THE
-
 2025 KAIST Global Entrepreneurship Summer School Concludes Successfully in Silicon Valley
< A group photo taken at the 2025 GESS Special Lecture.Vice President So Young Kim from the International Office, VC Jay Eum from GFT Ventures, Professor Byungchae Jin from the Impact MBA Program at the Business School, and Research Assistant Professor Sooa Lee from the Office of Global Initiative>
The “2025 KAIST Global Entrepreneurship Summer School (2025 KAIST GESS),” organized by the Office of Global Initiative of the KAIST International Office (Vice President So Young Kim), successfully concluded. Now in its fourth year, the program was designed to provide KAIST students with firsthand experience of the world’s leading startup ecosystem in Silicon Valley, USA, and to strengthen their practical capabilities to take on challenges on the global stage.
This year’s 2025 KAIST GESS welcomed approximately 40 participants, including 24 undergraduate and graduate students selected through document screening, interviews, team presentations, mentoring, and peer evaluations, as well as 16 Impact MBA students from the College of Business. The selected undergraduate and graduate participants underwent two months of pre-program training and received mentoring from experienced entrepreneurs to refine their business models and elevate their project ideas. Meanwhile, Impact MBA students joined the Silicon Valley program onsite, attending key lectures and networking sessions to broaden their understanding of the global startup ecosystem.
From June 22nd, participants spent seven days in Silicon Valley completing the global entrepreneurship curriculum. The program was operated in cooperation with major organizations including the KOTRA Silicon Valley IT Center, Korea-US AI Semiconductor Innovation Center (K-ASIC), and Plug and Play Tech Center. Local experts delivered lectures on topics such as “Startup Culture,” “Learning from Failures” and “Networks and Capital.”
Participants also had the opportunity to visit startups led by KAIST alumni and local entrepreneurs, gaining valuable insights from firsthand stories about global entrepreneurship. Companies visited included Medic Life Sciences (CEO Kyuho Han) and ImpriMed (CEO Sungwon Lim). Through these visits, participants received practical advice on market entry strategies and overcoming challenges in the global arena.
As part of their first onsite schedule, KAIST students attended an interactive fireside chat titled “Global Entrepreneurship and AI,” where they engaged in in-depth discussions on the future of AI-driven global startups. The session featured three distinguished speakers: Jay Kim, Head of US Business Development at Hyper Accel; Chandra Shekhar Dhir, AI/ML Director at JPMorgan Chase’s Machine Learning Center of Excellence; and Taesu Kim, co-founder of AI voice synthesis startup Neosapience and KAIST alumnus. Taesu Kim shared, “Facing serious health issues made me reflect on my life, and after recovering, I wanted to pursue something that could create a real impact on society, which led me to start my own company.” He also advised students to “take time at important turning points in life to deeply think about what you truly want to do and how you can contribute to society.
In line with the core value of ‘paying it forward’—a fundamental principle of global entrepreneurship learned in Silicon Valley—GESS participants engaged in a community service project titled “Let’s Play with AI+Tech,” organized in collaboration with the Sunnyvale community and Foothill College. Leveraging their strong foundation in AI, KAIST students designed and led a hands-on ‘Doodle AI’ educational program to make foundational AI concepts accessible and engaging for underrepresented local elementary school children and their parents, fostering meaningful community interaction.
On the final day of the 2025 KAIST GESS, a pitch competition was held with participation from Silicon Valley venture capitalists and accelerators. Participants presented their business models, developed over the two-month program, to a panel of judges. The winning team was eaureco, and Si Li Sara Aow (Civil and Environmental Engineering) shared, “GESS was a valuable opportunity to test and hone practical entrepreneurship skills beyond mere networking.” She added, “At first, I lacked confidence, but challenging myself to pitch in the final presentation gave me the courage to take one step closer to global entrepreneurship. Pitching in Silicon Valley, the heart of global startups, was an invaluable experience that will shape my path as a global entrepreneur.”
The program concluded with a special lecture by Jay Eum, a seasoned Silicon Valley venture capitalist and a judging panel member for GESS over the past three years. He shared key insights on startup success from an investor’s perspective, advising, “The journey of entrepreneurship is never easy, but the sooner you start, the better.” He further encouraged participants to “focus on solving problems in local markets, but do not fear challenging global markets,” inspiring them with courage and actionable advice.
So Young Kim, Director of the KAIST Office of Global Initiative, said, “We hope the 2025 KAIST GESS serves as a stepping stone for KAIST students to grow into influential entrepreneurs on the global stage,” adding, “This program is also expected to further enhance KAIST’s international reputation.”
Byungchae Jin, Faculty Chair of the KAIST Impact MBA, College of Business, highlighted the program's educational benefits, stating, “Engaging directly with local entrepreneurs and gaining practical experience in Silicon Valley's startup environment provide students with hands-on learning and significant inspiration.”
The 2025 KAIST GESS was jointly hosted by the KAIST Office of Global Initiative, Impact MBA, and Startup KAIST. Moving forward, KAIST plans to continue expanding its field-based global entrepreneurship education by linking with key global hubs like Silicon Valley, fostering next-generation global leaders who will lead innovation and challenge the status quo.
2025.07.01 View 135
2025 KAIST Global Entrepreneurship Summer School Concludes Successfully in Silicon Valley
< A group photo taken at the 2025 GESS Special Lecture.Vice President So Young Kim from the International Office, VC Jay Eum from GFT Ventures, Professor Byungchae Jin from the Impact MBA Program at the Business School, and Research Assistant Professor Sooa Lee from the Office of Global Initiative>
The “2025 KAIST Global Entrepreneurship Summer School (2025 KAIST GESS),” organized by the Office of Global Initiative of the KAIST International Office (Vice President So Young Kim), successfully concluded. Now in its fourth year, the program was designed to provide KAIST students with firsthand experience of the world’s leading startup ecosystem in Silicon Valley, USA, and to strengthen their practical capabilities to take on challenges on the global stage.
This year’s 2025 KAIST GESS welcomed approximately 40 participants, including 24 undergraduate and graduate students selected through document screening, interviews, team presentations, mentoring, and peer evaluations, as well as 16 Impact MBA students from the College of Business. The selected undergraduate and graduate participants underwent two months of pre-program training and received mentoring from experienced entrepreneurs to refine their business models and elevate their project ideas. Meanwhile, Impact MBA students joined the Silicon Valley program onsite, attending key lectures and networking sessions to broaden their understanding of the global startup ecosystem.
From June 22nd, participants spent seven days in Silicon Valley completing the global entrepreneurship curriculum. The program was operated in cooperation with major organizations including the KOTRA Silicon Valley IT Center, Korea-US AI Semiconductor Innovation Center (K-ASIC), and Plug and Play Tech Center. Local experts delivered lectures on topics such as “Startup Culture,” “Learning from Failures” and “Networks and Capital.”
Participants also had the opportunity to visit startups led by KAIST alumni and local entrepreneurs, gaining valuable insights from firsthand stories about global entrepreneurship. Companies visited included Medic Life Sciences (CEO Kyuho Han) and ImpriMed (CEO Sungwon Lim). Through these visits, participants received practical advice on market entry strategies and overcoming challenges in the global arena.
As part of their first onsite schedule, KAIST students attended an interactive fireside chat titled “Global Entrepreneurship and AI,” where they engaged in in-depth discussions on the future of AI-driven global startups. The session featured three distinguished speakers: Jay Kim, Head of US Business Development at Hyper Accel; Chandra Shekhar Dhir, AI/ML Director at JPMorgan Chase’s Machine Learning Center of Excellence; and Taesu Kim, co-founder of AI voice synthesis startup Neosapience and KAIST alumnus. Taesu Kim shared, “Facing serious health issues made me reflect on my life, and after recovering, I wanted to pursue something that could create a real impact on society, which led me to start my own company.” He also advised students to “take time at important turning points in life to deeply think about what you truly want to do and how you can contribute to society.
In line with the core value of ‘paying it forward’—a fundamental principle of global entrepreneurship learned in Silicon Valley—GESS participants engaged in a community service project titled “Let’s Play with AI+Tech,” organized in collaboration with the Sunnyvale community and Foothill College. Leveraging their strong foundation in AI, KAIST students designed and led a hands-on ‘Doodle AI’ educational program to make foundational AI concepts accessible and engaging for underrepresented local elementary school children and their parents, fostering meaningful community interaction.
On the final day of the 2025 KAIST GESS, a pitch competition was held with participation from Silicon Valley venture capitalists and accelerators. Participants presented their business models, developed over the two-month program, to a panel of judges. The winning team was eaureco, and Si Li Sara Aow (Civil and Environmental Engineering) shared, “GESS was a valuable opportunity to test and hone practical entrepreneurship skills beyond mere networking.” She added, “At first, I lacked confidence, but challenging myself to pitch in the final presentation gave me the courage to take one step closer to global entrepreneurship. Pitching in Silicon Valley, the heart of global startups, was an invaluable experience that will shape my path as a global entrepreneur.”
The program concluded with a special lecture by Jay Eum, a seasoned Silicon Valley venture capitalist and a judging panel member for GESS over the past three years. He shared key insights on startup success from an investor’s perspective, advising, “The journey of entrepreneurship is never easy, but the sooner you start, the better.” He further encouraged participants to “focus on solving problems in local markets, but do not fear challenging global markets,” inspiring them with courage and actionable advice.
So Young Kim, Director of the KAIST Office of Global Initiative, said, “We hope the 2025 KAIST GESS serves as a stepping stone for KAIST students to grow into influential entrepreneurs on the global stage,” adding, “This program is also expected to further enhance KAIST’s international reputation.”
Byungchae Jin, Faculty Chair of the KAIST Impact MBA, College of Business, highlighted the program's educational benefits, stating, “Engaging directly with local entrepreneurs and gaining practical experience in Silicon Valley's startup environment provide students with hands-on learning and significant inspiration.”
The 2025 KAIST GESS was jointly hosted by the KAIST Office of Global Initiative, Impact MBA, and Startup KAIST. Moving forward, KAIST plans to continue expanding its field-based global entrepreneurship education by linking with key global hubs like Silicon Valley, fostering next-generation global leaders who will lead innovation and challenge the status quo.
2025.07.01 View 135 -
 ‘InnoCORE Research Group’ Launched to Lead AI Convergence Innovation
KAIST announced on the 16th of June that it has launched the ‘InnoCORE (Innovation-Core) Research Group,’ which will lead advanced strategic research in AI convergence (AI+S&T), in cooperation with the Ministry of Science and ICT (Minister Yoo Sang-im, hereinafter referred to as MSIT) and DGIST, GIST, and UNIST*. Through this, the group plans to actively recruit up to 200 world-class postdoctoral researchers.
DGIST (Daegu Gyeongbuk Institute of Science & Technology), GIST (Gwangju Institute of Science & Technology), UNIST (Ulsan National Institute of Science and Technology)
The ‘InnoCORE Research Group’ aims to foster core research personnel who will lead innovation in the field of AI convergence, focusing on nurturing and attracting high-level research talent in AI+Science & Technology. This is a strategic response to prevent brain drain of domestic talent and attract excellent overseas talent amidst the accelerating global competition for AI talent.
Through this initiative, our university plans to accelerate AI-based science and technology innovation and disseminate research achievements across industries and the economy by supporting top domestic and international postdoctoral researchers to dedicate themselves to developing AI convergence technologies in an advanced collaborative research environment.
The InnoCORE project for advanced AI+S&T convergence research and global talent attraction is jointly promoted by four science and technology institutes, including KAIST. It is structured around AI core technologies (such as hyper-scale language models, AI semiconductors) and AI convergence technologies (such as bio, manufacturing, energy, and aerospace).
As the leading institution, our university operates the following four research groups:
Hyper-scale Language Model Innovation Research Group: Advancement of LLM technology and research on generative AI, multimodal AI, and ensuring reliability.
AI-based Intelligent Design-Manufacturing Integration Research Group: Establishment of an AI platform for the entire lifecycle of the manufacturing industry and innovation in design and processes.
AI-Innovation Drug Research Group: Securing AI-based drug development technologies across the entire lifecycle and overcoming intractable diseases.
AI-Transformed Aerospace Research Group: AI transformation of aerospace systems throughout their lifecycle and development of new technologies such as autonomous flight and space communication.
< Poster on the InnoCORE Global Jobfair for Recruitment of Postdoctoral Researchers >
In addition, a total of eight research groups are formed to promote global collaborative convergence research, including those led by DGIST, GIST, and UNIST: ▲Bio-Integrated Physical AI, ▲Early Diagnosis of Brain Diseases AI+Nano Convergence, ▲Intelligent Hydrogen Technology Innovation, and ▲AI-Space Solar Power Research Group.
Starting in 2025, the four science and technology institutes, including KAIST, will officially begin recruiting 400 postdoctoral researchers in the AI+S&T fields. Selected postdoctoral researchers will be guaranteed high-level treatment with an annual salary of over 90 million KRW, and additional support through matching with companies and research projects is also planned.
In particular, global recruitment fairs will be held in major US regions to expand the attraction of excellent overseas talent. Local recruitment fairs will be held in Boston (Harvard, MIT), New York (NYU), and Silicon Valley (Stanford) in June, along with promotions through global academic journals such as Nature and Science, and LinkedIn.
KAIST plans to provide multiple mentor programs, global joint research opportunities, and excellent infrastructure (such as supercomputers, semiconductor fabs, and AI research platforms) within the research groups to enable postdoctoral researchers to collaborate with experts from various academic and industrial fields.
President Kwang Hyung Lee emphasized, “Through this InnoCORE project, KAIST will leap forward as a Global Hub for AI+S&T convergence research. Young researchers from around the world will challenge themselves and grow at KAIST, and our country will play a pivotal role in establishing itself as a leading nation in global AI convergence research and industry. To achieve this, we will spare no effort in providing the best research environment and active support.”
KAIST plans to actively pursue the InnoCORE project to secure global competitiveness in AI convergence research and contribute to the development of advanced industries. The eight selected research groups will finalize their detailed research plans by the end of June and commence full-scale research in July.
2025.06.19 View 1061
‘InnoCORE Research Group’ Launched to Lead AI Convergence Innovation
KAIST announced on the 16th of June that it has launched the ‘InnoCORE (Innovation-Core) Research Group,’ which will lead advanced strategic research in AI convergence (AI+S&T), in cooperation with the Ministry of Science and ICT (Minister Yoo Sang-im, hereinafter referred to as MSIT) and DGIST, GIST, and UNIST*. Through this, the group plans to actively recruit up to 200 world-class postdoctoral researchers.
DGIST (Daegu Gyeongbuk Institute of Science & Technology), GIST (Gwangju Institute of Science & Technology), UNIST (Ulsan National Institute of Science and Technology)
The ‘InnoCORE Research Group’ aims to foster core research personnel who will lead innovation in the field of AI convergence, focusing on nurturing and attracting high-level research talent in AI+Science & Technology. This is a strategic response to prevent brain drain of domestic talent and attract excellent overseas talent amidst the accelerating global competition for AI talent.
Through this initiative, our university plans to accelerate AI-based science and technology innovation and disseminate research achievements across industries and the economy by supporting top domestic and international postdoctoral researchers to dedicate themselves to developing AI convergence technologies in an advanced collaborative research environment.
The InnoCORE project for advanced AI+S&T convergence research and global talent attraction is jointly promoted by four science and technology institutes, including KAIST. It is structured around AI core technologies (such as hyper-scale language models, AI semiconductors) and AI convergence technologies (such as bio, manufacturing, energy, and aerospace).
As the leading institution, our university operates the following four research groups:
Hyper-scale Language Model Innovation Research Group: Advancement of LLM technology and research on generative AI, multimodal AI, and ensuring reliability.
AI-based Intelligent Design-Manufacturing Integration Research Group: Establishment of an AI platform for the entire lifecycle of the manufacturing industry and innovation in design and processes.
AI-Innovation Drug Research Group: Securing AI-based drug development technologies across the entire lifecycle and overcoming intractable diseases.
AI-Transformed Aerospace Research Group: AI transformation of aerospace systems throughout their lifecycle and development of new technologies such as autonomous flight and space communication.
< Poster on the InnoCORE Global Jobfair for Recruitment of Postdoctoral Researchers >
In addition, a total of eight research groups are formed to promote global collaborative convergence research, including those led by DGIST, GIST, and UNIST: ▲Bio-Integrated Physical AI, ▲Early Diagnosis of Brain Diseases AI+Nano Convergence, ▲Intelligent Hydrogen Technology Innovation, and ▲AI-Space Solar Power Research Group.
Starting in 2025, the four science and technology institutes, including KAIST, will officially begin recruiting 400 postdoctoral researchers in the AI+S&T fields. Selected postdoctoral researchers will be guaranteed high-level treatment with an annual salary of over 90 million KRW, and additional support through matching with companies and research projects is also planned.
In particular, global recruitment fairs will be held in major US regions to expand the attraction of excellent overseas talent. Local recruitment fairs will be held in Boston (Harvard, MIT), New York (NYU), and Silicon Valley (Stanford) in June, along with promotions through global academic journals such as Nature and Science, and LinkedIn.
KAIST plans to provide multiple mentor programs, global joint research opportunities, and excellent infrastructure (such as supercomputers, semiconductor fabs, and AI research platforms) within the research groups to enable postdoctoral researchers to collaborate with experts from various academic and industrial fields.
President Kwang Hyung Lee emphasized, “Through this InnoCORE project, KAIST will leap forward as a Global Hub for AI+S&T convergence research. Young researchers from around the world will challenge themselves and grow at KAIST, and our country will play a pivotal role in establishing itself as a leading nation in global AI convergence research and industry. To achieve this, we will spare no effort in providing the best research environment and active support.”
KAIST plans to actively pursue the InnoCORE project to secure global competitiveness in AI convergence research and contribute to the development of advanced industries. The eight selected research groups will finalize their detailed research plans by the end of June and commence full-scale research in July.
2025.06.19 View 1061 -
 KAIST Holds a Ceremony to Declare their Renewed Commitment for Ethical Management
KAIST held a ceremony to declare their renewed "Commitment for Ethical Management" to raise awareness and solidify the commitment its members to faithfully fulfill ethical responsibilities and duties.
Last March, the university established the 'Special Committee for Ethical Management,' chaired by the Provost, and under the leadership of this committee, a new 'Code of Ethics' and 'Code of Conduct' were prepared, containing ethical standards that members must adhere to across all areas of education, research, and administration.
< Photo 1. Attendees pledge to practice ethics during the declaration for the ethical management. >
This ceremony was arranged as an occasion for the president, key executives, and representatives from each university constituent to share the purpose and direction of the newly established ethical standards and to pledge their commitment to practicing them.
The Ethical Management Declaration consisted of: ▲ a progress report by the KAIST Special Committee for Ethical Management, ▲ a commemorative address by the president, ▲ an oath of the Code of Ethics and Code of Conduct, and ▲ the presentation of the 'Excellent Ethics Professor Award' organized by the Graduate Student Human Rights Center. Attendees shared the values and meaning of ethical management pursued by KAIST.
Particularly at this ceremony, six representatives – faculty, staff, and students – selected to reflect KAIST's values encompassing diversity in position, role, gender, and future generations, took the oath for the Code of Ethics and Code of Conduct.
< Photo 2. Attendees pledge to practice ethics during the Ethical Management Declaration. >
Also introduced at the ceremony was the "Ethical Excellence Award for Professors". It is an award that was organized by the Graduate Student Human Rights Center under the KAIST Student Council to recognize the faculty members for their outstanding ethical conduct in the laboratory setting. The 2025 recipients of the newly established award were the honored at the declaration ceremony for added significance.
Taking this declaration ceremony as an example, KAIST plans to actively encourage each departments, divisions and offices to also hold ethical management declarations of their own to establish a trustworthy, healthy, and transparent organizational culture through the daily practice of ethical responsibilities, and to continuously spread the practice of ethical management among all members.
President Kwang Hyung Lee emphasized, "Adhering to research and social ethics must be the foundation for KAIST to become a university trusted globally," and expressed, "I hope this ceremony serves as a turning point for all members to more faithfully practice their ethical responsibilities and duties."
2025.06.16 View 694
KAIST Holds a Ceremony to Declare their Renewed Commitment for Ethical Management
KAIST held a ceremony to declare their renewed "Commitment for Ethical Management" to raise awareness and solidify the commitment its members to faithfully fulfill ethical responsibilities and duties.
Last March, the university established the 'Special Committee for Ethical Management,' chaired by the Provost, and under the leadership of this committee, a new 'Code of Ethics' and 'Code of Conduct' were prepared, containing ethical standards that members must adhere to across all areas of education, research, and administration.
< Photo 1. Attendees pledge to practice ethics during the declaration for the ethical management. >
This ceremony was arranged as an occasion for the president, key executives, and representatives from each university constituent to share the purpose and direction of the newly established ethical standards and to pledge their commitment to practicing them.
The Ethical Management Declaration consisted of: ▲ a progress report by the KAIST Special Committee for Ethical Management, ▲ a commemorative address by the president, ▲ an oath of the Code of Ethics and Code of Conduct, and ▲ the presentation of the 'Excellent Ethics Professor Award' organized by the Graduate Student Human Rights Center. Attendees shared the values and meaning of ethical management pursued by KAIST.
Particularly at this ceremony, six representatives – faculty, staff, and students – selected to reflect KAIST's values encompassing diversity in position, role, gender, and future generations, took the oath for the Code of Ethics and Code of Conduct.
< Photo 2. Attendees pledge to practice ethics during the Ethical Management Declaration. >
Also introduced at the ceremony was the "Ethical Excellence Award for Professors". It is an award that was organized by the Graduate Student Human Rights Center under the KAIST Student Council to recognize the faculty members for their outstanding ethical conduct in the laboratory setting. The 2025 recipients of the newly established award were the honored at the declaration ceremony for added significance.
Taking this declaration ceremony as an example, KAIST plans to actively encourage each departments, divisions and offices to also hold ethical management declarations of their own to establish a trustworthy, healthy, and transparent organizational culture through the daily practice of ethical responsibilities, and to continuously spread the practice of ethical management among all members.
President Kwang Hyung Lee emphasized, "Adhering to research and social ethics must be the foundation for KAIST to become a university trusted globally," and expressed, "I hope this ceremony serves as a turning point for all members to more faithfully practice their ethical responsibilities and duties."
2025.06.16 View 694 -
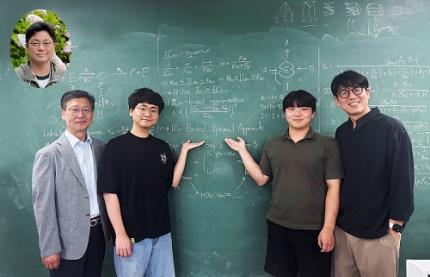 “One Experiment Is All It Takes”: KAIST Team Revolutionizes Drug Interaction Testing, Replacing 60,000 Studies
A groundbreaking new method developed by researchers at KAIST and Chungnam National University could drastically streamline drug interaction testing — replacing dozens of traditional experiments with just one.
The research, led by Professor Jae Kyoung Kim of KAIST Department of Mathematical Sciences & IBS Biomedical Mathematics Group and Professor Sang Kyum Kim of Chungnam National University's College of Pharmacy, introduces a novel analysis technique called 50-BOA, published in Nature Communications on June 5, 2025.
< Photo 1. (From left) Professor Sang Kyum Kim (Chungnam National University College of Pharmacy, co-corresponding author), Dr. Yun Min Song (IBS Biomedical Mathematics Group, formerly KAIST Department of Mathematical Sciences, co-first author), undergraduate student Hyeong Jun Jang (KAIST, co-first author), Professor Jae Kyoung Kim (KAIST and IBS Biomedical Mathematics Group, co-corresponding author) (Top left in the bubble) Professor Hwi-yeol Yun (Chungnam National University College of Pharmacy, co-author) >
For decades, scientists have had to repeat drug inhibition experiments across a wide range of concentrations to estimate inhibition constants — a process seen in over 60,000 scientific publications. But the KAIST-led team discovered that a single, well-chosen inhibitor concentration can yield even more accurate results.
< Figure 1. Graphical summary of 50-BOA. 50-BOA improves the accuracy and efficiency of inhibition constant estimation by using only a single inhibitor concentration instead of the traditionally used method of employing multiple inhibitor concentrations. >
“This approach challenges long-standing assumptions in experimental pharmacology,” says Prof. Kim. “It shows how mathematics can fundamentally redesign life science experiments.”
By mathematically analyzing the sources of error in conventional methods, the team found that over half the data typically collected adds no value or even skews results. Their new method not only cuts experimental effort by over 75%, but also enhances reproducibility and accuracy.
To help researchers adopt the method quickly, the team developed a user-friendly tool that takes simple Excel files as input, now freely available on GitHub:
☞ https://github.com/Mathbiomed/50-BOA
< Figure 2. The MATLAB and R package of 50-BOA at GitHub >
The work holds promise for faster and more reliable drug development, especially in assessing potential interactions in combination therapies. The U.S. FDA already emphasizes the importance of accurate enzyme inhibition assessment during early-stage drug evaluation — and this method could soon become a new gold standard.
2025.06.16 View 1449
“One Experiment Is All It Takes”: KAIST Team Revolutionizes Drug Interaction Testing, Replacing 60,000 Studies
A groundbreaking new method developed by researchers at KAIST and Chungnam National University could drastically streamline drug interaction testing — replacing dozens of traditional experiments with just one.
The research, led by Professor Jae Kyoung Kim of KAIST Department of Mathematical Sciences & IBS Biomedical Mathematics Group and Professor Sang Kyum Kim of Chungnam National University's College of Pharmacy, introduces a novel analysis technique called 50-BOA, published in Nature Communications on June 5, 2025.
< Photo 1. (From left) Professor Sang Kyum Kim (Chungnam National University College of Pharmacy, co-corresponding author), Dr. Yun Min Song (IBS Biomedical Mathematics Group, formerly KAIST Department of Mathematical Sciences, co-first author), undergraduate student Hyeong Jun Jang (KAIST, co-first author), Professor Jae Kyoung Kim (KAIST and IBS Biomedical Mathematics Group, co-corresponding author) (Top left in the bubble) Professor Hwi-yeol Yun (Chungnam National University College of Pharmacy, co-author) >
For decades, scientists have had to repeat drug inhibition experiments across a wide range of concentrations to estimate inhibition constants — a process seen in over 60,000 scientific publications. But the KAIST-led team discovered that a single, well-chosen inhibitor concentration can yield even more accurate results.
< Figure 1. Graphical summary of 50-BOA. 50-BOA improves the accuracy and efficiency of inhibition constant estimation by using only a single inhibitor concentration instead of the traditionally used method of employing multiple inhibitor concentrations. >
“This approach challenges long-standing assumptions in experimental pharmacology,” says Prof. Kim. “It shows how mathematics can fundamentally redesign life science experiments.”
By mathematically analyzing the sources of error in conventional methods, the team found that over half the data typically collected adds no value or even skews results. Their new method not only cuts experimental effort by over 75%, but also enhances reproducibility and accuracy.
To help researchers adopt the method quickly, the team developed a user-friendly tool that takes simple Excel files as input, now freely available on GitHub:
☞ https://github.com/Mathbiomed/50-BOA
< Figure 2. The MATLAB and R package of 50-BOA at GitHub >
The work holds promise for faster and more reliable drug development, especially in assessing potential interactions in combination therapies. The U.S. FDA already emphasizes the importance of accurate enzyme inhibition assessment during early-stage drug evaluation — and this method could soon become a new gold standard.
2025.06.16 View 1449 -
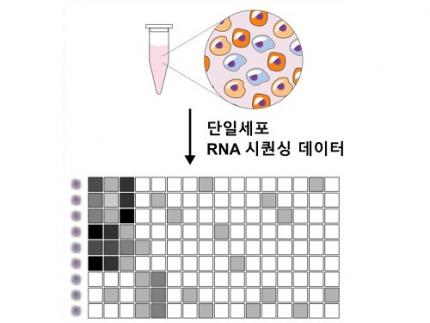 Revolutionary 'scLENS' Unveiled to Decode Complex Single-Cell Genomic Data
Unlocking biological information from complex single-cell genomic data has just become easier and more precise, thanks to the innovative 'scLENS' tool developed by the Biomedical Mathematics Group within the IBS Center for Mathematical and Computational Sciences led by Chief Investigator Jae Kyoung Kim, who is also a professor at KAIST. This new finding represents a significant leap forward in the field of single-cell transcriptomics.
Single-cell genomic analysis is an advanced technique that measures gene expression at the individual cell level, revealing cellular changes and interactions that are not observable with traditional genomic analysis methods. When applied to cancer tissues, this analysis can delineate the composition of diverse cell types within a tumor, providing insights into how cancer progresses and identifying key genes involved during each stage of progression.
Despite the immense potential of single-cell genomic analysis, handling the vast amount of data that it generates has always been challenging. The amount of data covers the expression of tens of thousands of genes across hundreds to thousands of individual cells. This not only results in large datasets but also introduces noise-related distortions, which arise in part due to current measurement limitations.
< Figure 1. Overview of scLENS (single-cell Low-dimensional embedding using the effective Noise Subtract) >
(Left) Current dimensionality reduction methods for scRNA-seq data involve conventional data preprocessing steps, such as log normalization, followed by manual selection of signals from the scaled data. However, this study reveals that the high levels of sparsity and variability in scRNA-seq data can lead to signal distortion during the data preprocessing, compromising the accuracy of downstream analyses.
(Right) To address this issue, the researchers integrated L2 normalization into the conventional preprocessing pipeline, effectively mitigating signal distortion. Moreover, they developed a novel signal detection algorithm that eliminates the need for user intervention by leveraging random matrix theory-based noise filtering and signal robustness testing. By incorporating these techniques, scLENS enables accurate and automated analysis of scRNA-seq data, overcoming the limitations of existing dimensionality reduction methods.
Corresponding author Jae Kyoung Kim highlighted, “There has been a remarkable advancement in experimental technologies for analyzing single-cell transcriptomes over the past decade. However, due to limitations in data analysis methods, there has been a struggle to fully utilize valuable data obtained through extensive cost and time."
Researchers have developed numerous analysis methods over the years to discern biological signals from this noise. However, the accuracy of these methods has been less than satisfactory. A critical issue is that determining signal and noise thresholds often depends on subjective decisions from the users.
The newly developed scLENS tool harnesses Random Matrix Theory and Signal robustness test to automatically differentiate signals from noise without relying on subjective user input.
First author Hyun Kim stated, "Previously, users had to arbitrarily decide the threshold for signal and noise, which compromised the reproducibility of analysis results and introduced subjectivity. scLENS eliminates this problem by automatically detecting signals using only the inherent structure of the data."
During the development of scLENS, researchers identified the fundamental reasons for inaccuracies in existing analysis methods. They found that commonly used data preprocessing methods distort both biological signals and noise. The new preprocessing approach that scLENS offers is free from such distortions.
By resolving issues related to noise threshold determined by subjective user choice and signal distortion in conventional data preprocessing, scLENS significantly outperforms existing methods in accuracy. Additionally, scLENS automates the laborious process of signal dimension selection, allowing researchers to extract biological signals conveniently and automatically.
CI Kim added, "scLENS solves major issues in single-cell transcriptome data analysis, substantially improving the accuracy and efficiency throughout the analysis process. This is a prime example of how fundamental mathematical theories can drive innovation in life sciences research, allowing researchers to more quickly and accurately answer biological questions and uncover secrets of life that were previously hidden."
This research was published in the international journal 'Nature Communications' on April 27.
Terminology
* Single-cell RNA sequencing (scRNA-seq): A technique used to measure gene expression levels in individual cells, providing insights into cell heterogeneity and rare cell types.
* Dimensionality reduction: A method to reduce the number of features or variables in a dataset while preserving the most important information, making data analysis more manageable and interpretable.
* Random matrix theory: A mathematical framework used to model and analyze the properties of large, random matrices, which can be applied to filter out noise in high-dimensional data.
* Signal robustness test: Among the signals, this test selects signals that are robust to the slight perturbation in data because real biological signals should be invariant for such slight modification in the data.
2024.05.09 View 6178
Revolutionary 'scLENS' Unveiled to Decode Complex Single-Cell Genomic Data
Unlocking biological information from complex single-cell genomic data has just become easier and more precise, thanks to the innovative 'scLENS' tool developed by the Biomedical Mathematics Group within the IBS Center for Mathematical and Computational Sciences led by Chief Investigator Jae Kyoung Kim, who is also a professor at KAIST. This new finding represents a significant leap forward in the field of single-cell transcriptomics.
Single-cell genomic analysis is an advanced technique that measures gene expression at the individual cell level, revealing cellular changes and interactions that are not observable with traditional genomic analysis methods. When applied to cancer tissues, this analysis can delineate the composition of diverse cell types within a tumor, providing insights into how cancer progresses and identifying key genes involved during each stage of progression.
Despite the immense potential of single-cell genomic analysis, handling the vast amount of data that it generates has always been challenging. The amount of data covers the expression of tens of thousands of genes across hundreds to thousands of individual cells. This not only results in large datasets but also introduces noise-related distortions, which arise in part due to current measurement limitations.
< Figure 1. Overview of scLENS (single-cell Low-dimensional embedding using the effective Noise Subtract) >
(Left) Current dimensionality reduction methods for scRNA-seq data involve conventional data preprocessing steps, such as log normalization, followed by manual selection of signals from the scaled data. However, this study reveals that the high levels of sparsity and variability in scRNA-seq data can lead to signal distortion during the data preprocessing, compromising the accuracy of downstream analyses.
(Right) To address this issue, the researchers integrated L2 normalization into the conventional preprocessing pipeline, effectively mitigating signal distortion. Moreover, they developed a novel signal detection algorithm that eliminates the need for user intervention by leveraging random matrix theory-based noise filtering and signal robustness testing. By incorporating these techniques, scLENS enables accurate and automated analysis of scRNA-seq data, overcoming the limitations of existing dimensionality reduction methods.
Corresponding author Jae Kyoung Kim highlighted, “There has been a remarkable advancement in experimental technologies for analyzing single-cell transcriptomes over the past decade. However, due to limitations in data analysis methods, there has been a struggle to fully utilize valuable data obtained through extensive cost and time."
Researchers have developed numerous analysis methods over the years to discern biological signals from this noise. However, the accuracy of these methods has been less than satisfactory. A critical issue is that determining signal and noise thresholds often depends on subjective decisions from the users.
The newly developed scLENS tool harnesses Random Matrix Theory and Signal robustness test to automatically differentiate signals from noise without relying on subjective user input.
First author Hyun Kim stated, "Previously, users had to arbitrarily decide the threshold for signal and noise, which compromised the reproducibility of analysis results and introduced subjectivity. scLENS eliminates this problem by automatically detecting signals using only the inherent structure of the data."
During the development of scLENS, researchers identified the fundamental reasons for inaccuracies in existing analysis methods. They found that commonly used data preprocessing methods distort both biological signals and noise. The new preprocessing approach that scLENS offers is free from such distortions.
By resolving issues related to noise threshold determined by subjective user choice and signal distortion in conventional data preprocessing, scLENS significantly outperforms existing methods in accuracy. Additionally, scLENS automates the laborious process of signal dimension selection, allowing researchers to extract biological signals conveniently and automatically.
CI Kim added, "scLENS solves major issues in single-cell transcriptome data analysis, substantially improving the accuracy and efficiency throughout the analysis process. This is a prime example of how fundamental mathematical theories can drive innovation in life sciences research, allowing researchers to more quickly and accurately answer biological questions and uncover secrets of life that were previously hidden."
This research was published in the international journal 'Nature Communications' on April 27.
Terminology
* Single-cell RNA sequencing (scRNA-seq): A technique used to measure gene expression levels in individual cells, providing insights into cell heterogeneity and rare cell types.
* Dimensionality reduction: A method to reduce the number of features or variables in a dataset while preserving the most important information, making data analysis more manageable and interpretable.
* Random matrix theory: A mathematical framework used to model and analyze the properties of large, random matrices, which can be applied to filter out noise in high-dimensional data.
* Signal robustness test: Among the signals, this test selects signals that are robust to the slight perturbation in data because real biological signals should be invariant for such slight modification in the data.
2024.05.09 View 6178 -
 Afternoon chemotherapy proved to deliver more desirable results for female lymphoma patients
Chemotherapy is a commonly used regimen for cancer treatment, but it is also a double-edged sword. While the drugs are highly effective at killing cancer cells, they are also notorious for killing healthy cells in the body. As such, minimizing the drug’s damage to the patient’s body is necessary for improving the prognosis of chemotherapy.
Recently, “chrono-chemotherapy” have been gaining interest in the research community. As the name suggests, the aim is timing the delivery of the drugs when the body is least vulnerable to their harmful effects and while the cancer cells are at their most vulnerable.
< Figure 1. Chrono-chemotherapy considering circadian rhythm >
Chrono-chemotherapy exploits the fact that human physiological processes, including cell proliferation and differentiation, are regulated by an endogenous timer called the circadian clock. However, this has not been widely exploited in real-world clinical settings because, as of now, there is no systematic method for finding the optimal chemotherapy delivery time.
This problem was tackled by an interdisciplinary team of researchers from South Korea. They were led by principal investigators Jae Kyoung Kim (a mathematician from the Biomedical Mathematics Group, Institute for Basic Science) and Youngil Koh (an oncologist at Seoul National University Hospital). The researchers studied a group of patients suffering from diffuse large B-cell lymphoma (DLBCL).
Terminology
* Diffuse large B-cell lymphoma (DLBCL): Lymphoma is a type of blood cancer caused by the malignant transformation of lymphoid tissue cells. Lymphoma is divided into Hodgkin's lymphoma and non-Hodgkin's lymphoma (malignant lymphoma), and diffuse large B-cell lymphoma accounts for about 30 to 40% of non-Hodgkin's lymphoma.
The research team noticed that DLBCL patients at Seoul National University Hospital received chemotherapy on two different schedules, with some patients receiving morning treatment (8:30 a.m.) and others taking the drugs in the afternoon (2:30 p.m.). All patients received the same cancer treatment (R-CHOP), which is a combination of targeted therapy and chemotherapy, four to six times in the morning or afternoon at intervals of about three weeks.
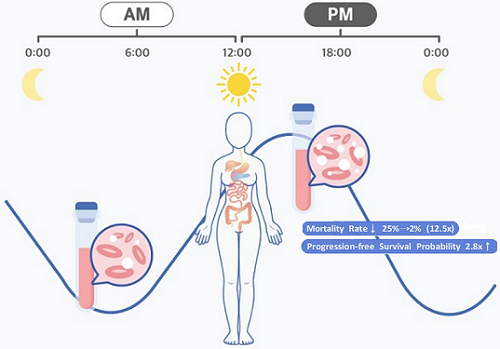
They analyzed 210 patients to investigate whether there was any difference between morning and afternoon treatments. It was found that female patients who received the afternoon treatment had a 12.5 times reduced mortality rate (25% to 2%), while the cancer recurrence after 60 months decreased by 2.8 times (37% to 13%). In addition, chemotherapy side effects such as neutropenia were more common in female patients who received the morning treatment.
Surprisingly, there was no differences found in treatment efficiency depending on the treatment schedule in the cases of male patients.
To understand the cause of the gender differences, the research team analyzed upto 14,000 blood samples from the Seoul National University Hospital Health Examination Center. It was found that in females, white blood cell counts tended to decrease in the morning and increase in the afternoon. This indicates that the bone marrow proliferation rate was higher in the morning than in the afternoon because there is a upto 12 hour delay between bone marrow proliferation and blood cell production.
This means that if a female patient receives chemotherapy in the morning when bone marrow is actively producing blood cells, the possibility of adverse side effects becomes greater. These results are consistent with the findings from recent randomized clinical trials that showed female colorectal cancer patients treated with irinotecan in the morning suffered from higher drug toxicities.
One confounding variable was the drug dose. Since the morning female patients suffered from greater adverse side effects, oftentimes the dose had to be reduced for these patients. On average, the drug dose was reduced by upto 10% compared to the dose intensity given to female patients receiving the afternoon treatment.
Unlike the female patients, it was found that male patients did not show a significant difference in white blood cell count and bone marrow cell proliferation activity throughout the day, which explains why the timing of the treatment had no impact.
Professor Youngil Koh said, “We plan to verify the conclusions of this study again with a large-scale follow-up study that completely controls for the confounding variables, and to confirm whether chrono-chemotherapy has similar effects on other cancers.”
CI Jae Kyoung Kim said, “Because the time of the internal circadian clock can vary greatly depending on the individual's sleep-wake patterns, we are currently developing a technology to estimate a patient’s circadian clock from their sleep pattern. We hope that this can be used to develop an individualized anti-cancer chronotherapy schedule.”
< Figure 2. Chemotherapy in the afternoon can improve treatment outcomes. >
The daily fluctuation of proliferative activity of bone marrow is larger in females than in males, and it becomes higher in the morning (left). Thus, chemotherapy in the morning strongly inhibits proliferative activity in female lymphoma patients, resulting in a higher incidence of adverse events such as neutropenia and infections. This forced the clinicians to reduce the dose intensity (center). Consequently, female patients undergoing the morning treatment showed a lower survival probability than those undergoing the afternoon treatment (right). Specifically, only ~13% of female patients treated in the afternoon had a worse outcome and ~2% of them died while ~37% of female patients treated in the morning had a worse outcome and ~25% of them died. Male patients did not show any difference in treatment outcomes depending on the chemotherapy delivery time.
2023.01.27 View 8009
Afternoon chemotherapy proved to deliver more desirable results for female lymphoma patients
Chemotherapy is a commonly used regimen for cancer treatment, but it is also a double-edged sword. While the drugs are highly effective at killing cancer cells, they are also notorious for killing healthy cells in the body. As such, minimizing the drug’s damage to the patient’s body is necessary for improving the prognosis of chemotherapy.
Recently, “chrono-chemotherapy” have been gaining interest in the research community. As the name suggests, the aim is timing the delivery of the drugs when the body is least vulnerable to their harmful effects and while the cancer cells are at their most vulnerable.
< Figure 1. Chrono-chemotherapy considering circadian rhythm >
Chrono-chemotherapy exploits the fact that human physiological processes, including cell proliferation and differentiation, are regulated by an endogenous timer called the circadian clock. However, this has not been widely exploited in real-world clinical settings because, as of now, there is no systematic method for finding the optimal chemotherapy delivery time.
This problem was tackled by an interdisciplinary team of researchers from South Korea. They were led by principal investigators Jae Kyoung Kim (a mathematician from the Biomedical Mathematics Group, Institute for Basic Science) and Youngil Koh (an oncologist at Seoul National University Hospital). The researchers studied a group of patients suffering from diffuse large B-cell lymphoma (DLBCL).
Terminology
* Diffuse large B-cell lymphoma (DLBCL): Lymphoma is a type of blood cancer caused by the malignant transformation of lymphoid tissue cells. Lymphoma is divided into Hodgkin's lymphoma and non-Hodgkin's lymphoma (malignant lymphoma), and diffuse large B-cell lymphoma accounts for about 30 to 40% of non-Hodgkin's lymphoma.
The research team noticed that DLBCL patients at Seoul National University Hospital received chemotherapy on two different schedules, with some patients receiving morning treatment (8:30 a.m.) and others taking the drugs in the afternoon (2:30 p.m.). All patients received the same cancer treatment (R-CHOP), which is a combination of targeted therapy and chemotherapy, four to six times in the morning or afternoon at intervals of about three weeks.
They analyzed 210 patients to investigate whether there was any difference between morning and afternoon treatments. It was found that female patients who received the afternoon treatment had a 12.5 times reduced mortality rate (25% to 2%), while the cancer recurrence after 60 months decreased by 2.8 times (37% to 13%). In addition, chemotherapy side effects such as neutropenia were more common in female patients who received the morning treatment.
Surprisingly, there was no differences found in treatment efficiency depending on the treatment schedule in the cases of male patients.
To understand the cause of the gender differences, the research team analyzed upto 14,000 blood samples from the Seoul National University Hospital Health Examination Center. It was found that in females, white blood cell counts tended to decrease in the morning and increase in the afternoon. This indicates that the bone marrow proliferation rate was higher in the morning than in the afternoon because there is a upto 12 hour delay between bone marrow proliferation and blood cell production.
This means that if a female patient receives chemotherapy in the morning when bone marrow is actively producing blood cells, the possibility of adverse side effects becomes greater. These results are consistent with the findings from recent randomized clinical trials that showed female colorectal cancer patients treated with irinotecan in the morning suffered from higher drug toxicities.
One confounding variable was the drug dose. Since the morning female patients suffered from greater adverse side effects, oftentimes the dose had to be reduced for these patients. On average, the drug dose was reduced by upto 10% compared to the dose intensity given to female patients receiving the afternoon treatment.
Unlike the female patients, it was found that male patients did not show a significant difference in white blood cell count and bone marrow cell proliferation activity throughout the day, which explains why the timing of the treatment had no impact.
Professor Youngil Koh said, “We plan to verify the conclusions of this study again with a large-scale follow-up study that completely controls for the confounding variables, and to confirm whether chrono-chemotherapy has similar effects on other cancers.”
CI Jae Kyoung Kim said, “Because the time of the internal circadian clock can vary greatly depending on the individual's sleep-wake patterns, we are currently developing a technology to estimate a patient’s circadian clock from their sleep pattern. We hope that this can be used to develop an individualized anti-cancer chronotherapy schedule.”
< Figure 2. Chemotherapy in the afternoon can improve treatment outcomes. >
The daily fluctuation of proliferative activity of bone marrow is larger in females than in males, and it becomes higher in the morning (left). Thus, chemotherapy in the morning strongly inhibits proliferative activity in female lymphoma patients, resulting in a higher incidence of adverse events such as neutropenia and infections. This forced the clinicians to reduce the dose intensity (center). Consequently, female patients undergoing the morning treatment showed a lower survival probability than those undergoing the afternoon treatment (right). Specifically, only ~13% of female patients treated in the afternoon had a worse outcome and ~2% of them died while ~37% of female patients treated in the morning had a worse outcome and ~25% of them died. Male patients did not show any difference in treatment outcomes depending on the chemotherapy delivery time.
2023.01.27 View 8009 -
 Scientists re-writes FDA-recommended equation to improve estimation of drug-drug interaction
Drugs absorbed into the body are metabolized and thus removed by enzymes from several organs like the liver. How fast a drug is cleared out of the system can be affected by other drugs that are taken together because added substance can increase the amount of enzyme secretion in the body. This dramatically decreases the concentration of a drug, reducing its efficacy, often leading to the failure of having any effect at all. Therefore, accurately predicting the clearance rate in the presence of drug-drug interaction* is critical in the process of drug prescription and development of a new drug in order to ensure its efficacy and/or to avoid unwanted side-effects.
*Drug-drug interaction: In terms of metabolism, drug-drug interaction is a phenomenon in which one drug changes the metabolism of another drug to promote or inhibit its excretion from the body when two or more drugs are taken together. As a result, it increases the toxicity of medicines or causes loss of efficacy.
Since it is practically impossible to evaluate all interactions between new drug candidates and all marketed drugs during the development process, the FDA recommends indirect evaluation of drug interactions using a formula suggested in their guidance, first published in 1997, revised in January of 2020, in order to evaluate drug interactions and minimize side effects of having to use more than one type of drugs at once.
The formula relies on the 110-year-old Michaelis-Menten (MM) model, which has a fundamental limit of making a very broad and groundless assumption on the part of the presence of the enzymes that metabolizes the drug. While MM equation has been one of the most widely known equations in biochemistry used in more than 220,000 published papers, the MM equation is accurate only when the concentration of the enzyme that metabolizes the drug is almost non-existent, causing the accuracy of the equation highly unsatisfactory – only 38 percent of the predictions had less than two-fold errors.
“To make up for the gap, researcher resorted to plugging in scientifically unjustified constants into the equation,” Professor Jung-woo Chae of Chungnam National University College of Pharmacy said. “This is comparable to having to have the epicyclic orbits introduced to explain the motion of the planets back in the days in order to explain the now-defunct Ptolemaic theory, because it was 'THE' theory back then.”
< (From left) Ph.D. student Yun Min Song (KAIST, co-first authors), Professor Sang Kyum Kim (Chungnam National University, co-corresponding author), Jae Kyoung Kim, CI (KAIST, co-corresponding author), Professor Jung-woo Chae (Chungnam National University, co-corresponding author), Ph.D. students Quyen Thi Tran and Ngoc-Anh Thi Vu (Chungnam National University, co-first authors) >
A joint research team composed of mathematicians from the Biomedical Mathematics Group within the Institute for Basic Science (IBS) and the Korea Advanced Institute of Science and Technology (KAIST) and pharmacological scientists from the Chungnam National University reported that they identified the major causes of the FDA-recommended equation’s inaccuracies and presented a solution.
