29 patents and applying 16 patents overseas. Also, he transferred four technologies in 2017.
Professor Kim is recognized as one of the researchers who is leading nanofiber technology. On January 17, he made a keynote speech at the 5th International Conference on Electrospinning, which was his fourth keynote speech at that conference.
Moreover, he received the Technology Innovation Award at the College of Engineering, KAIST on December 19, 2017.
Professor Kim said, “It is my great honor to receive the Song-gok Science and Technology Award. I would like to bring distinction to KAIST by taking the lead in the commercializing a nanofiber-based highly sensitive nanosensors, diversifying and commercializing technology using nanofiber.”
2018.02.13 View 9666