CO2
-
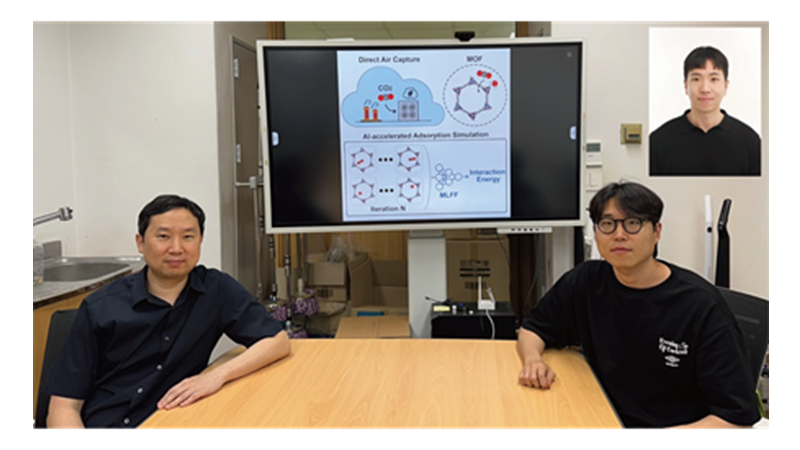 KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 219
KAIST Develops AI to Easily Find Promising Materials That Capture Only CO₂
< Photo 1. (From left) Professor Jihan Kim, Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of the Department of Chemical and Biomolecular Engineering >
In order to help prevent the climate crisis, actively reducing already-emitted CO₂ is essential. Accordingly, direct air capture (DAC) — a technology that directly extracts only CO₂ from the air — is gaining attention. However, effectively capturing pure CO₂ is not easy due to water vapor (H₂O) present in the air. KAIST researchers have successfully used AI-driven machine learning techniques to identify the most promising CO₂-capturing materials among metal-organic frameworks (MOFs), a key class of materials studied for this technology.
KAIST (President Kwang Hyung Lee) announced on the 29th of June that a research team led by Professor Jihan Kim from the Department of Chemical and Biomolecular Engineering, in collaboration with a team at Imperial College London, has developed a machine-learning-based simulation method that can quickly and accurately screen MOFs best suited for atmospheric CO₂ capture.
< Figure 1. Concept diagram of Direct Air Capture (DAC) technology and carbon capture using Metal-Organic Frameworks (MOFs). MOFs are promising porous materials capable of capturing carbon dioxide from the atmosphere, drawing attention as a core material for DAC technology. >
To overcome the difficulty of discovering high-performance materials due to the complexity of structures and the limitations of predicting intermolecular interactions, the research team developed a machine learning force field (MLFF) capable of precisely predicting the interactions between CO₂, water (H₂O), and MOFs. This new method enables calculations of MOF adsorption properties with quantum-mechanics-level accuracy at vastly faster speeds than before.
Using this system, the team screened over 8,000 experimentally synthesized MOF structures, identifying more than 100 promising candidates for CO₂ capture. Notably, this included new candidates that had not been uncovered by traditional force-field-based simulations. The team also analyzed the relationships between MOF chemical structure and adsorption performance, proposing seven key chemical features that will help in designing new materials for DAC.
< Figure 2. Concept diagram of adsorption simulation using Machine Learning Force Field (MLFF). The developed MLFF is applicable to various MOF structures and allows for precise calculation of adsorption properties by predicting interaction energies during repetitive Widom insertion simulations. It is characterized by simultaneously achieving high accuracy and low computational cost compared to conventional classical force fields. >
This research is recognized as a significant advance in the DAC field, greatly enhancing materials design and simulation by precisely predicting MOF-CO₂ and MOF-H₂O interactions.
The results of this research, with Ph.D. candidate Yunsung Lim and Dr. Hyunsoo Park of KAIST as co-first authors, were published in the international academic journal Matter on June 12.
※Paper Title: Accelerating CO₂ direct air capture screening for metal–organic frameworks with a transferable machine learning force field
※DOI: 10.1016/j.matt.2025.102203
This research was supported by the Saudi Aramco-KAIST CO₂ Management Center and the Ministry of Science and ICT's Global C.L.E.A.N. Project.
2025.06.29 View 219 -
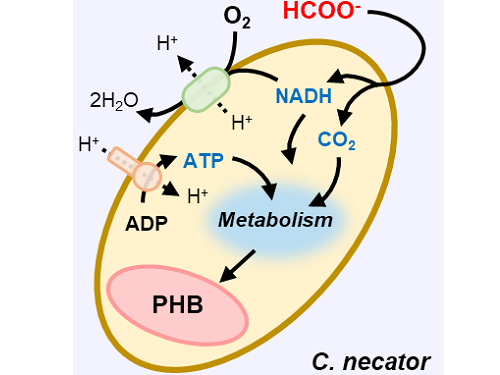 A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11842
A biohybrid system to extract 20 times more bioplastic from CO2 developed by KAIST researchers
As the issues surrounding global climate change intensify, more attention and determined efforts are required to re-grasp the issue as a state of “crisis” and respond to it properly. Among the various methods of recycling CO2, the electrochemical CO2 conversion technology is a technology that can convert CO2 into useful chemical substances using electrical energy. Since it is easy to operate facilities and can use the electricity from renewable sources like the solar cells or the wind power, it has received a lot of attention as an eco-friendly technology can contribute to reducing greenhouse gases and achieve carbon neutrality.
KAIST (President Kwang Hyung Lee) announced on the 30th that the joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering succeeded in developing a technology that produces bioplastics from CO2 with high efficiency by developing a hybrid system that interlinked the electrochemical CO2 conversion and microbial bio conversion methods together. The results of the research, which showed the world's highest productivity by more than 20 times compared to similar systems, were published online on March 27th in the "Proceedings of the National Academy of Sciences (PNAS)".
※ Paper title: Biohybrid CO2 electrolysis for the direct synthesis of polyesters from CO2
※ Author information: Jinkyu Lim (currently at Stanford Linear Accelerator Center, co-first author), So Young Choi (KAIST, co-first author), Jae Won Lee (KAIST, co-first author), Hyunjoo Lee (KAIST, corresponding author), Sang Yup Lee (KAIST, corresponding author)
For the efficient conversion of CO2, high-efficiency electrode catalysts and systems are actively being developed. As conversion products, only compounds containing one or up to three carbon atoms are produced on a limited basis. Compounds of one carbon, such as CO, formic acid, and ethylene, are produced with relatively high efficiency. Liquid compounds of several carbons, such as ethanol, acetic acid, and propanol, can also be produced by these systems, but due to the nature of the chemical reaction that requires more electrons, there are limitations involving the conversion efficiency and the product selection.
Accordingly, a joint research team led by Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee of the Department of Chemical and Biomolecular Engineering at KAIST developed a technology to produce bioplastics from CO2 by linking electrochemical conversion technology with bioconversion method that uses microorganisms.
This electrochemical-bio hybrid system is in the form of having an electrolyzer, in which electrochemical conversion reactions occur, connected to a fermenter, in which microorganisms are cultured. When CO2 is converted to formic acid in the electrolyzer, and it is fed into the fermenter in which the microbes like the Cupriavidus necator, in this case, consumes the carbon source to produce polyhydroxyalkanoate (PHA), a microbial-derived bioplastic.
According to the research results of the existing hybrid concepts, there was a disadvantage of having low productivity or stopping at a non-continuous process due to problems of low efficiency of the electrolysis and irregular results arising from the culturing conditions of the microbes.
In order to overcome these problems, the joint research team made formic acid with a gas diffusion electrode using gaseous CO2. In addition, the team developed a 'physiologically compatible catholyte' that can be used as a culture medium for microorganisms as well as an electrolyte that allows the electrolysis to occur sufficiently without inhibiting the growth of microorganisms, without having to have a additional separation and purification process, which allowed the acide to be supplied directly to microorganisms.
Through this, the electrolyte solution containing formic acid made from CO2 enters the fermentation tank, is used for microbial culture, and enters the electrolyzer to be circulated, maximizing the utilization of the electrolyte solution and remaining formic acid. In addition, a filter was installed to ensure that only the electrolyte solution with any and all microorganisms that can affect the electrosis filtered out is supplied back to the electrolyzer, and that the microorganisms exist only in the fermenter, designing the two system to work well together with utmost efficiency.
Through the developed hybrid system, the produced bioplastic, poly-3-hydroxybutyrate (PHB), of up to 83% of the cell dry weight was produced from CO2, which produced 1.38g of PHB from a 4 cm2 electrode, which is the world's first gram(g) level production and is more than 20 times more productive than previous research. In addition, the hybrid system is expected to be applied to various industrial processes in the future as it shows promises of the continuous culture system.
The corresponding authors, Professor Hyunjoo Lee and Distinguished Professor Sang Yup Lee noted that “The results of this research are technologies that can be applied to the production of various chemical substances as well as bioplastics, and are expected to be used as key parts needed in achieving carbon neutrality in the future.”
This research was received and performed with the supports from the CO2 Reduction Catalyst and Energy Device Technology Development Project, the Heterogeneous Atomic Catalyst Control Project, and the Next-generation Biorefinery Source Technology Development Project to lead the Biochemical Industry of the Oil-replacement Eco-friendly Chemical Technology Development Program by the Ministry of Science and ICT.
Figure 1. Schematic diagram and photo of the biohybrid CO2 electrolysis system.
(A) A conceptual scheme and (B) a photograph of the biohybrid CO2 electrolysis system. (C) A detailed scheme of reaction inside the system. Gaseous CO2 was converted to formate in the electrolyzer, and the formate was converted to PHB by the cells in the fermenter. The catholyte was developed so that it is compatible with both CO2 electrolysis and fermentation and was continuously circulated.
