x-ray
-
 KAIST Scientifically Identifies a Method to Prevent Dental Erosion from Carbonated Drinks
A Korean research team, which had previously visualized and scientifically proven the harmful effects of carbonated drinks like cola on dental health using nanotechnology, has now identified a mechanism for an effective method to prevent tooth damage caused by these beverages.
KAIST (represented by President Kwang Hyung Lee) announced on the 5th of December that a team led by Professor Seungbum Hong from the Department of Materials Science and Engineering, in collaboration with Seoul National University's School of Dentistry (Departments of Pediatric Dentistry and Oral Microbiology) and Professor Hye Ryung Byon’s research team from the Department of Chemistry, has revealed through nanotechnology that silver diamine fluoride (SDF)* forms a fluoride-containing protective layer on the tooth surface, effectively inhibiting cola-induced erosion.
*SDF (Silver Diamine Fluoride): A dental agent primarily used for the treatment and prevention of tooth decay. SDF strengthens carious lesions, suppresses bacterial growth, and halts the progression of cavities.
The team analyzed the surface morphology and mechanical properties of tooth enamel on a nanoscale using atomic force microscopy (AFM). They also examined the chemical properties of the nano-film formed by SDF treatment using X-ray photoelectron spectroscopy (XPS)* and Fourier-transform infrared spectroscopy (FTIR)*.
*XPS (X-ray Photoelectron Spectroscopy): A powerful surface analysis technique used to investigate the chemical composition and electronic structure of materials.
*FTIR (Fourier-Transform Infrared Spectroscopy): An analytical method that identifies the molecular structure and composition of materials by analyzing how they absorb or transmit infrared light.
The findings showed significant differences in surface roughness and elastic modulus between teeth exposed to cola with and without SDF treatment. Teeth treated with SDF exhibited minimal changes in surface roughness due to erosion (from 64 nm to 70 nm) and maintained a high elastic modulus (from 215 GPa to 205 GPa).
This was attributed to the formation of a fluoroapatite* layer by SDF, which acted as a protective shield.
*Fluoroapatite: A phosphate mineral with the chemical formula Ca₅(PO₄)₃F (calcium fluoro-phosphate). It can occur naturally or be synthesized biologically/artificially and plays a crucial role in strengthening teeth and bones.
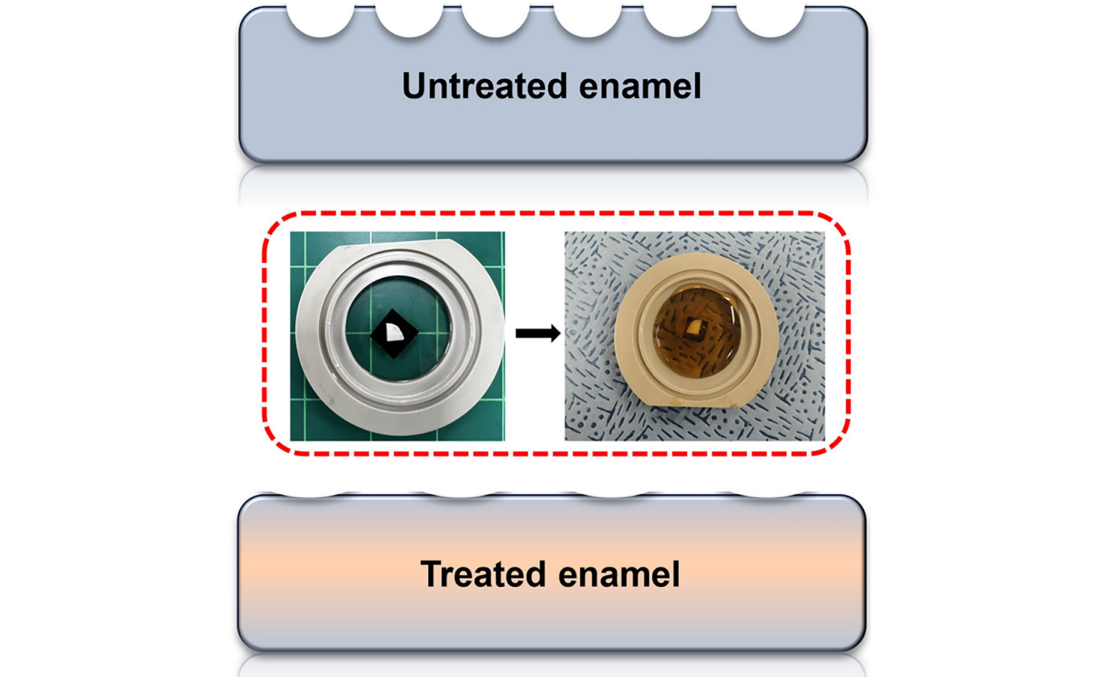
< Figure 1. Schematic of the workflow. Surface morphology and mechanical properties of untreated and treated silver diamine fluoride (SDF) treated enamel exposed to cola were analyzed over time using atomic force microscopy (AFM). >
Professor Young J. Kim from Seoul National University's Department of Pediatric Dentistry noted, "This technology could be applied to prevent dental erosion and strengthen teeth for both children and adults. It is a cost-effective and accessible dental treatment."
< Figure 2. Changes in surface roughness and elastic modulus according to time of exposure to cola for SDF untreated and treated teeth. After 1 hour, the surface roughness of the SDF untreated teeth rapidly became rougher from 83 nm to 287 nm and the elastic modulus weakened from 125 GPa to 13 GPa, whereas the surface roughness of the SDF treated teeth changed only slightly from 64 nm to 70 nm and the elastic modulus barely changed from 215 GPa to 205 GPa, maintaining a similar state to the initial state. >
Professor Seungbum Hong emphasized, "Dental health significantly impacts quality of life. This research offers an effective non-invasive method to prevent early dental erosion, moving beyond traditional surgical treatments. By simply applying SDF, dental erosion can be prevented and enamel strengthened, potentially reducing pain and costs associated with treatment."
This study, led by the first author Aditi Saha, a PhD student in KAIST’s Department of Materials Science and Engineering, was published in the international journal Biomaterials Research on November 7 under the title "Nanoscale Study on Noninvasive Prevention of Dental Erosion of Enamel by Silver Diamine Fluoride". The research was supported by the National Research Foundation of Korea.
2024.12.11 View 3469
KAIST Scientifically Identifies a Method to Prevent Dental Erosion from Carbonated Drinks
A Korean research team, which had previously visualized and scientifically proven the harmful effects of carbonated drinks like cola on dental health using nanotechnology, has now identified a mechanism for an effective method to prevent tooth damage caused by these beverages.
KAIST (represented by President Kwang Hyung Lee) announced on the 5th of December that a team led by Professor Seungbum Hong from the Department of Materials Science and Engineering, in collaboration with Seoul National University's School of Dentistry (Departments of Pediatric Dentistry and Oral Microbiology) and Professor Hye Ryung Byon’s research team from the Department of Chemistry, has revealed through nanotechnology that silver diamine fluoride (SDF)* forms a fluoride-containing protective layer on the tooth surface, effectively inhibiting cola-induced erosion.
*SDF (Silver Diamine Fluoride): A dental agent primarily used for the treatment and prevention of tooth decay. SDF strengthens carious lesions, suppresses bacterial growth, and halts the progression of cavities.
The team analyzed the surface morphology and mechanical properties of tooth enamel on a nanoscale using atomic force microscopy (AFM). They also examined the chemical properties of the nano-film formed by SDF treatment using X-ray photoelectron spectroscopy (XPS)* and Fourier-transform infrared spectroscopy (FTIR)*.
*XPS (X-ray Photoelectron Spectroscopy): A powerful surface analysis technique used to investigate the chemical composition and electronic structure of materials.
*FTIR (Fourier-Transform Infrared Spectroscopy): An analytical method that identifies the molecular structure and composition of materials by analyzing how they absorb or transmit infrared light.
The findings showed significant differences in surface roughness and elastic modulus between teeth exposed to cola with and without SDF treatment. Teeth treated with SDF exhibited minimal changes in surface roughness due to erosion (from 64 nm to 70 nm) and maintained a high elastic modulus (from 215 GPa to 205 GPa).
This was attributed to the formation of a fluoroapatite* layer by SDF, which acted as a protective shield.
*Fluoroapatite: A phosphate mineral with the chemical formula Ca₅(PO₄)₃F (calcium fluoro-phosphate). It can occur naturally or be synthesized biologically/artificially and plays a crucial role in strengthening teeth and bones.
< Figure 1. Schematic of the workflow. Surface morphology and mechanical properties of untreated and treated silver diamine fluoride (SDF) treated enamel exposed to cola were analyzed over time using atomic force microscopy (AFM). >
Professor Young J. Kim from Seoul National University's Department of Pediatric Dentistry noted, "This technology could be applied to prevent dental erosion and strengthen teeth for both children and adults. It is a cost-effective and accessible dental treatment."
< Figure 2. Changes in surface roughness and elastic modulus according to time of exposure to cola for SDF untreated and treated teeth. After 1 hour, the surface roughness of the SDF untreated teeth rapidly became rougher from 83 nm to 287 nm and the elastic modulus weakened from 125 GPa to 13 GPa, whereas the surface roughness of the SDF treated teeth changed only slightly from 64 nm to 70 nm and the elastic modulus barely changed from 215 GPa to 205 GPa, maintaining a similar state to the initial state. >
Professor Seungbum Hong emphasized, "Dental health significantly impacts quality of life. This research offers an effective non-invasive method to prevent early dental erosion, moving beyond traditional surgical treatments. By simply applying SDF, dental erosion can be prevented and enamel strengthened, potentially reducing pain and costs associated with treatment."
This study, led by the first author Aditi Saha, a PhD student in KAIST’s Department of Materials Science and Engineering, was published in the international journal Biomaterials Research on November 7 under the title "Nanoscale Study on Noninvasive Prevention of Dental Erosion of Enamel by Silver Diamine Fluoride". The research was supported by the National Research Foundation of Korea.
2024.12.11 View 3469 -
 KAIST researchers find the key to overcome the limits in X-ray microscopy
X-ray microscopes have the advantage of penetrating most substances, so internal organs and skeletons can be observed non-invasively through chest X-rays or CT scans. Recently, studies to increase the resolution of X-ray imaging technology are being actively conducted in order to precisely observe the internal structure of semiconductors and batteries at the nanoscale.
KAIST (President Kwang Hyung Lee) announced on April 12th that a joint research team led by Professor YongKeun Park of the Department of Physics and Dr. Jun Lim of the Pohang Accelerator Laboratory has succeeded in developing a core technology that can overcome the resolution limitations of existing X-ray microscopes.
d
This study, in which Dr. KyeoReh Lee participated as the first author, was published on 6th of April in “Light: Science and Application”, a world-renowned academic journal in optics and photonics. (Paper title: Direct high-resolution X-ray imaging exploiting pseudorandomness).
X-ray nanomicroscopes do not have refractive lenses. In an X-ray microscope, a circular grating called a concentric zone plate is used instead of a lens. The resolution of an image obtained using the zone plate is determined by the quality of the nanostructure that comprises the plate. There are several difficulties in fabricating and maintaining these nanostructures, which set the limit to the level of resolution for X-ray microscopy.
The research team developed a new X-ray nanomicroscopy technology to overcome this problem. The X-ray lens proposed by the research team is in the form of numerous holes punched in a thin tungsten film, and generates random diffraction patterns by diffracting incident X-rays. The research team mathematically identified that, paradoxically, the high-resolution information of the sample was fully contained in these random diffraction patterns, and actually succeeded in extracting the information and imaging the internal states of the samples.
The imaging method using the mathematical properties of random diffraction was proposed and implemented in the visible light band for the first time by Dr. KyeoReh Lee and Professor YongKeun Park in 2016*. This study uses the results of previous studies to solve the difficult, lingering problem in the field of the X-ray imaging. ※ "Exploiting the speckle-correlation scattering matrix for a compact reference-free holographic image sensor." Nature communications 7.1 (2016): 13359.
The resolution of the image of the constructed sample has no direct correlation with the size of the pattern etched on the random lens used. Based on this idea, the research team succeeded in acquiring images with 14 nm resolution (approximately 1/7 the size of the coronavirus) by using random lenses made in a circular pattern with a diameter of 300 nm.
The imaging technology developed by this research team is a key fundamental technology that can enhance the resolution of X-ray nanomicroscopy, which has been blocked by limitations of the production of existing zone plates.
The first author and one of the co-corresponding author, Dr. KyeoReh Lee of KAIST Department of Physics, said, “In this study, the resolution was limited to 14 nm, but if the next-generation X-ray light source and high-performance X-ray detector are used, the resolution would exceed that of the conventional X-ray nano-imaging and approach the resolution of an electron microscope.” and added, “Unlike an electron microscope, X-rays can observe the internal structure without damaging the sample, so it will be able to present a new standard for non-invasive nanostructure observation processes such as quality inspections for semiconductors.”.
The co-corresponding author, Dr. Jun Lim of the Pohang Accelerator Laboratory, said, “In the same context, the developed image technology is expected to greatly increase the performance in the 4th generation multipurpose radiation accelerator which is set to be established in Ochang of the Northern Chungcheong Province.”
This research was conducted with the support through the Research Leader Program and the Sejong Science Fellowship of the National Research Foundation of Korea.
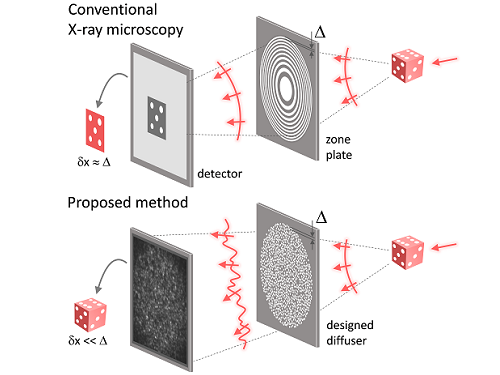
Fig. 1. Designed diffuser as X-ray imaging lens. a, Schematic of full-field transmission X-ray microscopy. The attenuation (amplitude) map of a sample is measured. The image resolution (dx) is limited by the outermost zone width of the zone plate (D). b, Schematic of the proposed method. A designed diffuser is used instead of a zone plate. The image resolution is finer than the hole size of the diffuser (dx << D).
Fig. 2. The left panel is a surface electron microscopy (SEM) image of the X-ray diffuser used in the experiment. The middle panel shows the design of the X-ray diffuser, and there is an inset in the middle of the panel that shows a corresponding part of the SEM image. The right panel shows an experimental random X-ray diffraction pattern, also known as a speckle pattern, obtained from the X-ray diffuser.
Fig. 3. Images taken from the proposed randomness-based X-ray imaging (bottom) and the corresponding surface electron microscope (SEM) images (top).
2023.04.12 View 7330
KAIST researchers find the key to overcome the limits in X-ray microscopy
X-ray microscopes have the advantage of penetrating most substances, so internal organs and skeletons can be observed non-invasively through chest X-rays or CT scans. Recently, studies to increase the resolution of X-ray imaging technology are being actively conducted in order to precisely observe the internal structure of semiconductors and batteries at the nanoscale.
KAIST (President Kwang Hyung Lee) announced on April 12th that a joint research team led by Professor YongKeun Park of the Department of Physics and Dr. Jun Lim of the Pohang Accelerator Laboratory has succeeded in developing a core technology that can overcome the resolution limitations of existing X-ray microscopes.
d
This study, in which Dr. KyeoReh Lee participated as the first author, was published on 6th of April in “Light: Science and Application”, a world-renowned academic journal in optics and photonics. (Paper title: Direct high-resolution X-ray imaging exploiting pseudorandomness).
X-ray nanomicroscopes do not have refractive lenses. In an X-ray microscope, a circular grating called a concentric zone plate is used instead of a lens. The resolution of an image obtained using the zone plate is determined by the quality of the nanostructure that comprises the plate. There are several difficulties in fabricating and maintaining these nanostructures, which set the limit to the level of resolution for X-ray microscopy.
The research team developed a new X-ray nanomicroscopy technology to overcome this problem. The X-ray lens proposed by the research team is in the form of numerous holes punched in a thin tungsten film, and generates random diffraction patterns by diffracting incident X-rays. The research team mathematically identified that, paradoxically, the high-resolution information of the sample was fully contained in these random diffraction patterns, and actually succeeded in extracting the information and imaging the internal states of the samples.
The imaging method using the mathematical properties of random diffraction was proposed and implemented in the visible light band for the first time by Dr. KyeoReh Lee and Professor YongKeun Park in 2016*. This study uses the results of previous studies to solve the difficult, lingering problem in the field of the X-ray imaging. ※ "Exploiting the speckle-correlation scattering matrix for a compact reference-free holographic image sensor." Nature communications 7.1 (2016): 13359.
The resolution of the image of the constructed sample has no direct correlation with the size of the pattern etched on the random lens used. Based on this idea, the research team succeeded in acquiring images with 14 nm resolution (approximately 1/7 the size of the coronavirus) by using random lenses made in a circular pattern with a diameter of 300 nm.
The imaging technology developed by this research team is a key fundamental technology that can enhance the resolution of X-ray nanomicroscopy, which has been blocked by limitations of the production of existing zone plates.
The first author and one of the co-corresponding author, Dr. KyeoReh Lee of KAIST Department of Physics, said, “In this study, the resolution was limited to 14 nm, but if the next-generation X-ray light source and high-performance X-ray detector are used, the resolution would exceed that of the conventional X-ray nano-imaging and approach the resolution of an electron microscope.” and added, “Unlike an electron microscope, X-rays can observe the internal structure without damaging the sample, so it will be able to present a new standard for non-invasive nanostructure observation processes such as quality inspections for semiconductors.”.
The co-corresponding author, Dr. Jun Lim of the Pohang Accelerator Laboratory, said, “In the same context, the developed image technology is expected to greatly increase the performance in the 4th generation multipurpose radiation accelerator which is set to be established in Ochang of the Northern Chungcheong Province.”
This research was conducted with the support through the Research Leader Program and the Sejong Science Fellowship of the National Research Foundation of Korea.
Fig. 1. Designed diffuser as X-ray imaging lens. a, Schematic of full-field transmission X-ray microscopy. The attenuation (amplitude) map of a sample is measured. The image resolution (dx) is limited by the outermost zone width of the zone plate (D). b, