Materials+chemistry
-
 KAIST develops biocompatible adhesive applicable to hair transplants
Aside from being used as a new medical adhesive, the new material can be applied to developing a new method of hair transplants, which cannot be repeated multiple times using current method of implanting the wholly intact follicles into the skin.
Medical adhesives are materials that can be applied to various uses such as wound healing, hemostasis, vascular anastomosis, and tissue engineering, and is expected to contribute greatly to the development of minimally invasive surgery and organ transplants. However, adhesives with high adhesion, low toxicity, and capable of decomposing in the body are rare. Adhesives based on natural proteins, such as fibrin and collagen, have high biocompatibility but insufficient adhesive strength. Synthetic polymer adhesives based on urethane or acrylic have greater adhesion but do not decompose well and may cause an inflammatory reaction in the body.
A joint research team led by Professor Myungeun Seo and Professor Haeshin Lee from the KAIST Department of Chemistry developed a bio-friendly adhesive from biocompatible polymers using tannic acid, the source of astringency in wine.
The research team focused on tannic acid, a natural polyphenolic product. Tannic acid is a polyphenol present in large amounts in fruit peels, nuts, and cacao. It has a high affinity and coating ability on other substances, and we sense the astringent taste in wine when tannic acid sticks to the surface of our tongue. When tannic acid is mixed with hydrophilic polymers, they form coacervates, or small droplets of jelly-like fluids that sink. If the polymers used are biocompatible, the mixture can be applied as a medical adhesive with low toxicity. However, coacervates are fundamentally fluid-like and cannot withstand large forces, which limits their adhesive capabilities. Thus, while research to utilize it as an adhesive has been actively discussed, a biodegradable material exhibiting strong adhesion due to its high shear strength has not yet been developed.
The research team figured out a way to enhance adhesion by mixing two biocompatible FDA-approved polymers, polyethylene glycol (PEG) and polylactic acid (PLA). While PEG, which is used widely in eyedrops and cream, is hydrophilic, PLA, a well-known bioplastic derived from lactic acid, is insoluble in water. The team combined the two into a block copolymer, which forms hydrophilic PLA aggregates in water with PEG blocks surrounding them. A coacervate created by mixing the micelles and tannic acid would behave like a solid due to the hard PLA components, and show an elastic modulus improved by a thousand times compared to PEG, enabling it to withstand much greater force as an adhesive.
Figure 1. (Above) Principle of biodegradable adhesive made by mixing poly(ethylene glycol)-poly(lactic acid) diblock copolymer and tannic acid in water. Yellow coacervate is precipitated through hydrogen bonding between the block copolymer micelles and tannic acid, and exhibits adhesion. After heat treatment, hydrogen bonds are rearranged to further improve adhesion. (Bottom) Adhesion comparison. Compared to using poly(ethylene glycol) polymer (d), it can support 10 times more weight when using block copolymer (e) and 60 times more weight after heat treatment (f). The indicated G' values represent the elastic modulus of the material.
Furthermore, the research team observed that the material’s mechanical properties can be improved by over a hundred times through a heating and cooling process that is used to heat-treat metals. They also discovered that this is due to the enforced interactions between micelle and tannic acid arrays.
The research team used the fact that the material shows minimal irritation to the skin and decomposes well in the body to demonstrate its possible application as an adhesive for hair transplantation through an animal experiment. Professor Haeshin Lee, who has pioneered various application fields including medical adhesives, hemostatic agents, and browning shampoo, focused on the adhesive capacities and low toxicity of polyphenols like tannic acid, and now looks forward to it improving the limitations of current hair transplant methods, which still involve follicle transfer and are difficult to be repeated multiple times.
Figure 2. (a) Overview of a hair transplantation method using a biodegradable adhesive (right) compared to a conventional hair transplantation method (left) that transplants hair containing hair follicles. After applying an adhesive to the tip of the hair, it is fixed to the skin by implanting it through a subcutaneous injection, and repeated treatment is possible. (b) Initial animal test results. One day after 15 hair transplantation, 12 strands of hair remain. If you pull the 3 strands of hair, you can see that the whole body is pulled up, indicating that it is firmly implanted into the skin. All strands of hair applied without the new adhesive material fell off, and in the case of adhesive without heat treatment, the efficiency was 1/7.