2023.03.30 View 11842 -
 A New Strategy for Early Evaluations of CO2 Utilization Technologies
- A three-step evaluation procedure based on technology readiness levels helps find the most efficient technology before allocating R&D manpower and investments in CO2 utilization technologies. -
Researchers presented a unified framework for early-stage evaluations of a variety of emerging CO2 utilization (CU) technologies. The three-step procedure allows a large number of potential CU technologies to be screened in order to identify the most promising ones, including those at low level of technical maturity, before allocating R&D manpower and investments.
When evaluating new technology, various aspects of the new technology should be considered. Its feasibility, efficiency, economic competitiveness, and environmental friendliness are crucial, and its level of technical maturity is also an important component for further consideration. However, most technology evaluation procedures are data-driven, and the amount of reliable data in the early stages of technology development has been often limited.
A research team led by Professor Jay Hyung Lee from the Department of Chemical and Biomolecular Engineering at KAIST proposed a new procedure for evaluating the early development stages of emerging CU technologies which are applicable at various technology readiness levels (TRLs).
The procedure obtains performance indicators via primary data preparation, secondary data calculation, and performance indicator calculation, and the lead author of the study Dr. Kosan Roh and his colleagues presented a number of databases, methods, and computer-aided tools that can effectively facilitate the procedure.
The research team demonstrated the procedure through four case studies involving novel CU technologies of different types and at various TRLs. They confirmed the electrochemical CO2 reduction for the production of ten chemicals, the co-electrolysis of CO2 and water for ethylene production, the direct oxidation of CO2 -based methanol for oxymethylene dimethyl production, and the microalgal biomass co-firing for power generation.
The expected range of the performance indicators for low TRL technologies is broader than that for high TRL technologies, however, it is not the case for high TRL technologies as they are already at an optimized state. The research team believes that low TRL technologies will be significantly improved through future R&D until they are commercialized. “We plan to develop a systematic approach for such a comparison to help avoid misguided decision-making,” Professor Lee explained.
Professor Lee added, “This procedure allows us to conduct a comprehensive and systematic evaluation of new technology. On top of that, it helps make efficient and reliable assessment possible.”
The research team collaborated with Professor Alexander Mitsos, Professor André Bardow, and Professor Matthias Wessling at RWTH Aachen University in Germany. Their findings were reported in Green Chemistry on May 21. This work was supported by the Korea Carbon Capture and Sequestration R&D Center (KCRC).
Publications:
Roh, K., et al. (2020) ‘Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels’ Green Chemistry. Available online at https://doi.org/10.1039/c9gc04440j
Profile: Jay Hyung Lee, Ph.D.
Professor
jayhlee@kaist.ac.kr
http://lense.kaist.ac.kr/
Laboratory for Energy System Engineering (LENSE)
Department of Chemical and Biomolecular Engineering
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.22 View 11584
A New Strategy for Early Evaluations of CO2 Utilization Technologies
- A three-step evaluation procedure based on technology readiness levels helps find the most efficient technology before allocating R&D manpower and investments in CO2 utilization technologies. -
Researchers presented a unified framework for early-stage evaluations of a variety of emerging CO2 utilization (CU) technologies. The three-step procedure allows a large number of potential CU technologies to be screened in order to identify the most promising ones, including those at low level of technical maturity, before allocating R&D manpower and investments.
When evaluating new technology, various aspects of the new technology should be considered. Its feasibility, efficiency, economic competitiveness, and environmental friendliness are crucial, and its level of technical maturity is also an important component for further consideration. However, most technology evaluation procedures are data-driven, and the amount of reliable data in the early stages of technology development has been often limited.
A research team led by Professor Jay Hyung Lee from the Department of Chemical and Biomolecular Engineering at KAIST proposed a new procedure for evaluating the early development stages of emerging CU technologies which are applicable at various technology readiness levels (TRLs).
The procedure obtains performance indicators via primary data preparation, secondary data calculation, and performance indicator calculation, and the lead author of the study Dr. Kosan Roh and his colleagues presented a number of databases, methods, and computer-aided tools that can effectively facilitate the procedure.
The research team demonstrated the procedure through four case studies involving novel CU technologies of different types and at various TRLs. They confirmed the electrochemical CO2 reduction for the production of ten chemicals, the co-electrolysis of CO2 and water for ethylene production, the direct oxidation of CO2 -based methanol for oxymethylene dimethyl production, and the microalgal biomass co-firing for power generation.
The expected range of the performance indicators for low TRL technologies is broader than that for high TRL technologies, however, it is not the case for high TRL technologies as they are already at an optimized state. The research team believes that low TRL technologies will be significantly improved through future R&D until they are commercialized. “We plan to develop a systematic approach for such a comparison to help avoid misguided decision-making,” Professor Lee explained.
Professor Lee added, “This procedure allows us to conduct a comprehensive and systematic evaluation of new technology. On top of that, it helps make efficient and reliable assessment possible.”
The research team collaborated with Professor Alexander Mitsos, Professor André Bardow, and Professor Matthias Wessling at RWTH Aachen University in Germany. Their findings were reported in Green Chemistry on May 21. This work was supported by the Korea Carbon Capture and Sequestration R&D Center (KCRC).