When estimating the gut bioavailability (Fg), which is the key parameter of the equation, the fraction absorbed from the gut lumen (Fa) is usually assumed to be 1. However, many experiments have shown that Fa is less than 1, obviously since it can’t be expected that all of the orally taken drugs to be completely absorbed by the intestines. To solve this problem, the research team used an “estimated Fa” value based on factors such as the drug’s transit time, intestine radius, and permeability values and used it to re-calculate Fg.
Also, taking a different approach from the MM equation, the team used an alternative model they derived in a previous study back in 2020, which can more accurately predict the drug metabolism rate regardless of the enzyme concentration. Combining these changes, the modified equation with re-calculated Fg had a dramatically increased accuracy of the resulting estimate. The existing FDA formula predicted drug interactions within a 2-fold margin of error at the rate of 38%, whereas the accuracy rate of the revised formula reached 80%.
“Such drastic improvement in drug-drug interaction prediction accuracy is expected to make great contribution to increasing the success rate of new drug development and drug efficacy in clinical trials. As the results of this study were published in one of the top clinical pharmacology journal, it is expected that the FDA guidance will be revised according to the results of this study.” said Professor Sang Kyum Kim from Chungnam National University College of Pharmacy.
Furthermore, this study highlights the importance of collaborative research between research groups in vastly different disciplines, in a field that is as dynamic as drug interactions.
“Thanks to the collaborative research between mathematics and pharmacy, we were able to recify the formula that we have accepted to be the right answer for so long to finally grasp on the leads toward healthier life for mankind.,” said Professor Jae Kyung Kim. He continued, “I hope seeing a ‘K-formula’ entered into the US FDA guidance one day.”
The results of this study were published in the online edition of Clinical Pharmacology and Therapeutics (IF 7.051), an authoritative journal in the field of clinical pharmacology, on December 15, 2022 (Korean time).
Thesis Title: Beyond the Michaelis-Menten: Accurate Prediction of Drug Interactions through Cytochrome P450 3A4 Induction (doi: 10.1002/cpt.2824)
< Figure 1. The formula proposed by the FDA guidance for predicting drug-drug interactions (top) and the formula newly derived by the researchers (bottom). AUCR (the ratio of substrate area under the plasma concentration-time curve) represents the rate of change in drug concentration due to drug interactions. The research team more than doubled the accuracy of drug interaction prediction compared to the existing formula. >
< Figure 2. Existing FDA formulas tend to underestimate the extent of drug-drug interactions (gray dots) than the actual measured values. On the other hand, the newly derived equation (red dot) has a prediction rate that is within the error range of 2 times (0.5 to 2 times) of the measured value, and is more than twice as high as the existing equation. The solid line in the figure represents the predicted value that matches the measured value. The dotted line represents the predicted value with an error of 0.5 to 2 times. >
For further information or to request media assistance, please contact Jae Kyoung Kim at Biomedical Mathematics Group, Institute for Basic Science (IBS) (jaekkim@ibs.re.kr) or William I. Suh at the IBS Communications Team (willisuh@ibs.re.kr).
- About the Institute for Basic Science (IBS)
IBS was founded in 2011 by the government of the Republic of Korea with the sole purpose of driving forward the development of basic science in South Korea. IBS has 4 research institutes and 33 research centers as of January 2023. There are eleven physics, three mathematics, five chemistry, nine life science, two earth science, and three interdisciplinary research centers.
2023.01.18 View 14606
Scientists re-writes FDA-recommended equation to improve estimation of drug-drug interaction
Drugs absorbed into the body are metabolized and thus removed by enzymes from several organs like the liver. How fast a drug is cleared out of the system can be affected by other drugs that are taken together because added substance can increase the amount of enzyme secretion in the body. This dramatically decreases the concentration of a drug, reducing its efficacy, often leading to the failure of having any effect at all. Therefore, accurately predicting the clearance rate in the presence of drug-drug interaction* is critical in the process of drug prescription and development of a new drug in order to ensure its efficacy and/or to avoid unwanted side-effects.
*Drug-drug interaction: In terms of metabolism, drug-drug interaction is a phenomenon in which one drug changes the metabolism of another drug to promote or inhibit its excretion from the body when two or more drugs are taken together. As a result, it increases the toxicity of medicines or causes loss of efficacy.
Since it is practically impossible to evaluate all interactions between new drug candidates and all marketed drugs during the development process, the FDA recommends indirect evaluation of drug interactions using a formula suggested in their guidance, first published in 1997, revised in January of 2020, in order to evaluate drug interactions and minimize side effects of having to use more than one type of drugs at once.
The formula relies on the 110-year-old Michaelis-Menten (MM) model, which has a fundamental limit of making a very broad and groundless assumption on the part of the presence of the enzymes that metabolizes the drug. While MM equation has been one of the most widely known equations in biochemistry used in more than 220,000 published papers, the MM equation is accurate only when the concentration of the enzyme that metabolizes the drug is almost non-existent, causing the accuracy of the equation highly unsatisfactory – only 38 percent of the predictions had less than two-fold errors.
“To make up for the gap, researcher resorted to plugging in scientifically unjustified constants into the equation,” Professor Jung-woo Chae of Chungnam National University College of Pharmacy said. “This is comparable to having to have the epicyclic orbits introduced to explain the motion of the planets back in the days in order to explain the now-defunct Ptolemaic theory, because it was 'THE' theory back then.”
< (From left) Ph.D. student Yun Min Song (KAIST, co-first authors), Professor Sang Kyum Kim (Chungnam National University, co-corresponding author), Jae Kyoung Kim, CI (KAIST, co-corresponding author), Professor Jung-woo Chae (Chungnam National University, co-corresponding author), Ph.D. students Quyen Thi Tran and Ngoc-Anh Thi Vu (Chungnam National University, co-first authors) >
A joint research team composed of mathematicians from the Biomedical Mathematics Group within the Institute for Basic Science (IBS) and the Korea Advanced Institute of Science and Technology (KAIST) and pharmacological scientists from the Chungnam National University reported that they identified the major causes of the FDA-recommended equation’s inaccuracies and presented a solution.
When estimating the gut bioavailability (Fg), which is the key parameter of the equation, the fraction absorbed from the gut lumen (Fa) is usually assumed to be 1. However, many experiments have shown that Fa is less than 1, obviously since it can’t be expected that all of the orally taken drugs to be completely absorbed by the intestines. To solve this problem, the research team used an “estimated Fa” value based on factors such as the drug’s transit time, intestine radius, and permeability values and used it to re-calculate Fg.
Also, taking a different approach from the MM equation, the team used an alternative model they derived in a previous study back in 2020, which can more accurately predict the drug metabolism rate regardless of the enzyme concentration. Combining these changes, the modified equation with re-calculated Fg had a dramatically increased accuracy of the resulting estimate. The existing FDA formula predicted drug interactions within a 2-fold margin of error at the rate of 38%, whereas the accuracy rate of the revised formula reached 80%.
“Such drastic improvement in drug-drug interaction prediction accuracy is expected to make great contribution to increasing the success rate of new drug development and drug efficacy in clinical trials. As the results of this study were published in one of the top clinical pharmacology journal, it is expected that the FDA guidance will be revised according to the results of this study.” said Professor Sang Kyum Kim from Chungnam National University College of Pharmacy.
Furthermore, this study highlights the importance of collaborative research between research groups in vastly different disciplines, in a field that is as dynamic as drug interactions.
“Thanks to the collaborative research between mathematics and pharmacy, we were able to recify the formula that we have accepted to be the right answer for so long to finally grasp on the leads toward healthier life for mankind.,” said Professor Jae Kyung Kim. He continued, “I hope seeing a ‘K-formula’ entered into the US FDA guidance one day.”
The results of this study were published in the online edition of Clinical Pharmacology and Therapeutics (IF 7.051), an authoritative journal in the field of clinical pharmacology, on December 15, 2022 (Korean time).
Thesis Title: Beyond the Michaelis-Menten: Accurate Prediction of Drug Interactions through Cytochrome P450 3A4 Induction (doi: 10.1002/cpt.2824)
< Figure 1. The formula proposed by the FDA guidance for predicting drug-drug interactions (top) and the formula newly derived by the researchers (bottom). AUCR (the ratio of substrate area under the plasma concentration-time curve) represents the rate of change in drug concentration due to drug interactions. The research team more than doubled the accuracy of drug interaction prediction compared to the existing formula. >
< Figure 2. Existing FDA formulas tend to underestimate the extent of drug-drug interactions (gray dots) than the actual measured values. On the other hand, the newly derived equation (red dot) has a prediction rate that is within the error range of 2 times (0.5 to 2 times) of the measured value, and is more than twice as high as the existing equation. The solid line in the figure represents the predicted value that matches the measured value. The dotted line represents the predicted value with an error of 0.5 to 2 times. >
For further information or to request media assistance, please contact Jae Kyoung Kim at Biomedical Mathematics Group, Institute for Basic Science (IBS) (jaekkim@ibs.re.kr) or William I. Suh at the IBS Communications Team (willisuh@ibs.re.kr).
- About the Institute for Basic Science (IBS)
IBS was founded in 2011 by the government of the Republic of Korea with the sole purpose of driving forward the development of basic science in South Korea. IBS has 4 research institutes and 33 research centers as of January 2023. There are eleven physics, three mathematics, five chemistry, nine life science, two earth science, and three interdisciplinary research centers.
2023.01.18 View 14606 -
 Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11489
Mathematicians Identify a Key Source of Cell-to-Cell Variability in Cell Signaling
Systematic inferences identify a major source of heterogeneity in cell signaling dynamics
Why do genetically identical cells respond differently to the same external stimuli, such as antibiotics? This long-standing mystery has been solved by KAIST and IBS mathematicians who have developed a new framework for analyzing cell responses to some stimuli. The team found that the cell-to-cell variability in antibiotic stress response increases as the effective length of the cell signaling pathway (i.e., the number of rate-limiting steps) increases. This finding could identify more effective chemotherapies to overcome the fractional killing of cancer cells caused by cell-to-cell variability.