This research was conducted by first co-authors Dr. Jongmin Park (currently a senior researcher at the Korea Research Institute of Chemical Technology) from Professor Myeongeun Seo’s team and Dr. Eunsook Park from Professor Haeshin Lee’s team in the KAIST Department of Chemistry, and through joint research with the teams led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Siyoung Choi from the Department of Chemical and Biomolecular Engineering. The research was published online on August 22 in the international journal Au (JACS Au) under the title Biodegradable Block Copolymer-Tannic Acid Glue.
This study was funded by the Support Research Under Protection Project of the National Research Foundation (NRF), Leading Research Center Support Project (Research Center for Multiscale Chiral Structure), Biodegradable Plastics Commercialization and Demonstration Project by the Ministry of Trade and Industry, and institutional funding from the Korea Research Institute of Chemical Technology.
2022.10.07 View 12941
KAIST develops biocompatible adhesive applicable to hair transplants
Aside from being used as a new medical adhesive, the new material can be applied to developing a new method of hair transplants, which cannot be repeated multiple times using current method of implanting the wholly intact follicles into the skin.
Medical adhesives are materials that can be applied to various uses such as wound healing, hemostasis, vascular anastomosis, and tissue engineering, and is expected to contribute greatly to the development of minimally invasive surgery and organ transplants. However, adhesives with high adhesion, low toxicity, and capable of decomposing in the body are rare. Adhesives based on natural proteins, such as fibrin and collagen, have high biocompatibility but insufficient adhesive strength. Synthetic polymer adhesives based on urethane or acrylic have greater adhesion but do not decompose well and may cause an inflammatory reaction in the body.
A joint research team led by Professor Myungeun Seo and Professor Haeshin Lee from the KAIST Department of Chemistry developed a bio-friendly adhesive from biocompatible polymers using tannic acid, the source of astringency in wine.
The research team focused on tannic acid, a natural polyphenolic product. Tannic acid is a polyphenol present in large amounts in fruit peels, nuts, and cacao. It has a high affinity and coating ability on other substances, and we sense the astringent taste in wine when tannic acid sticks to the surface of our tongue. When tannic acid is mixed with hydrophilic polymers, they form coacervates, or small droplets of jelly-like fluids that sink. If the polymers used are biocompatible, the mixture can be applied as a medical adhesive with low toxicity. However, coacervates are fundamentally fluid-like and cannot withstand large forces, which limits their adhesive capabilities. Thus, while research to utilize it as an adhesive has been actively discussed, a biodegradable material exhibiting strong adhesion due to its high shear strength has not yet been developed.
The research team figured out a way to enhance adhesion by mixing two biocompatible FDA-approved polymers, polyethylene glycol (PEG) and polylactic acid (PLA). While PEG, which is used widely in eyedrops and cream, is hydrophilic, PLA, a well-known bioplastic derived from lactic acid, is insoluble in water. The team combined the two into a block copolymer, which forms hydrophilic PLA aggregates in water with PEG blocks surrounding them. A coacervate created by mixing the micelles and tannic acid would behave like a solid due to the hard PLA components, and show an elastic modulus improved by a thousand times compared to PEG, enabling it to withstand much greater force as an adhesive.
Figure 1. (Above) Principle of biodegradable adhesive made by mixing poly(ethylene glycol)-poly(lactic acid) diblock copolymer and tannic acid in water. Yellow coacervate is precipitated through hydrogen bonding between the block copolymer micelles and tannic acid, and exhibits adhesion. After heat treatment, hydrogen bonds are rearranged to further improve adhesion. (Bottom) Adhesion comparison. Compared to using poly(ethylene glycol) polymer (d), it can support 10 times more weight when using block copolymer (e) and 60 times more weight after heat treatment (f). The indicated G' values represent the elastic modulus of the material.
Furthermore, the research team observed that the material’s mechanical properties can be improved by over a hundred times through a heating and cooling process that is used to heat-treat metals. They also discovered that this is due to the enforced interactions between micelle and tannic acid arrays.