Publications:
Roh, K., et al. (2020) ‘Early-stage evaluation of emerging CO2 utilization technologies at low technology readiness levels’ Green Chemistry. Available online at https://doi.org/10.1039/c9gc04440j
Profile: Jay Hyung Lee, Ph.D.
Professor
jayhlee@kaist.ac.kr
http://lense.kaist.ac.kr/
Laboratory for Energy System Engineering (LENSE)
Department of Chemical and Biomolecular Engineering
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.22 View 11584 -
 A New Strategy for the Optimal Electroreduction of CO2 to High-Value Products
-Researchers suggest that modulation of local CO2 concentration improves the selectivity, conversion rate, and electrode stability, and shed a new light on the electrochemical CO2 reduction technology for controlling emissions at a low cost.-
A KAIST research team presented three novel approaches for modulating local carbon dioxide (CO2) concentration in gas-diffusion electrode (GDE)-based flow electrolyzers. Their study also empirically demonstrated that providing a moderate local CO2 concentration is effective in promoting Carbon–Carbon (C–C) coupling reactions toward the production of multi-carbon molecules. This work, featured in the May 20th issue of Joule, serves as a rational guide to tune CO2 mass transport for the optimal production of valuable multi-carbon products.
Amid global efforts to reduce and recycle anthropogenic CO2 emissions, CO2 electrolysis holds great promise for converting CO2 into useful chemicals that were traditionally derived from fossil fuels. Many researches have been attempting to improve the selectivity of CO2 for commercially and industrially high-value multi-carbon products such as ethylene, ethanol, and 1-propanol, due to their high energy density and large market size.
In order to achieve the highly-selective conversion of CO2 into valuable multi-carbon products, past studies have focused on the design of catalysts and the tuning of local environment related to pH, cations, and molecular additives.
Conventional CO2 electrolytic systems relied heavily on an alkaline electrolyte that is often consumed in large quantities when reacting with CO2, and thus led to an increase in the operational costs. Moreover, the life span of a catalyst electrode was short, due to its inherent chemical reactivity.
In their recent study, a group of KAIST researchers led by Professor Jihun Oh from the Department of Materials Science and Engineering reported that the local CO2 concentration has been an overlooked factor that largely affects the selectivity toward multi-carbon products.
Professor Oh and his researchers Dr. Ying Chuan Tan, Hakhyeon Song, and Kelvin Berm Lee proposed that there is an intimate relation between local CO2 and multi-carbon product selectivity during electrochemical CO2 reduction reactions. The team employed the mass-transport modeling of a GDE-based flow electrolyzer that utilizes copper oxide (Cu2O) nanoparticles as model catalysts. They then identified and applied three approaches to modulate the local CO2 concentration within a GDE-based electrolytic system, including 1) controlling the catalyst layer structure, 2) CO2 feed concentration, and 3) feed flow rate.
Contrary to common intuition, the study showed that providing a maximum CO2 transport leads to suboptimal multi-carbon product faradaic efficiency. Instead, by restricting and providing a moderate local CO2 concentration, C–C coupling can be significantly enhanced.
The researchers demonstrated experimentally that the selectivity rate increased from 25.4% to 61.9%, and from 5.9% to 22.6% for the CO2 conversion rate. When a cheap milder near-neutral electrolyte was used, the stability of the CO2 electrolytic system improved to a great extent, allowing over 10 hours of steady selective production of multi-carbon products.
Dr. Tan, the lead author of the paper, said, “Our research clearly revealed that the optimization of the local CO2 concentration is the key to maximizing the efficiency of converting CO2 into high-value multi-carbon products.”
Professor Oh added, “This finding is expected to deliver new insights to the research community that variables affecting local CO2 concentration are also influential factors in the electrochemical CO2 reduction reaction performance. My colleagues and I hope that our study becomes a cornerstone for related technologies and their industrial applications.”
This work was supported by the Korean Ministry of Science and ICT (MSIT) Creative Materials Discovery Program.
Publication:
Tan, Y. C et al. (2020) ‘Modulating Local CO2 Concentration as a General Strategy for Enhancing C−C Coupling in CO2 Electroreduction’, Joule, Vol. 4, Issue 5, pp. 1104-1120. Available online at https://doi.org/10.1016/j.joule.2020.03.013
Profile: Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr/
Laboratory for Energy and Sustainability (LE&S)
Department of Materials Science and Engineering (MSE)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Republic of Korea
Profile: Ying Chuan Tan, PhD
tanyc@kaist.ac.kr
LE&S, MSE, KAIST
Profile: Hakhyeon Song, PhD Candidate
hyeon0401@kaist.ac.kr
LE&S, MSE, KAIST
Profile: Kelvin Berm Lee, M.S. Candidate
kbl9105@kaist.ac.kr
LE&S, MSE, KAIST
(END)
2020.06.03 View 13092
A New Strategy for the Optimal Electroreduction of CO2 to High-Value Products
-Researchers suggest that modulation of local CO2 concentration improves the selectivity, conversion rate, and electrode stability, and shed a new light on the electrochemical CO2 reduction technology for controlling emissions at a low cost.-
A KAIST research team presented three novel approaches for modulating local carbon dioxide (CO2) concentration in gas-diffusion electrode (GDE)-based flow electrolyzers. Their study also empirically demonstrated that providing a moderate local CO2 concentration is effective in promoting Carbon–Carbon (C–C) coupling reactions toward the production of multi-carbon molecules. This work, featured in the May 20th issue of Joule, serves as a rational guide to tune CO2 mass transport for the optimal production of valuable multi-carbon products.
