Nanoporous+materials
-
 Expanding Gas Storage Capacity of Nanoporous Materials
A KAIST research team led by Professor Jihan Kim of the Department of Chemical and Biomolecular Engineering has successfully proposed a rational defect engineering methodology that can greatly enhance the gas storage capacity of nanoporous materials. The team conducted a high-throughput computational screening of a large experimental metal-organic framework database to identify 13 candidate materials that could experience significant methane uptake enhancement with only a small proportion of linker vacancy defects.
This research was published online on November 16 in Nature Communications, with M.S. candidate Sanggyu Chong from KAIST as the first author and post-doctorate researcher Günther Thiele from the Department of Chemistry at UC Berkeley as a contributing author.
Metal-organic frameworks, hereinafter MOF, are crystalline nanoporous materials that are comprised of metal clusters and organic linkers continuously bound together by coordination bonds. Due to their ultrahigh surface areas and pore volumes, they have been widely studied for various energy and environment applications.
Similar to other crystalline materials, MOFs are never perfectly crystalline and are likely to contain several different types of defects within their crystalline structures. Among these defects, linker vacancy defects, or the random absence of linker vacancies in their designated bonding positions, are known to be controllable by practicing careful control over the synthesis conditions.
The research team combined the concepts of rational defect engineering over the linker vacancy defects and the potential presence of inaccessible pores within MOFs to propose a methodology where controlled the introduction of linker vacancy defects could lead to a dramatic enhancement in gas adsorption and storage capacities.
The study utilized a Graphic Processing Unit (GPU) code developed by Professor Kim in a high-throughput computational screening of 12,000 experimentally synthesized MOFs to identify the structures with significant amounts of pores that were inaccessible for methane. In determining the presence of inaccessible pores, a flood-fill algorithm was performed over the energy-low regions of the structure, which is the same algorithm used for filling an area with color in Microsoft Paint.
For the MOFs with significant amounts of inaccessible pores, as determined from the screening, the research team emulated linker vacancy defects in their crystalline structures so that the previously inaccessible pores would be newly merged into the main adsorption channel with the introduction of defects for additional surface area and pore volume available for adsorption. The research team successfully identified 13 structures that would experience up to a 55.56% increase in their methane uptake with less than 8.33% of the linker vacancy defects.
The research team believes that this rational defect engineering scheme can be further utilized for many other applications in areas such as selective adsorption of an adsorbate from a gas mixture and the semi-permanent capture of gas molecules.
This research was conducted with the support of the Mid-career Research Program of the National Research Foundation of Korea.
Figure1. A diagram for flood fill algorithm and example of identification of inaccessible regions within the MOFs, using the flood fill algorithm
Figure2. Methane energy contours before and after detect introduction
2017.12.04 View 9109
Expanding Gas Storage Capacity of Nanoporous Materials
A KAIST research team led by Professor Jihan Kim of the Department of Chemical and Biomolecular Engineering has successfully proposed a rational defect engineering methodology that can greatly enhance the gas storage capacity of nanoporous materials. The team conducted a high-throughput computational screening of a large experimental metal-organic framework database to identify 13 candidate materials that could experience significant methane uptake enhancement with only a small proportion of linker vacancy defects.
This research was published online on November 16 in Nature Communications, with M.S. candidate Sanggyu Chong from KAIST as the first author and post-doctorate researcher Günther Thiele from the Department of Chemistry at UC Berkeley as a contributing author.
Metal-organic frameworks, hereinafter MOF, are crystalline nanoporous materials that are comprised of metal clusters and organic linkers continuously bound together by coordination bonds. Due to their ultrahigh surface areas and pore volumes, they have been widely studied for various energy and environment applications.
Similar to other crystalline materials, MOFs are never perfectly crystalline and are likely to contain several different types of defects within their crystalline structures. Among these defects, linker vacancy defects, or the random absence of linker vacancies in their designated bonding positions, are known to be controllable by practicing careful control over the synthesis conditions.
The research team combined the concepts of rational defect engineering over the linker vacancy defects and the potential presence of inaccessible pores within MOFs to propose a methodology where controlled the introduction of linker vacancy defects could lead to a dramatic enhancement in gas adsorption and storage capacities.