The research team used the fact that the material shows minimal irritation to the skin and decomposes well in the body to demonstrate its possible application as an adhesive for hair transplantation through an animal experiment. Professor Haeshin Lee, who has pioneered various application fields including medical adhesives, hemostatic agents, and browning shampoo, focused on the adhesive capacities and low toxicity of polyphenols like tannic acid, and now looks forward to it improving the limitations of current hair transplant methods, which still involve follicle transfer and are difficult to be repeated multiple times.
Figure 2. (a) Overview of a hair transplantation method using a biodegradable adhesive (right) compared to a conventional hair transplantation method (left) that transplants hair containing hair follicles. After applying an adhesive to the tip of the hair, it is fixed to the skin by implanting it through a subcutaneous injection, and repeated treatment is possible. (b) Initial animal test results. One day after 15 hair transplantation, 12 strands of hair remain. If you pull the 3 strands of hair, you can see that the whole body is pulled up, indicating that it is firmly implanted into the skin. All strands of hair applied without the new adhesive material fell off, and in the case of adhesive without heat treatment, the efficiency was 1/7.
This research was conducted by first co-authors Dr. Jongmin Park (currently a senior researcher at the Korea Research Institute of Chemical Technology) from Professor Myeongeun Seo’s team and Dr. Eunsook Park from Professor Haeshin Lee’s team in the KAIST Department of Chemistry, and through joint research with the teams led by Professor Hyungjun Kim from the KAIST Department of Chemistry and Professor Siyoung Choi from the Department of Chemical and Biomolecular Engineering. The research was published online on August 22 in the international journal Au (JACS Au) under the title Biodegradable Block Copolymer-Tannic Acid Glue.
This study was funded by the Support Research Under Protection Project of the National Research Foundation (NRF), Leading Research Center Support Project (Research Center for Multiscale Chiral Structure), Biodegradable Plastics Commercialization and Demonstration Project by the Ministry of Trade and Industry, and institutional funding from the Korea Research Institute of Chemical Technology.
2022.10.07 View 12941 -
 Synthesized Microporous 3D Graphene-like Carbons
Distinguished Professor Ryong Ryoo of the Chemistry Department at KAIST, who is also the Director of the Center for Nanomaterials and Carbon Materials at the Institute for Basic Science (IBS), and his research team have recently published their research results entitled "Lanthanum-catalysed Synthesis of Microporous 3D Graphene-like Carbons in a Zeolite Template" on June 29, 2016 in Nature on a new method to synthesize carbons having graphene structures with 3D periodic micropores, a trait resulted from using a zeolite as a template for the synthesis. The research team expects this technology to find a range of useful applications such as in batteries and catalysts.
Graphene, an allotrope of carbon, which was discovered more than a decade ago, has led to myriad research that seeks to unlock its vast potential. Zeolites, commonly used microporous solid catalysts in the petrochemical industry, have recently attracted attention in the field of material science as a template for carbon synthesis. Zeolites’ individual crystal is distinguished by its unique 1 nanometer (nm)-size pore structures. These structures facilitate the accommodation of carbon nanotubes inside zeolites. In their paper, the research team showed that these nanoporous systems are an ideal template for the carbon synthesis of three-dimensional (3D) graphene architecture, but zeolite pores are too small to accommodate bulky molecular compounds like polyaromatic and furfuryl alcohol that are often used in carbon synthesis. Small molecules like ethylene and acetylene can be used as a carbon source to achieve successful carbonization within zeolite pores, but it comes at a great cost. The high temperatures required for the synthesis cause reactions of carbons being deposited randomly on the external surfaces of zeolites as well as their internal pore walls, resulting in coke deposition and consequently, causing serious diffusion limitations in the zeolite pores.
The team from the IBS Center for Nanomaterials and Carbon Materials solved this conundrum with a novel approach. First author Dr. KIM Kyoungsoo explains: “The zeolite-template carbon synthesis has existed for a long time, but the problem with temperatures has foiled many scientists from extracting their full potential. Here, our team sought to find the answer by embedding lanthanum ions (La3+), a silvery-white metal element, in zeolite pores. This lowers the temperature required for the carbonization of ethylene or acetylene. Graphene-like sp2 carbon structures can be selectively formed inside the zeolite template, without carbon deposition at the external surfaces. After the zeolite template is removed, the carbon framework exhibits the electrical conductivity two orders of magnitude higher than amorphous mesoporous carbon, which is a pretty astonishing result. This highly efficient synthesis strategy based on the lanthanum ions renders the carbon framework to be formed in pores with a less than 1 nm diameter, just like as easily reproducible as in mesoporous templates. This provides a general method to synthesize carbon nanostructures with various topologies corresponding to the template zeolite pore topologies, such as FAU, EMT, beta, LTL, MFI, and LTA. Also, all the synthesis can be readily scaled up, which is important for practical applications in areas of batteries, fuel storage, and other zeolite-like catalyst supports.”