Amid global efforts to reduce and recycle anthropogenic CO2 emissions, CO2 electrolysis holds great promise for converting CO2 into useful chemicals that were traditionally derived from fossil fuels. Many researches have been attempting to improve the selectivity of CO2 for commercially and industrially high-value multi-carbon products such as ethylene, ethanol, and 1-propanol, due to their high energy density and large market size.
In order to achieve the highly-selective conversion of CO2 into valuable multi-carbon products, past studies have focused on the design of catalysts and the tuning of local environment related to pH, cations, and molecular additives.
Conventional CO2 electrolytic systems relied heavily on an alkaline electrolyte that is often consumed in large quantities when reacting with CO2, and thus led to an increase in the operational costs. Moreover, the life span of a catalyst electrode was short, due to its inherent chemical reactivity.
In their recent study, a group of KAIST researchers led by Professor Jihun Oh from the Department of Materials Science and Engineering reported that the local CO2 concentration has been an overlooked factor that largely affects the selectivity toward multi-carbon products.
Professor Oh and his researchers Dr. Ying Chuan Tan, Hakhyeon Song, and Kelvin Berm Lee proposed that there is an intimate relation between local CO2 and multi-carbon product selectivity during electrochemical CO2 reduction reactions. The team employed the mass-transport modeling of a GDE-based flow electrolyzer that utilizes copper oxide (Cu2O) nanoparticles as model catalysts. They then identified and applied three approaches to modulate the local CO2 concentration within a GDE-based electrolytic system, including 1) controlling the catalyst layer structure, 2) CO2 feed concentration, and 3) feed flow rate.
Contrary to common intuition, the study showed that providing a maximum CO2 transport leads to suboptimal multi-carbon product faradaic efficiency. Instead, by restricting and providing a moderate local CO2 concentration, C–C coupling can be significantly enhanced.
The researchers demonstrated experimentally that the selectivity rate increased from 25.4% to 61.9%, and from 5.9% to 22.6% for the CO2 conversion rate. When a cheap milder near-neutral electrolyte was used, the stability of the CO2 electrolytic system improved to a great extent, allowing over 10 hours of steady selective production of multi-carbon products.
Dr. Tan, the lead author of the paper, said, “Our research clearly revealed that the optimization of the local CO2 concentration is the key to maximizing the efficiency of converting CO2 into high-value multi-carbon products.”
Professor Oh added, “This finding is expected to deliver new insights to the research community that variables affecting local CO2 concentration are also influential factors in the electrochemical CO2 reduction reaction performance. My colleagues and I hope that our study becomes a cornerstone for related technologies and their industrial applications.”
This work was supported by the Korean Ministry of Science and ICT (MSIT) Creative Materials Discovery Program.
Publication:
Tan, Y. C et al. (2020) ‘Modulating Local CO2 Concentration as a General Strategy for Enhancing C−C Coupling in CO2 Electroreduction’, Joule, Vol. 4, Issue 5, pp. 1104-1120. Available online at https://doi.org/10.1016/j.joule.2020.03.013
Profile: Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr/
Laboratory for Energy and Sustainability (LE&S)
Department of Materials Science and Engineering (MSE)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Republic of Korea
Profile: Ying Chuan Tan, PhD
tanyc@kaist.ac.kr
LE&S, MSE, KAIST
Profile: Hakhyeon Song, PhD Candidate
hyeon0401@kaist.ac.kr
LE&S, MSE, KAIST
Profile: Kelvin Berm Lee, M.S. Candidate
kbl9105@kaist.ac.kr
LE&S, MSE, KAIST
(END)
2020.06.03 View 13092 -
 3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 19423
3D Hierarchically Porous Nanostructured Catalyst Helps Efficiently Reduce CO2
- This new catalyst will bring CO2 one step closer to serving as a sustainable energy source. -
KAIST researchers developed a three-dimensional (3D) hierarchically porous nanostructured catalyst with carbon dioxide (CO2) to carbon monoxide (CO) conversion rate up to 3.96 times higher than that of conventional nanoporous gold catalysts. This new catalyst helps overcome the existing limitations of the mass transport that has been a major cause of decreases in the CO2 conversion rate, holding a strong promise for the large-scale and cost-effective electrochemical conversion of CO2 into useful chemicals.
As CO2 emissions increase and fossil fuels deplete globally, reducing and converting CO2 to clean energy electrochemically has attracted a great deal of attention as a promising technology. Especially due to the fact that the CO2 reduction reaction occurs competitively with hydrogen evolution reactions (HER) at similar redox potentials, the development of an efficient electrocatalyst for selective and robust CO2 reduction reactions has remained a key technological issue.
Gold (Au) is one of the most commonly used catalysts in CO2 reduction reactions, but the high cost and scarcity of Au pose obstacles for mass commercial applications. The development of nanostructures has been extensively studied as a potential approach to improving the selectivity for target products and maximizing the number of active stable sites, thus enhancing the energy efficiency.
However, the nanopores of the previously reported complex nanostructures were easily blocked by gaseous CO bubbles during aqueous reactions. The CO bubbles hindered mass transport of the reactants through the electrolyte, resulting in low CO2 conversion rates.
In the study published in the Proceedings of the National Academy of Sciences of the USA (PNAS) on March 4, a research group at KAIST led by Professor Seokwoo Jeon and Professor Jihun Oh from the Department of Materials Science and Engineering designed a 3D hierarchically porous Au nanostructure with two different sizes of macropores and nanopores. The team used proximity-field nanopatterning (PnP) and electroplating techniques that are effective for fabricating the 3D well-ordered nanostructures.
The proposed nanostructure, comprised of interconnected macroporous channels 200 to 300 nanometers (nm) wide and 10 nm nanopores, induces efficient mass transport through the interconnected macroporous channels as well as high selectivity by producing highly active stable sites from numerous nanopores.
As a result, its electrodes show a high CO selectivity of 85.8% at a low overpotential of 0.264 V and efficient mass activity that is up to 3.96 times higher than that of de-alloyed nanoporous Au electrodes.
“These results are expected to solve the problem of mass transfer in the field of similar electrochemical reactions and can be applied to a wide range of green energy applications for the efficient utilization of electrocatalysts,” said the researchers.
This work was supported by the National Research Foundation (NRF) of Korea.
Image credit: Professor Seokwoo Jeon and Professor Jihun Oh, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Hyun et al. (2020) Hierarchically porous Au nanostructures with interconnected channels for efficient mass transport in electrocatalytic CO2 reduction. Proceedings of the National Academy of Sciences of the USA (PNAS). Available online at https://doi.org/10.1073/pnas.1918837117
Profile:
Seokwoo Jeon, PhD
Professor
jeon39@kaist.ac.kr
http://fdml.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
https://www.kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)Daejeon, Republic of Korea
Profile:
Jihun Oh, PhD
Associate Professor
jihun.oh@kaist.ac.kr
http://les.kaist.ac.kr
Department of Materials Science and Engineering (MSE)
Department of Energy, Environment, Water and Sustainability (EEWS)
KAIST
Profile:
Gayea Hyun
PhD Candidate
cldywkd93@kaist.ac.kr
http://fdml.kaist.ac.kr
Flexible Devices and Metamaterials Laboratory (FDML)
Department of Materials Science and Engineering (MSE)
KAIST
Profile:
Jun Tae Song, PhD
Assistant Professor
song.juntae@cstf.kyushu-u.ac.jp
http://www.cstf.kyushu-u.ac.jp/~ishihara-lab/
Department of Applied Chemistry
https://www.kyushu-u.ac.jp
Kyushu UniversityFukuoka, Japan
(END)
2020.03.13 View 19423 -
 New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19065
New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) >
Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 14 in Science.
“We set out to develop an effective catalyst that can convert large amounts of the greenhouse gases carbon dioxide and methane without failure,” said Cafer T. Yavuz, paper author and associate professor of chemical and biomolecular engineering and of chemistry at KAIST.
The catalyst, made from inexpensive and abundant nickel, magnesium, and molybdenum, initiates and speeds up the rate of reaction that converts carbon dioxide and methane into hydrogen gas. It can work efficiently for more than a month.
This conversion is called ‘dry reforming’, where harmful gases, such as carbon dioxide, are processed to produce more useful chemicals that could be refined for use in fuel, plastics, or even pharmaceuticals. It is an effective process, but it previously required rare and expensive metals such as platinum and rhodium to induce a brief and inefficient chemical reaction.
Other researchers had previously proposed nickel as a more economical solution, but carbon byproducts would build up and the surface nanoparticles would bind together on the cheaper metal, fundamentally changing the composition and geometry of the catalyst and rendering it useless.
“The difficulty arises from the lack of control on scores of active sites over the bulky catalysts surfaces because any refinement procedures attempted also change the nature of the catalyst itself,” Yavuz said.
The researchers produced nickel-molybdenum nanoparticles under a reductive environment in the presence of a single crystalline magnesium oxide. As the ingredients were heated under reactive gas, the nanoparticles moved on the pristine crystal surface seeking anchoring points. The resulting activated catalyst sealed its own high-energy active sites and permanently fixed the location of the nanoparticles — meaning that the nickel-based catalyst will not have a carbon build up, nor will the surface particles bind to one another.
“It took us almost a year to understand the underlying mechanism,” said first author Youngdong Song, a graduate student in the Department of Chemical and Biomolecular Engineering at KAIST. “Once we studied all the chemical events in detail, we were shocked.”