The study utilized a Graphic Processing Unit (GPU) code developed by Professor Kim in a high-throughput computational screening of 12,000 experimentally synthesized MOFs to identify the structures with significant amounts of pores that were inaccessible for methane. In determining the presence of inaccessible pores, a flood-fill algorithm was performed over the energy-low regions of the structure, which is the same algorithm used for filling an area with color in Microsoft Paint.
For the MOFs with significant amounts of inaccessible pores, as determined from the screening, the research team emulated linker vacancy defects in their crystalline structures so that the previously inaccessible pores would be newly merged into the main adsorption channel with the introduction of defects for additional surface area and pore volume available for adsorption. The research team successfully identified 13 structures that would experience up to a 55.56% increase in their methane uptake with less than 8.33% of the linker vacancy defects.
The research team believes that this rational defect engineering scheme can be further utilized for many other applications in areas such as selective adsorption of an adsorbate from a gas mixture and the semi-permanent capture of gas molecules.
This research was conducted with the support of the Mid-career Research Program of the National Research Foundation of Korea.
Figure1. A diagram for flood fill algorithm and example of identification of inaccessible regions within the MOFs, using the flood fill algorithm
Figure2. Methane energy contours before and after detect introduction
2017.12.04 View 9109 -
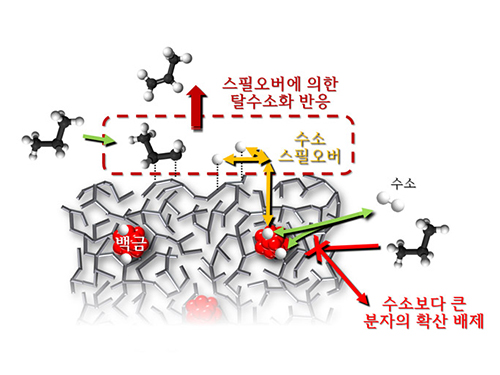 Spillover Phenomenon Identified Using Model Catalyst System
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 12213
Spillover Phenomenon Identified Using Model Catalyst System
Researchers at KAIST have identified spillover phenomenon, which has remained controversial since its discovery in the early 1960s.
KAIST Department of Chemical and Biomolecular Engineering’s Professor Min-Gi Choi and his team has explained the "spillover phenomenon," using their own model catalyst system where platinum is selectively located within the amorphous aluminosilicate.
The research results were published on the 25th February online edition of Nature Communications.
Spillover refers to a phenomenon that occurs when hydrogen atoms that have been activated on the surface of metals, such as platinum, move to the surface of the catalyst. It was predicted that this phenomenon can be used to design a catalyst with high activity and stability, and thus has been actively studied over the last 50 years.
However, many cases of the known catalysts involved competing reactions on the exposed metal surface, which made it impossible to directly identify the presence and formation mechanism of spillover.
The catalysts developed by the researchers at KAIST used platinum nanoparticles covered with aluminosilicate. This only allowed the hydrogen molecules to pass through and has effectively blocked the competing reactions, enabling the research team to study the spillover phenomenon.
Through various catalyst structure and reactivity analysis, as well as computer modeling, the team has discovered that Brönsted acid sites present on the aluminosilicate plays a crucial role in spillover phenomenon.
In addition, the spillover-based hydrogenation catalyst proposed by the research team showed very high hydrogenation and dehydrogenation activity. The ability of the catalyst to significantly inhibit unwanted hydrogenolysis reaction during the petrochemical processes also suggested a large industrial potential.
Professor Min-Gi Choi said, “This particular catalyst, which can trigger the reaction only by spillover phenomenon, can be properly designed to exceed the capacity of the conventional metal catalysts. The future goal is to make a catalyst with much higher activity and selectivity.”
The research was conducted through funds subsidized by SK Innovation and Ministry of Science, ICT and Future Planning.
The senior research fellow of SK Innovation Seung-Hun Oh said, “SK Innovation will continue to develop a new commercial catalyst based on the technology from this research.”
Juh-Wan Lim and Hye-Yeong Shin led the research as joint first authors under supervision of Professor Min-Gi Choi and computer modeling works were conducted by KAIST EEWS (environment, energy, water, and sustainability) graduate school’s Professor Hyeong-Jun Kim.
2014.03.03 View 12213