The research team began their experiment by utilizing La3+ ions. Dr. KIM elucidates why this silvery-white element proved so beneficial to the team, “La3+ ions are unreducible under carbonization process condition, so they can stay inside the zeolite pores instead of moving to the outer zeolite surface in the form of reduced metal particles. Within the pores, they can stabilize ethylene and the pyrocondensation intermediately to form a carbon framework in zeolites.”
In order to test this hypothesis, the team compared the amount of carbon deposited in La3+-containing form of Y zeolite (LaY) sample against a host of other samples such as NaY and HY. The experimental results indicate that all the LaY, NaY, and HY zeolite samples show rapid carbon deposition at 800°C. However, as the temperature decreases, there appears to be a dramatic difference between the different ionic forms of zeolites. At 600°C, the LaY zeolite is still active as a carbon deposition template. In contrast, both NaY and HY lose their carbon deposition functions almost completely.
The results, according to their paper published in Nature, highlight a catalytic effect of lanthanum for carbonization. By making graphene with 3D periodic nanoporous architectures, it promises a wide range of useful applications such as in batteries and catalysts but due to the lack of efficient synthetic strategies, such applications have not yet been successful. By taking advantage of the pore-selective carbon filling at decreased temperatures, the synthesis can readily be scaled up for studies requiring bulk quantities of carbon, in particular high electrical conductivity, which is a highly sought aspect for the production of batteries.
YouTube Link: https://youtu.be/lkNiHiB8lBk
Image 1: (Top to Bottom) Zeolite Template: Microporous Aluminosilicate; Zeolite ion exchanged with La3+ ions in aqueous solution; and Zeolite Template with La3+ ions
Image 2: (Top to Bottom) Catalytic carbonization progressed at La3+ ions-exchanged sites using ethylene as a carbon precursor. Carbon is highlighted in grey; Zeolite template removed in an acid solution (HF/ HCl); Microporous 3D graphene-like carbon
2016.07.01 View 10275
Synthesized Microporous 3D Graphene-like Carbons
Distinguished Professor Ryong Ryoo of the Chemistry Department at KAIST, who is also the Director of the Center for Nanomaterials and Carbon Materials at the Institute for Basic Science (IBS), and his research team have recently published their research results entitled "Lanthanum-catalysed Synthesis of Microporous 3D Graphene-like Carbons in a Zeolite Template" on June 29, 2016 in Nature on a new method to synthesize carbons having graphene structures with 3D periodic micropores, a trait resulted from using a zeolite as a template for the synthesis. The research team expects this technology to find a range of useful applications such as in batteries and catalysts.
Graphene, an allotrope of carbon, which was discovered more than a decade ago, has led to myriad research that seeks to unlock its vast potential. Zeolites, commonly used microporous solid catalysts in the petrochemical industry, have recently attracted attention in the field of material science as a template for carbon synthesis. Zeolites’ individual crystal is distinguished by its unique 1 nanometer (nm)-size pore structures. These structures facilitate the accommodation of carbon nanotubes inside zeolites. In their paper, the research team showed that these nanoporous systems are an ideal template for the carbon synthesis of three-dimensional (3D) graphene architecture, but zeolite pores are too small to accommodate bulky molecular compounds like polyaromatic and furfuryl alcohol that are often used in carbon synthesis. Small molecules like ethylene and acetylene can be used as a carbon source to achieve successful carbonization within zeolite pores, but it comes at a great cost. The high temperatures required for the synthesis cause reactions of carbons being deposited randomly on the external surfaces of zeolites as well as their internal pore walls, resulting in coke deposition and consequently, causing serious diffusion limitations in the zeolite pores.