The researchers dubbed the catalyst Nanocatalysts on Single Crystal Edges (NOSCE). The magnesium-oxide nanopowder comes from a finely structured form of magnesium oxide, where the molecules bind continuously to the edge. There are no breaks or defects in the surface, allowing for uniform and predictable reactions.
“Our study solves a number of challenges the catalyst community faces,” Yavuz said. “We believe the NOSCE mechanism will improve other inefficient catalytic reactions and provide even further savings of greenhouse gas emissions.”
This work was supported, in part, by the Saudi-Aramco-KAIST CO2 Management Center and the National Research Foundation of Korea.
Other contributors include Ercan Ozdemir, Sreerangappa Ramesh, Aldiar Adishev, and Saravanan Subramanian, all of whom are affiliated with the Graduate School of Energy, Environment, Water and Sustainability at KAIST; Aadesh Harale, Mohammed Albuali, Bandar Abdullah Fadhel, and Aqil Jamal, all of whom are with the Research and Development Center in Saudi Arabia; and Dohyun Moon and Sun Hee Choi, both of whom are with the Pohang Accelerator Laboratory in Korea. Ozdemir is also affiliated with the Institute of Nanotechnology at the Gebze Technical University in Turkey; Fadhel and Jamal are also affiliated with the Saudi-Armco-KAIST CO2 Management Center in Korea.
<Newly developed catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas and other chemicals.>
Publication:
Song et al. (2020) Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science, Vol. 367, Issue 6479, pp. 777-781. Available online at http://dx.doi.org/10.1126/science.aav2412
Profile: Prof. Cafer T. Yavuz, MA, PhD
yavuz@kaist.ac.kr
http://yavuz.kaist.ac.kr/
Associate Professor
Oxide and Organic Nanomaterials for the Environment (ONE) Laboratory
Graduate School of Energy, Environment, Water and Sustainability (EEWS)
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Youngdong Song ydsong88@kaist.ac.kr
Ph.D. Candidate
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.17 View 19065 -
 KAIST and Saudi Aramco agreed to establish a joint CO2 research center in Korea
The Korea Advanced Institute of Science and Technology (KAIST) and Saudi Aramco, a global energy and petrochemicals enterprise, signed a memorandum of understanding (MOU) on January 6, 2013 in Dhahran, Saudi Arabia and pledged to jointly collaborate in research and development of innovative technologies and solutions to address the world"s energy challenges.
Under the MOU, the two entities agreed to establish a research center, Saudi Aramco-KAIST CO2 Research Center, near KAIST"s main campus in Daejeon, Korea. The research center, to be jointly managed by KAIST and Saudi Aramco, will foster and facilitate research collaborations in areas such as tackling carbon dioxide (CO2) emissions by removal or capture of CO2, conversing CO2 into useful products, developing efficiency improvements in energy production, sharing carbon management technologies, establishing exchange programs, and conducting joint projects.
According to Saudi Aramco, the company"s collaboration with KAIST is the first partnership established in Asia. Khalid A. Al-Falih, President and CEO of Saudi Aramco, said,
"The CO2 Research Center represents a major step in Saudi Aramco"s research and technology strategy to partner with top global institutions to help address and find sustainable solutions to the world’s energy challenge both domestically and internationally."
2013.03.19 View 11462
KAIST and Saudi Aramco agreed to establish a joint CO2 research center in Korea
The Korea Advanced Institute of Science and Technology (KAIST) and Saudi Aramco, a global energy and petrochemicals enterprise, signed a memorandum of understanding (MOU) on January 6, 2013 in Dhahran, Saudi Arabia and pledged to jointly collaborate in research and development of innovative technologies and solutions to address the world"s energy challenges.
Under the MOU, the two entities agreed to establish a research center, Saudi Aramco-KAIST CO2 Research Center, near KAIST"s main campus in Daejeon, Korea. The research center, to be jointly managed by KAIST and Saudi Aramco, will foster and facilitate research collaborations in areas such as tackling carbon dioxide (CO2) emissions by removal or capture of CO2, conversing CO2 into useful products, developing efficiency improvements in energy production, sharing carbon management technologies, establishing exchange programs, and conducting joint projects.
According to Saudi Aramco, the company"s collaboration with KAIST is the first partnership established in Asia. Khalid A. Al-Falih, President and CEO of Saudi Aramco, said,
"The CO2 Research Center represents a major step in Saudi Aramco"s research and technology strategy to partner with top global institutions to help address and find sustainable solutions to the world’s energy challenge both domestically and internationally."
2013.03.19 View 11462 -
 Launched the Saudi Aramco-KAIST CO2 Management Center in Korea
KAIST and Saudi Aramco, a global energy and petrochemicals enterprise, signed on February 20, 2013 the Master Research and Collaboration Agreement (the Agreement) on joint collaborations in research and development of carbon management between the two entities.
The Agreement was subsequently concluded upon the signing of the Memorandum of Understanding (MOU) between KAIST and Saudi Aramco, dated January 7th, 2013. In the Agreement, the two organizations specified terms and conditions necessary to conduct joint research projects and stipulated governing body for the operation of the Saudi Aramco-KAIST CO2 Management Center.
