T+Cell
-
 KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 6213
KAIST Unveils New Possibilities for Treating Intractable Brain Tumors
< Photo 1. (From left) Professor Heung Kyu Lee, KAIST Department of Biological Sciences, and Dr. Keun Bon Ku >
Immunotherapy, which enhances the immune system's T cell response to eliminate cancer cells, has emerged as a key approach in cancer treatment. However, in the case of glioblastoma, an aggressive and treatment-resistant brain tumor, numerous clinical trials have failed to confirm their efficacy. Korean researchers have recently analyzed the mechanisms that cause T cell exhaustion, which is characterized by a loss of function or a weakened response following prolonged exposure to antigens in such intractable cancers, identifying key control factors in T cell activation and clarifying the mechanisms that enhance therapeutic effectiveness.
KAIST (represented by President Kwang Hyung Lee) announced on the 6th of November that Professor Heung Kyu Lee’s team from the Department of Biological Sciences, in collaboration with the Korea Research Institute of Chemical Technology (represented by President Young Kuk Lee), has confirmed improved survival rates in a glioblastoma mouse model. By removing the inhibitory Fc gamma receptor (FcγRIIB), the research team was able to restore the responsiveness of cytotoxic T cells to immune checkpoint inhibitors, leading to enhanced anticancer activity.
The research team examined the effect of FcγRIIB, an inhibitory receptor recently found in cytotoxic T cells, on tumor-infiltrating T cells and the therapeutic effectiveness of the anti-PD-1 immune checkpoint inhibitor.
< Figure 1. Study results on improved survival rate due to increased antitumor activity of anti-PD-1 treatment in inhibitory Fc gamma receptor(Fcgr2b) ablation mice with murine glioblastoma. >
Their findings showed that deleting FcγRIIB induced the increase of tumor antigen-specific memory T cells, which helps to suppress exhaustion, enhances stem-like qualities, and reactivates T cell-mediated antitumor immunity, particularly in response to anti-PD-1 treatment. Furthermore, FcγRIIB deletion led to an increase in antigen-specific memory T cells that maintained continuous infiltration into the tumor tissue.
This study presents a new therapeutic target for tumors unresponsive to immune checkpoint inhibitors and demonstrates that combining FcγRIIB inhibition with anti-PD-1 treatment can produce synergistic effects, potentially improving therapeutic outcomes for tumors like glioblastoma, which typically show resistance to anti-PD-1 therapy.
< Figure 2. Overview of the study on the enhanced response to anti-PD-1 therapy for glioblastoma brain tumors upon deletion of the inhibitory Fc gamma receptor (FcγRIIB) in tumor microenvironment. When the inhibitory Fc gamma receptor (FcγRIIB) of cytotoxic T cells is deleted, an increase in tumor-specific memory T cells (Ttsms) was observed. In addition, this T cell subset is identified as originating from the tumor-draining lymph nodes(TdLNs) and leads to persistent infiltration into the tumor tissue. Anti-PD-1 therapy leads to an increased anti-tumor immune response via Ttsms, which is confirmed by increased tumor cell toxicity and increased cell division and decreased cell de-migration indices. Ultimately, the increased cytotoxic T cell immune response leads to an increase in the survival rate of glioblastoma. >
Professor Heung Kyu Lee explained, "This study offers a way to overcome clinical failures in treating brain tumors with immune checkpoint therapy and opens possibilities for broader applications to other intractable cancers. It also highlights the potential of utilizing cytotoxic T cells for tumor cell therapy."
The study, led by Dr. Keun Bon Ku of KAIST (currently a senior researcher at the Korea Research Institute of Chemical Technology's Center for Infectious Disease Diagnosis and Prevention), along with Chae Won Kim, Yumin Kim, Byeong Hoon Kang, Jeongwoo La, In Kang, Won Hyung Park, Stephen Ahn, and Sung Ki Lee, was published online on October 26 in the Journal for ImmunoTherapy of Cancer, an international journal in tumor immunology and therapy from the Society for Immunotherapy of Cancer. (Paper title: “Inhibitory Fcγ receptor deletion enhances CD8 T cell stemness increasing anti-PD-1 therapy responsiveness against glioblastoma,” http://dx.doi.org/10.1136/jitc-2024-009449).
This research received support from the National Research Foundation of Korea, the Bio & Medical Technology Development Program, and the Samsung Science & Technology Foundation.
2024.11.15 View 6213 -
 The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 10764
The cause of disability in aged brain meningeal membranes identified
Due to the increase in average age, studies on changes in the brain following general aging process without serious brain diseases have also become an issue that requires in-depth studies. Regarding aging research, as aging progresses, ‘sugar’ accumulates in the body, and the accumulated sugar becomes a causative agent for various diseases such as aging-related inflammation and vascular disease. In the end, “surplus” sugar molecules attach to various proteins in the body and interfere with their functions.
KAIST (President Kwang Hyung Lee), a joint research team of Professor Pilnam Kim and Professor Yong Jeong of the Department of Bio and Brain Engineering, revealed on the 15th that it was confirmed that the function of being the “front line of defense” for the cerebrocortex of the brain meninges, the layers of membranes that surrounds the brain, is hindered when 'sugar' begins to build up on them as aging progresses.
Professor Kim's research team confirmed excessive accumulation of sugar molecules in the meninges of the elderly and also confirmed that sugar accumulation occurs mouse models in accordance with certain age levels. The meninges are thin membranes that surround the brain and exist at the boundary between the cerebrospinal fluid and the cortex and play an important role in protecting the brain. In this study, it was revealed that the dysfunction of these brain membranes caused by aging is induced by 'excess' sugar in the brain. In particular, as the meningeal membrane becomes thinner and stickier due to aging, a new paradigm has been provided for the discovery of the principle of the decrease in material exchange between the cerebrospinal fluid and the cerebral cortex.
This research was conducted by the Ph.D. candidate Hyo Min Kim and Dr. Shinheun Kim as the co-first authors to be published online on February 28th in the international journal, Aging Cell. (Paper Title: Glycation-mediated tissue-level remodeling of brain meningeal membrane by aging)
The meninges, which are in direct contact with the cerebrospinal fluid, are mainly composed of collagen, an extracellular matrix (ECM) protein, and are composed of fibroblasts, which are cells that produce this protein. The cells that come in contact with collagen proteins that are attached with sugar have a low collagen production function, while the meningeal membrane continuously thins and collapses as the expression of collagen degrading enzymes increases.
Studies on the relationship between excess sugar molecules accumulation in the brain due to continued sugar intake and the degeneration of neurons and brain diseases have been continuously conducted. However, this study was the first to identify meningeal degeneration and dysfunction caused by glucose accumulation with the focus on the meninges itself, and the results are expected to present new ideas for research into approach towards discoveries of new treatments for brain disease.
Researcher Hyomin Kim, the first author, introduced the research results as “an interesting study that identified changes in the barriers of the brain due to aging through a convergent approach, starting from the human brain and utilizing an animal model with a biomimetic meningeal model”.
Professor Pilnam Kim's research team is conducting research and development to remove sugar that accumulated throughout the human body, including the meninges. Advanced glycation end products, which are waste products formed when proteins and sugars meet in the human body, are partially removed by macrophages. However, glycated products bound to extracellular matrix proteins such as collagen are difficult to remove naturally. Through the KAIST-Ceragem Research Center, this research team is developing a healthcare medical device to remove 'sugar residue' in the body.
This study was carried out with the National Research Foundation of Korea's collective research support.
Figure 1. Schematic diagram of proposed mechanism showing aging‐related ECM remodeling through meningeal fibroblasts on the brain leptomeninges. Meningeal fibroblasts in the young brain showed dynamic COL1A1 synthetic and COL1‐interactive function on the collagen membrane. They showed ITGB1‐mediated adhesion on the COL1‐composed leptomeningeal membrane and induction of COL1A1 synthesis for maintaining the collagen membrane. With aging, meningeal fibroblasts showed depletion of COL1A1 synthetic function and altered cell–matrix interaction.
Figure 2. Representative rat meningeal images observed in the study. Compared to young rats, it was confirmed that type 1 collagen (COL1) decreased along with the accumulation of glycated end products (AGE) in the brain membrane of aged rats, and the activity of integrin beta 1 (ITGB1), a representative receptor corresponding to cell-collagen interaction. Instead, it was observed that the activity of discoidin domain receptor 2 (DDR2), one of the tyrosine kinases, increased.
Figure 3. Substance flux through the brain membrane decreases with aging. It was confirmed that the degree of adsorption of fluorescent substances contained in cerebrospinal fluid (CSF) to the brain membrane increased and the degree of entry into the periphery of the cerebral blood vessels decreased in the aged rats. In this study, only the influx into the brain was confirmed during the entry and exit of substances, but the degree of outflow will also be confirmed through future studies.
2023.03.15 View 10764 -
 Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 15929
Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 15929 -
 Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14820
Simulations Open a New Way to Reverse Cell Aging
Turning off a newly identified enzyme could reverse a natural aging process in cells.
Research findings by a KAIST team provide insight into the complex mechanism of cellular senescence and present a potential therapeutic strategy for reducing age-related diseases associated with the accumulation of senescent cells.
Simulations that model molecular interactions have identified an enzyme that could be targeted to reverse a natural aging process called cellular senescence. The findings were validated with laboratory experiments on skin cells and skin equivalent tissues, and published in the Proceedings of the National Academy of Sciences (PNAS).
“Our research opens the door for a new generation that perceives aging as a reversible biological phenomenon,” says Professor Kwang-Hyun Cho of the Department of Bio and Brain engineering at the Korea Advanced Institute of Science and Technology (KAIST), who led the research with colleagues from KAIST and Amorepacific Corporation in Korea.
Cells respond to a variety of factors, such as oxidative stress, DNA damage, and shortening of the telomeres capping the ends of chromosomes, by entering a stable and persistent exit from the cell cycle. This process, called cellular senescence, is important, as it prevents damaged cells from proliferating and turning into cancer cells. But it is also a natural process that contributes to aging and age-related diseases. Recent research has shown that cellular senescence can be reversed. But the laboratory approaches used thus far also impair tissue regeneration or have the potential to trigger malignant transformations.
Professor Cho and his colleagues used an innovative strategy to identify molecules that could be targeted for reversing cellular senescence. The team pooled together information from the literature and databases about the molecular processes involved in cellular senescence. To this, they added results from their own research on the molecular processes involved in the proliferation, quiescence (a non-dividing cell that can re-enter the cell cycle) and senescence of skin fibroblasts, a cell type well known for repairing wounds. Using algorithms, they developed a model that simulates the interactions between these molecules. Their analyses allowed them to predict which molecules could be targeted to reverse cell senescence.
They then investigated one of the molecules, an enzyme called PDK1, in incubated senescent skin fibroblasts and three-dimensional skin equivalent tissue models. They found that blocking PDK1 led to the inhibition of two downstream signalling molecules, which in turn restored the cells’ ability to enter back into the cell cycle. Notably, the cells retained their capacity to regenerate wounded skin without proliferating in a way that could lead to malignant transformation.
The scientists recommend investigations are next done in organs and organisms to determine the full effect of PDK1 inhibition. Since the gene that codes for PDK1 is overexpressed in some cancers, the scientists expect that inhibiting it will have both anti-aging and anti-cancer effects.
-Profile
Professor Kwang-Hyun Cho
Laboratory for Systems Biology and Bio-Inspired Engineering
http://sbie.kaist.ac.kr
Department of Bio and Brain Engineering
KAIST
2020.11.26 View 14820 -
 Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
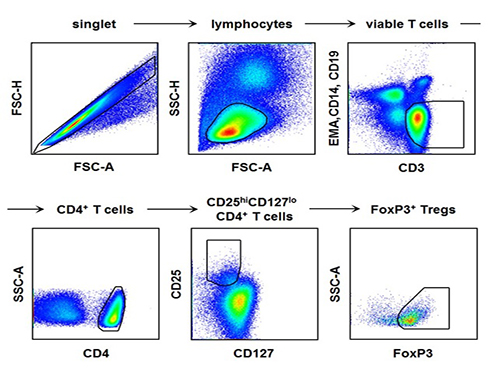
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11586
Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 11586