The team from the IBS Center for Nanomaterials and Carbon Materials solved this conundrum with a novel approach. First author Dr. KIM Kyoungsoo explains: “The zeolite-template carbon synthesis has existed for a long time, but the problem with temperatures has foiled many scientists from extracting their full potential. Here, our team sought to find the answer by embedding lanthanum ions (La3+), a silvery-white metal element, in zeolite pores. This lowers the temperature required for the carbonization of ethylene or acetylene. Graphene-like sp2 carbon structures can be selectively formed inside the zeolite template, without carbon deposition at the external surfaces. After the zeolite template is removed, the carbon framework exhibits the electrical conductivity two orders of magnitude higher than amorphous mesoporous carbon, which is a pretty astonishing result. This highly efficient synthesis strategy based on the lanthanum ions renders the carbon framework to be formed in pores with a less than 1 nm diameter, just like as easily reproducible as in mesoporous templates. This provides a general method to synthesize carbon nanostructures with various topologies corresponding to the template zeolite pore topologies, such as FAU, EMT, beta, LTL, MFI, and LTA. Also, all the synthesis can be readily scaled up, which is important for practical applications in areas of batteries, fuel storage, and other zeolite-like catalyst supports.”
The research team began their experiment by utilizing La3+ ions. Dr. KIM elucidates why this silvery-white element proved so beneficial to the team, “La3+ ions are unreducible under carbonization process condition, so they can stay inside the zeolite pores instead of moving to the outer zeolite surface in the form of reduced metal particles. Within the pores, they can stabilize ethylene and the pyrocondensation intermediately to form a carbon framework in zeolites.”
In order to test this hypothesis, the team compared the amount of carbon deposited in La3+-containing form of Y zeolite (LaY) sample against a host of other samples such as NaY and HY. The experimental results indicate that all the LaY, NaY, and HY zeolite samples show rapid carbon deposition at 800°C. However, as the temperature decreases, there appears to be a dramatic difference between the different ionic forms of zeolites. At 600°C, the LaY zeolite is still active as a carbon deposition template. In contrast, both NaY and HY lose their carbon deposition functions almost completely.
The results, according to their paper published in Nature, highlight a catalytic effect of lanthanum for carbonization. By making graphene with 3D periodic nanoporous architectures, it promises a wide range of useful applications such as in batteries and catalysts but due to the lack of efficient synthetic strategies, such applications have not yet been successful. By taking advantage of the pore-selective carbon filling at decreased temperatures, the synthesis can readily be scaled up for studies requiring bulk quantities of carbon, in particular high electrical conductivity, which is a highly sought aspect for the production of batteries.
YouTube Link: https://youtu.be/lkNiHiB8lBk
Image 1: (Top to Bottom) Zeolite Template: Microporous Aluminosilicate; Zeolite ion exchanged with La3+ ions in aqueous solution; and Zeolite Template with La3+ ions
Image 2: (Top to Bottom) Catalytic carbonization progressed at La3+ ions-exchanged sites using ethylene as a carbon precursor. Carbon is highlighted in grey; Zeolite template removed in an acid solution (HF/ HCl); Microporous 3D graphene-like carbon
2016.07.01 View 10275 -
 Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12172
Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12172 -
 Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12822
Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12822 -
 Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10717
Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10717 -
 Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 13868
Scientists develop highly efficient industrial catalyst
http://english.yonhapnews.co.kr/business/2011/07/14/48/0501000000AEN20110714009600320F.HTML
SEOUL, July 15 (Yonhap) -- South Korean scientists said Friday that they have developed a highly efficient nanoporous industrial catalyst that can have a considerable impact on chemical and oil-refining sectors.
The team of scientists led by Ryoo Ryong, a chemistry professor at the Korea Advanced Institute of Science and Technology (KAIST), said the solid zeolite compound developed in the laboratory has a reaction speed five to 10 times faster than that of conventional materials.
Zeolite, which is made from silica and aluminium, is frequently used as an absorbent, water purifier and in nuclear reprocessing, although it is mainly employed in the chemical industry.
The annual size of the zeolite market is estimated at US$2.5 billion with output using the material topping $30 billion. At present, 41 percent of all catalysts used in the chemical sector are nano-scale zeolite materials.
The KAIST team said that because the new zeolite is made up of different sized pores, the material can be used as a catalyst when existing materials are unable to act as a changing agent.
"Existing zeolites only have pores under 1 nanometer in diameter, but the new material has holes that range from 1 nanometer to 3.5 nanometers, which are all arranged in a regular honeycomb arrangement," Ryoo said. A nanometer is one-billionth of a meter.
He said the ability to have both micro- and meso-sized pores is key to the faster reaction speed that is an integral part of raising efficiency. The South Korean researchers used a so-called surfactant process to make the different sizes of pores.
The development is a breakthrough because researchers and companies such as Exxon Mobil Corp. have been trying to build zeolite with different sizes of pores for the past two decades without making serious headway. There are more than 200 different types of zeolites in the world.
Ryoo, who received funding from the government, has requested intellectual property rights for the discovery, which has been published in the latest issue of Science magazine. He also developed another zeolite in the past that can transform methanol to gasoline up to 10 times more efficiently than existing catalysts.
Exxon Mobil has expressed interest in the two zeolites made by Ryoo"s team. Undisclosed South Korean petrochemical companies have also made inquiries that may lead to commercial development in the future.
"There are some technical issues to resolve, mainly related with mass production and stability," the scientist said.
He said full-fledge production will be determined by how much companies are willing to spend on research to speed up development that can bring down overall production costs.
The KAIST team said it took two years to make the new zeolite, which can be custom made to meet specific needs.
(END)
2011.07.15 View 13868 -
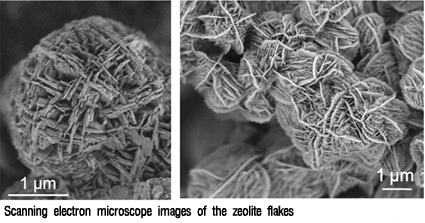 Prof. Ryoo's Team Discovers Breakthrough Method to Create New Zeolite
A group of scientists led by Prof. Ryong Ryoo of the Department of Chemistry, KAIST, has found a method to direct the growth of zeolite, a crystalline substance that is frequently used as catalyst in the chemical and petrochemical industries, the university authorities said on Thursday (Sept. 10).
Ryoo"s research team successfully created ultrathin nano-sheets, only two nano-meters thick, that are efficiently used as long-life catalysts for hydrocarbon cracking and other petrochemical applications. The breakthrough finding, which is credited with taking acidic zeolite catalysts to the limit in terms of thickness, was published in the latest edition of the peer-review journal, "Nature."
A team from the Polytechnic Univeristy of Valencia, Spain, also contributed to the research.
Zeolites are already widely used in the petrochemical industry, but making the catalysts very thin means that reactant molecules can easily diffuse into the zeolite structure and product molecules can get out quickly. This improves the efficiency of the catalyst and reduces unwanted side reactions that can produce polymeric hydrocarbon "coke" that clogs the zeolite pores and eventually kills the catalytic activity, Prof. Yoo said.
To make the thin sheets, Ryoo and his team used a surfactant as a template to direct the growth of the zeolite structure. The surfactant molecule has a polar "head" group - with two quaternary ammonium groups around which the aluminosilicate zeolite crystal grows - and a long hydrocarbon "tail," which prevents the sheets from aggregating together into larger, three dimensional crystals. When the surfactant is removed, these flakes pile up randomly with gaps in between which further aids diffusion to the catalyst sites.
"Zeolite could be used as a catalyst to convert heavy oil into gasoline. Our new zeolite could provide even more possibilities, such as being used as catalysts for transforming methanol into gasline," Ryoo said.
Prof. Ryoo, a Distinguished Professor of KAIST, has won a variety of academic awards, which included the Top Scientist Award given by the Korean government in 2005 and the 2001 KOSEF Science and Technology Award for his work on the synthesis and crystal structure of mezzoporous silica.
Ryoo obtained his bachelor"s degree from Seoul National University in 1977, master"s from KAIST in 1979, and doctorate from Stanford University in 1985.
In 2006, Ryoo and his research team announced the discovery of a form of zeolite that can catalyze petrochemical reactions much more effectively than previous zeolites. Because of the potential of this to streamline the gasoline refining process, it was greeted as a "magical substance" by the South Korean press.
2009.09.11 View 14043
Prof. Ryoo's Team Discovers Breakthrough Method to Create New Zeolite
A group of scientists led by Prof. Ryong Ryoo of the Department of Chemistry, KAIST, has found a method to direct the growth of zeolite, a crystalline substance that is frequently used as catalyst in the chemical and petrochemical industries, the university authorities said on Thursday (Sept. 10).
Ryoo"s research team successfully created ultrathin nano-sheets, only two nano-meters thick, that are efficiently used as long-life catalysts for hydrocarbon cracking and other petrochemical applications. The breakthrough finding, which is credited with taking acidic zeolite catalysts to the limit in terms of thickness, was published in the latest edition of the peer-review journal, "Nature."
A team from the Polytechnic Univeristy of Valencia, Spain, also contributed to the research.
Zeolites are already widely used in the petrochemical industry, but making the catalysts very thin means that reactant molecules can easily diffuse into the zeolite structure and product molecules can get out quickly. This improves the efficiency of the catalyst and reduces unwanted side reactions that can produce polymeric hydrocarbon "coke" that clogs the zeolite pores and eventually kills the catalytic activity, Prof. Yoo said.
To make the thin sheets, Ryoo and his team used a surfactant as a template to direct the growth of the zeolite structure. The surfactant molecule has a polar "head" group - with two quaternary ammonium groups around which the aluminosilicate zeolite crystal grows - and a long hydrocarbon "tail," which prevents the sheets from aggregating together into larger, three dimensional crystals. When the surfactant is removed, these flakes pile up randomly with gaps in between which further aids diffusion to the catalyst sites.
"Zeolite could be used as a catalyst to convert heavy oil into gasoline. Our new zeolite could provide even more possibilities, such as being used as catalysts for transforming methanol into gasline," Ryoo said.
Prof. Ryoo, a Distinguished Professor of KAIST, has won a variety of academic awards, which included the Top Scientist Award given by the Korean government in 2005 and the 2001 KOSEF Science and Technology Award for his work on the synthesis and crystal structure of mezzoporous silica.
Ryoo obtained his bachelor"s degree from Seoul National University in 1977, master"s from KAIST in 1979, and doctorate from Stanford University in 1985.
In 2006, Ryoo and his research team announced the discovery of a form of zeolite that can catalyze petrochemical reactions much more effectively than previous zeolites. Because of the potential of this to streamline the gasoline refining process, it was greeted as a "magical substance" by the South Korean press.
2009.09.11 View 14043 -
 Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo (Department of Chemistry) was selected as a scientist wished to resemble and to be 2006.
Professor Ryoo developed in 2000 world’s first nanoporous carbon material in which numberless several nanometer-sized holes were drilled. The development of this nanoporous material was introduced by international scientific journal NATURE in 2000 and 2001 and expected to contribute to the progress of mankind through the development of high efficiency fuel cell or ultra-light computer. Professor Ryoo also developed a new technology that can considerably improve the catalyst activation and stability of ‘Zeolite’, a main catalyst in the petrochemical industry, which was introduced by NATURE materials. The above achievements qualified Professor Ryoo for the selection.
‘Scientists wished to resemble and to be 2006’ were selected among scientists showing vigorous activities in the science and technology circle on the basis of their recent achievements, etc. by the Ministry of Science and Technology and the Korea Science Foundation, and total 10 scientists qualified to be the model of children and the youth were announced on August 24.
2006.09.06 View 17680
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo, selected as a scientist wished to resemble and to be 2006
Professor Ryong Ryoo (Department of Chemistry) was selected as a scientist wished to resemble and to be 2006.
Professor Ryoo developed in 2000 world’s first nanoporous carbon material in which numberless several nanometer-sized holes were drilled. The development of this nanoporous material was introduced by international scientific journal NATURE in 2000 and 2001 and expected to contribute to the progress of mankind through the development of high efficiency fuel cell or ultra-light computer. Professor Ryoo also developed a new technology that can considerably improve the catalyst activation and stability of ‘Zeolite’, a main catalyst in the petrochemical industry, which was introduced by NATURE materials. The above achievements qualified Professor Ryoo for the selection.
‘Scientists wished to resemble and to be 2006’ were selected among scientists showing vigorous activities in the science and technology circle on the basis of their recent achievements, etc. by the Ministry of Science and Technology and the Korea Science Foundation, and total 10 scientists qualified to be the model of children and the youth were announced on August 24.
2006.09.06 View 17680