KAIST and Saudi Aramco, a national oil company for Saudi Arabia, entered into the MOU, in which the two parties shared a common interest in addressing the issue of CO2 capture, CO2storage, CO2 avoidance using efficiency improvements, and converting CO2 into useful chemicals and other materials, and agreed to “create a major research center for CO2” in Korea.
As envisioned by the MOU and its subsequent agreement, KAIST and Saudi Aramco decided to operate an interim office of the Saudi Aramco-KAIST CO2 Management Center at KAIST campus in Daejeon, Korea, pending the establishment of the research center. The full-fledged, independent research facility will be built at a location and during a period to be agreed between the two parties.
Following the signing of the Agreement, there was a celebration event taken place, including a signboard hanging ceremony for the interim research office. A 10-member delegation from Saudi Aramco, which was headed by Vice President of Engineering Services Samir Al-Tubayyeb, Dr. Nam-Pyo Suh, former president of KAIST, Vice President of Research at KAIST Kyung-Wook Paik, and senior representatives from Korean oil and petrochemical companies such as S-Oil, Lotte Chemicals, SK Innovation, and STX attended the event.
Kyung-Wook Paik, Vice President of Research at KAIST, said,
“In order to help find solutions to carbon management, KAIST and Saudi Aramco will facilitate to exchange each party’s complementary technical expertise, gain insight into new research fields, and have access to key sources of talent, while promoting innovation for technology solutions and contributing to the lifelong learning agenda of both organizations.”
Samir Al-Tubayyeb, Vice President of Engineering Services at Saudi Aramco, added that “As a world-leading oil and gas company, Saudi Aramco’s mission is to promote the continued use of safe, environmentally-friendly petroleum products with a vision to becoming a global leader in research and technology. Building a strong and cooperative relationship with KAIST in our endeavor to search for alternative ways to better utilization of fossil fuels will expedite the creation of opportunities to make the world environmentally safer and sustainable.”
KAIST and Saudi Aramco will each chip in a maximum of USD 5 million annually for the establishment and operation of the Saudi Aramco-KAIST CO2 Management Center during the initial term of the Master Research and Collaboration Agreement, which starts in 2013 and continues through 2018.
2013.03.19 View 15408
Launched the Saudi Aramco-KAIST CO2 Management Center in Korea
KAIST and Saudi Aramco, a global energy and petrochemicals enterprise, signed on February 20, 2013 the Master Research and Collaboration Agreement (the Agreement) on joint collaborations in research and development of carbon management between the two entities.
The Agreement was subsequently concluded upon the signing of the Memorandum of Understanding (MOU) between KAIST and Saudi Aramco, dated January 7th, 2013. In the Agreement, the two organizations specified terms and conditions necessary to conduct joint research projects and stipulated governing body for the operation of the Saudi Aramco-KAIST CO2 Management Center.
KAIST and Saudi Aramco, a national oil company for Saudi Arabia, entered into the MOU, in which the two parties shared a common interest in addressing the issue of CO2 capture, CO2storage, CO2 avoidance using efficiency improvements, and converting CO2 into useful chemicals and other materials, and agreed to “create a major research center for CO2” in Korea.
As envisioned by the MOU and its subsequent agreement, KAIST and Saudi Aramco decided to operate an interim office of the Saudi Aramco-KAIST CO2 Management Center at KAIST campus in Daejeon, Korea, pending the establishment of the research center. The full-fledged, independent research facility will be built at a location and during a period to be agreed between the two parties.
Following the signing of the Agreement, there was a celebration event taken place, including a signboard hanging ceremony for the interim research office. A 10-member delegation from Saudi Aramco, which was headed by Vice President of Engineering Services Samir Al-Tubayyeb, Dr. Nam-Pyo Suh, former president of KAIST, Vice President of Research at KAIST Kyung-Wook Paik, and senior representatives from Korean oil and petrochemical companies such as S-Oil, Lotte Chemicals, SK Innovation, and STX attended the event.
Kyung-Wook Paik, Vice President of Research at KAIST, said,
“In order to help find solutions to carbon management, KAIST and Saudi Aramco will facilitate to exchange each party’s complementary technical expertise, gain insight into new research fields, and have access to key sources of talent, while promoting innovation for technology solutions and contributing to the lifelong learning agenda of both organizations.”
Samir Al-Tubayyeb, Vice President of Engineering Services at Saudi Aramco, added that “As a world-leading oil and gas company, Saudi Aramco’s mission is to promote the continued use of safe, environmentally-friendly petroleum products with a vision to becoming a global leader in research and technology. Building a strong and cooperative relationship with KAIST in our endeavor to search for alternative ways to better utilization of fossil fuels will expedite the creation of opportunities to make the world environmentally safer and sustainable.”
KAIST and Saudi Aramco will each chip in a maximum of USD 5 million annually for the establishment and operation of the Saudi Aramco-KAIST CO2 Management Center during the initial term of the Master Research and Collaboration Agreement, which starts in 2013 and continues through 2018.
2013.03.19 View 15408 -
 A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13628
A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13628