Cells in the human body contain signal transduction systems that respond to various external stimuli such as antibiotics and changes in osmotic pressure. When an external stimulus is detected, various biochemical reactions occur sequentially. This leads to the expression of relevant genes, allowing the cells to respond to the perturbed external environment. Furthermore, signal transduction leads to a drug response (e.g., antibiotic resistance genes are expressed when antibiotic drugs are given).
However, even when the same external stimuli are detected, the responses of individual cells are greatly heterogeneous. This leads to the emergence of persister cells that are highly resistant to drugs. To identify potential sources of this cell-to cell variability, many studies have been conducted. However, most of the intermediate signal transduction reactions are unobservable with current experimental techniques.
A group of researchers including Dae Wook Kim and Hyukpyo Hong and led by Professor Jae Kyoung Kim from the KAIST Department of Mathematical Sciences and IBS Biomedical Mathematics Group solved the mystery by exploiting queueing theory and Bayesian inference methodology. They proposed a queueing process that describes the signal transduction system in cells. Based on this, they developed Bayesian inference computational software using MBI (the Moment-based Bayesian Inference method). This enables the analysis of the signal transduction system without a direct observation of the intermediate steps. This study was published in Science Advances.
By analyzing experimental data from Escherichia coli using MBI, the research team found that cell-to-cell variability increases as the number of rate-limiting steps in the signaling pathway increases.
The rate-limiting steps denote the slowest steps (i.e., bottlenecks) in sequential biochemical reaction steps composing cell signaling pathways and thus dominates most of the signaling time. As the number of the rate-limiting steps increases, the intensity of the transduced signal becomes greatly heterogeneous even in a population of genetically identical cells. This finding is expected to provide a new paradigm for studying the heterogeneous antibiotic resistance of cells, which is a big challenge in cancer medicine.
Professor Kim said, “As a mathematician, I am excited to help advance the understanding of cell-to-cell variability in response to external stimuli. I hope this finding facilitates the development of more effective chemotherapies.”
This work was supported by the Samsung Science and Technology Foundation, the National Research Foundation of Korea, and the Institute for Basic Science.
-Publication:Dae Wook Kim, Hyukpyo Hong, and Jae Kyoung Kim (2022) “Systematic inference identifies a major source of heterogeneity in cell signaling dynamics: the rate-limiting step number,”Science Advances March 18, 2022 (DOI: 10.1126/sciadv.abl4598)
-Profile:Professor Jae Kyoung Kimhttp://mathsci.kaist.ac.kr/~jaekkim
jaekkim@kaist.ac.kr@umichkim on TwitterDepartment of Mathematical SciencesKAIST
2022.03.29 View 11489 -
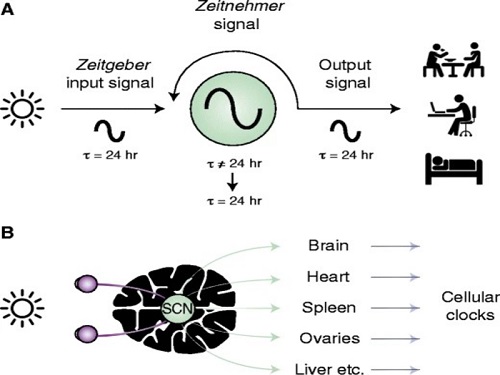 Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 11497
Scientist Discover How Circadian Rhythm Can Be Both Strong and Flexible
Study reveals that master and slave oscillators function via different molecular mechanisms
From tiny fruit flies to human beings, all animals on Earth maintain their daily rhythms based on their internal circadian clock. The circadian clock enables organisms to undergo rhythmic changes in behavior and physiology based on a 24-hour circadian cycle. For example, our own biological clock tells our brain to release melatonin, a sleep-inducing hormone, at night time.
The discovery of the molecular mechanism of the circadian clock was bestowed the Nobel Prize in Physiology or Medicine 2017. From what we know, no one centralized clock is responsible for our circadian cycles. Instead, it operates in a hierarchical network where there are “master pacemaker” and “slave oscillator”.
The master pacemaker receives various input signals from the environment such as light. The master then drives the slave oscillator that regulates various outputs such as sleep, feeding, and metabolism. Despite the different roles of the pacemaker neurons, they are known to share common molecular mechanisms that are well conserved in all lifeforms. For example, interlocked systems of multiple transcriptional-translational feedback loops (TTFLs) composed of core clock proteins have been deeply studied in fruit flies.
However, there is still much that we need to learn about our own biological clock. The hierarchically-organized nature of master and slave clock neurons leads to a prevailing belief that they share an identical molecular clockwork. At the same time, the different roles they serve in regulating bodily rhythms also raise the question of whether they might function under different molecular clockworks.
Research team led by Professor Kim Jae Kyoung from the Department of Mathematical Sciences, a chief investigator at the Biomedical Mathematics Group at the Institute for Basic Science, used a combination of mathematical and experimental approaches using fruit flies to answer this question. The team found that the master clock and the slave clock operate via different molecular mechanisms.
In both master and slave neurons of fruit flies, a circadian rhythm-related protein called PER is produced and degraded at different rates depending on the time of the day. Previously, the team found that the master clock neuron (sLNvs) and the slave clock neuron (DN1ps) have different profiles of PER in wild-type and Clk-Δ mutant Drosophila. This hinted that there might be a potential difference in molecular clockworks between the master and slave clock neurons.
However, due to the complexity of the molecular clockwork, it was challenging to identify the source of such differences. Thus, the team developed a mathematical model describing the molecular clockworks of the master and slave clocks. Then, all possible molecular differences between the master and slave clock neurons were systematically investigated by using computer simulations. The model predicted that PER is more efficiently produced and then rapidly degraded in the master clock compared to the slave clock neurons. This prediction was then confirmed by the follow-up experiments using animal.
Then, why do the master clock neurons have such different molecular properties from the slave clock neurons? To answer this question, the research team again used the combination of mathematical model simulation and experiments. It was found that the faster rate of synthesis of PER in the master clock neurons allows them to generate synchronized rhythms with a high level of amplitude. Generation of such a strong rhythm with high amplitude is critical to delivering clear signals to slave clock neurons.
However, such strong rhythms would typically be unfavorable when it comes to adapting to environmental changes. These include natural causes such as different daylight hours across summer and winter seasons, up to more extreme artificial cases such as jet lag that occurs after international travel. Thanks to the distinct property of the master clock neurons, it is able to undergo phase dispersion when the standard light-dark cycle is disrupted, drastically reducing the level of PER. The master clock neurons can then easily adapt to the new diurnal cycle. Our master pacemaker’s plasticity explains how we can quickly adjust to the new time zones after international flights after just a brief period of jet lag.
It is hoped that the findings of this study can have future clinical implications when it comes to treating various disorders that affect our circadian rhythm. Professor Kim notes, “When the circadian clock loses its robustness and flexibility, the circadian rhythms sleep disorders can occur. As this study identifies the molecular mechanism that generates robustness and flexibility of the circadian clock, it can facilitate the identification of the cause of and treatment strategy for the circadian rhythm sleep disorders.” This work was supported by the Human Frontier Science Program.
-PublicationEui Min Jeong, Miri Kwon, Eunjoo Cho, Sang Hyuk Lee, Hyun Kim, Eun Young Kim, and Jae Kyoung Kim, “Systematic modeling-driven experiments identify distinct molecularclockworks underlying hierarchically organized pacemaker neurons,” February 22, 2022, Proceedings of the National Academy of Sciences of the United States of America
-ProfileProfessor Jae Kyoung KimDepartment of Mathematical SciencesKAIST
2022.02.23 View 11497 -
 A Mathematical Model Shows High Viral Transmissions Reduce the Progression Rates for Severe Covid-19
The model suggests a clue as to when a pandemic will turn into an endemic
A mathematical model demonstrated that high transmission rates among highly vaccinated populations of COVID-19 ultimately reduce the numbers of severe cases. This model suggests a clue as to when this pandemic will turn into an endemic.
With the future of the pandemic remaining uncertain, a research team of mathematicians and medical scientists analyzed a mathematical model that may predict how the changing transmission rate of COVID-19 would affect the settlement process of the virus as a mild respiratory virus.
The team led by Professor Jae Kyoung Kim from the Department of Mathematical Science and Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering used a new approach by dividing the human immune responses to SARS-CoV-2 into a shorter-term neutralizing antibody response and a longer-term T-cell immune response, and applying them each to a mathematical model. Additionally, the analysis was based on the fact that although breakthrough infection may occur frequently, the immune response of the patient will be boosted after recovery from each breakthrough infection.
The results showed that in an environment with a high vaccination rate, although COVID-19 cases may rise temporarily when the transmission rate increases, the ratio of critical cases would ultimately decline, thereby decreasing the total number of critical cases and in fact settling COVID-19 as a mild respiratory disease more quickly.
Conditions in which the number of cases may spike include relaxing social distancing measures or the rise of variants with higher transmission rates like the Omicron variant. This research did not take the less virulent characteristic of the Omicron variant into account but focused on the results of its high transmission rate, thereby predicting what may happen in the process of the endemic transition of COVID-19.
The research team pointed out the limitations of their mathematical model, such as the lack of consideration for age or patients with underlying diseases, and explained that the results of this study must be applied with care when compared against high-risk groups. Additionally, as medical systems may collapse when the number of cases rises sharply, this study must be interpreted with prudence and applied accordingly. The research team therefore emphasized that for policies that encourage a step-wise return to normality to succeed, the sustainable maintenance of public health systems is indispensable.
Professor Kim said, “We have drawn a counter-intuitive conclusion amid the unpredictable pandemic through an adequate mathematical model,” asserting the importance of applying mathematical models to medical research.
Professor Shin said, “Although the Omicron variant has become the dominant strain and the number of cases is rising rapidly in South Korea, it is important to use scientific approaches to predict the future and apply them to policies rather than fearing the current situation.”
The results of the research were published on medRxiv.org on February 11, under the title “Increasing viral transmission paradoxically reduces progression rates to severe COVID-19 during endemic transition.”
This research was funded by the Institute of Basic Science, the Korea Health Industry Development Institute, and the National Research Foundation of Korea.
-PublicationHyukpyo Hong, Ji Yun Noh, Hyojung Lee, Sunhwa Choi, Boseung Choi, Jae Kyung Kim, Eui-Cheol Shin, “Increasing viral transmission paradoxically reduces progression rates to
severe COVID-19 during endemic transition,” medRxiv, February 9, 2022 (doi.org/10.1101/2022.02.09.22270633)
-ProfileProfessor Jae Kyung KimDepartment of Mathematical SciencesKAIST
Professor Eui-Cheol ShinGraduate School of Medical Science and EngineeringKAIST
2022.02.22 View 11824
A Mathematical Model Shows High Viral Transmissions Reduce the Progression Rates for Severe Covid-19
The model suggests a clue as to when a pandemic will turn into an endemic
A mathematical model demonstrated that high transmission rates among highly vaccinated populations of COVID-19 ultimately reduce the numbers of severe cases. This model suggests a clue as to when this pandemic will turn into an endemic.
With the future of the pandemic remaining uncertain, a research team of mathematicians and medical scientists analyzed a mathematical model that may predict how the changing transmission rate of COVID-19 would affect the settlement process of the virus as a mild respiratory virus.
The team led by Professor Jae Kyoung Kim from the Department of Mathematical Science and Professor Eui-Cheol Shin from the Graduate School of Medical Science and Engineering used a new approach by dividing the human immune responses to SARS-CoV-2 into a shorter-term neutralizing antibody response and a longer-term T-cell immune response, and applying them each to a mathematical model. Additionally, the analysis was based on the fact that although breakthrough infection may occur frequently, the immune response of the patient will be boosted after recovery from each breakthrough infection.
The results showed that in an environment with a high vaccination rate, although COVID-19 cases may rise temporarily when the transmission rate increases, the ratio of critical cases would ultimately decline, thereby decreasing the total number of critical cases and in fact settling COVID-19 as a mild respiratory disease more quickly.
Conditions in which the number of cases may spike include relaxing social distancing measures or the rise of variants with higher transmission rates like the Omicron variant. This research did not take the less virulent characteristic of the Omicron variant into account but focused on the results of its high transmission rate, thereby predicting what may happen in the process of the endemic transition of COVID-19.
The research team pointed out the limitations of their mathematical model, such as the lack of consideration for age or patients with underlying diseases, and explained that the results of this study must be applied with care when compared against high-risk groups. Additionally, as medical systems may collapse when the number of cases rises sharply, this study must be interpreted with prudence and applied accordingly. The research team therefore emphasized that for policies that encourage a step-wise return to normality to succeed, the sustainable maintenance of public health systems is indispensable.
Professor Kim said, “We have drawn a counter-intuitive conclusion amid the unpredictable pandemic through an adequate mathematical model,” asserting the importance of applying mathematical models to medical research.
Professor Shin said, “Although the Omicron variant has become the dominant strain and the number of cases is rising rapidly in South Korea, it is important to use scientific approaches to predict the future and apply them to policies rather than fearing the current situation.”
The results of the research were published on medRxiv.org on February 11, under the title “Increasing viral transmission paradoxically reduces progression rates to severe COVID-19 during endemic transition.”
This research was funded by the Institute of Basic Science, the Korea Health Industry Development Institute, and the National Research Foundation of Korea.
-PublicationHyukpyo Hong, Ji Yun Noh, Hyojung Lee, Sunhwa Choi, Boseung Choi, Jae Kyung Kim, Eui-Cheol Shin, “Increasing viral transmission paradoxically reduces progression rates to
severe COVID-19 during endemic transition,” medRxiv, February 9, 2022 (doi.org/10.1101/2022.02.09.22270633)
-ProfileProfessor Jae Kyung KimDepartment of Mathematical SciencesKAIST
Professor Eui-Cheol ShinGraduate School of Medical Science and EngineeringKAIST
2022.02.22 View 11824 -
 Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 11935
Five Projects Ranked in the Top 100 for National R&D Excellence
Five KAIST research projects were selected as the 2021 Top 100 for National R&D Excellence by the Ministry of Science and ICT and the Korea Institute of Science & Technology Evaluation and Planning.
The five projects are:-The development of E. coli that proliferates with only formic acid and carbon dioxide by Distinguished Professor Sang Yup Lee from the Department of Chemical and Biomolecular Engineering
-An original reverse aging technology that restores an old human skin cell into a younger one by Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering-The development of next-generation high-efficiency perovskite-silicon tandem solar cells by Professor Byungha Shin from the Department of Materials Science and Engineering-Research on the effects of ultrafine dust in the atmosphere has on energy consumption by Professor Jiyong Eom from the School of Business and Technology Management-Research on a molecular trigger that controls the phase transformation of bio materials by Professor Myungchul Kim from the Department of Bio and Brain Engineering
Started in 2006, an Evaluation Committee composed of experts in industries, universities, and research institutes has made the preliminary selections of the most outstanding research projects based on their significance as a scientific and technological development and their socioeconomic effects. The finalists went through an open public evaluation. The final 100 studies are from six fields: 18 from mechanics & materials, 26 from biology & marine sciences, 19 from ICT & electronics, 10 from interdisciplinary research, and nine from natural science and infrastructure.
The selected 100 studies will receive a certificate and an award plaque from the minister of MSIT as well as additional points for business and institutional evaluations according to appropriate regulations, and the selected researchers will be strongly recommended as candidates for national meritorious awards.
In particular, to help the 100 selected research projects become more accessible for the general public, their main contents will be provided in a free e-book ‘The Top 100 for National R&D Excellence of 2021’ that will be available from online booksellers.
2022.02.17 View 11935 -
 KAIST KPC4IR Presents the AI Global Guide for Healthcare
The benchmark for the responsible usage of AI technology in the healthcare sector will promote clarity and high standards for technological applications
The KAIST Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) published 'Using AI to Support Healthcare Decisions: A Guide for Society.' This global guide is designed to serve as a benchmark for the responsible usage of AI technologies, and will promote clarity and high standards for technological applications in the healthcare sector. The guide details what should be considered when making clinical decisions to help reduce the chances of the AI giving false or misleading results.
The KPC4IR presented the guide in collaboration with the Lloyd’s Register Foundation Institute for the Public Understanding of Risk at the National University of Singapore (NUS IPUR) and Sense about Science, a non-profit organization in the UK specialized in science communication, during the 2021 SIG-KDD (Special Interest Group on Knowledge Discovery and Data Mining) Conference on August 15.
AI technology is being widely used in the healthcare sector and has already proved its accuracy and efficiency in diagnosing and predicting diseases. Despite its huge impact on our daily lives in every sector of society, AI technology has some drawbacks and comes with risks, especially due to biased algorithms.
“We focused on the ‘reliability’ of AI applications in the healthcare sector to make all data well represented, in good quality. The technology will eventually innovate to better serve the people’s new demand, especially critical demands for safety and precision in healthcare services. This global guide will help both developers and people’s understanding of the appropriate technology applications,” says Director So Young Kim at the KPC4IR.
The guide, for instance, says that to scrutinize quality and reliability, the source of the data must be clearly known; the data must have been collected or selected for the purpose it’s being used for; the limitations and assumptions for that purpose have been clearly stated; the biases have been addressed; and it has been properly tested in the real world. It also reflects the importance of the representativeness of data that will affect the accuracy of the AI applications.
“By being transparent and demonstrating the steps taken to check that the AI is reliable, researchers and developers can help give people confidence about providing their data,” the guide states.
For this guide, the KPC4IR and its collaborators collected data after working with numerous experts from the Graduate School of AI at KAIST, the Science and Technology Policy Institute in Korea, Asan Medical Center in Seoul, Seoul National University Bundang Hospital, and AI solution companies.
2021.08.17 View 9453
KAIST KPC4IR Presents the AI Global Guide for Healthcare
The benchmark for the responsible usage of AI technology in the healthcare sector will promote clarity and high standards for technological applications
The KAIST Korea Policy Center for the Fourth Industrial Revolution (KPC4IR) published 'Using AI to Support Healthcare Decisions: A Guide for Society.' This global guide is designed to serve as a benchmark for the responsible usage of AI technologies, and will promote clarity and high standards for technological applications in the healthcare sector. The guide details what should be considered when making clinical decisions to help reduce the chances of the AI giving false or misleading results.
The KPC4IR presented the guide in collaboration with the Lloyd’s Register Foundation Institute for the Public Understanding of Risk at the National University of Singapore (NUS IPUR) and Sense about Science, a non-profit organization in the UK specialized in science communication, during the 2021 SIG-KDD (Special Interest Group on Knowledge Discovery and Data Mining) Conference on August 15.
AI technology is being widely used in the healthcare sector and has already proved its accuracy and efficiency in diagnosing and predicting diseases. Despite its huge impact on our daily lives in every sector of society, AI technology has some drawbacks and comes with risks, especially due to biased algorithms.
“We focused on the ‘reliability’ of AI applications in the healthcare sector to make all data well represented, in good quality. The technology will eventually innovate to better serve the people’s new demand, especially critical demands for safety and precision in healthcare services. This global guide will help both developers and people’s understanding of the appropriate technology applications,” says Director So Young Kim at the KPC4IR.
The guide, for instance, says that to scrutinize quality and reliability, the source of the data must be clearly known; the data must have been collected or selected for the purpose it’s being used for; the limitations and assumptions for that purpose have been clearly stated; the biases have been addressed; and it has been properly tested in the real world. It also reflects the importance of the representativeness of data that will affect the accuracy of the AI applications.
“By being transparent and demonstrating the steps taken to check that the AI is reliable, researchers and developers can help give people confidence about providing their data,” the guide states.
For this guide, the KPC4IR and its collaborators collected data after working with numerous experts from the Graduate School of AI at KAIST, the Science and Technology Policy Institute in Korea, Asan Medical Center in Seoul, Seoul National University Bundang Hospital, and AI solution companies.
2021.08.17 View 9453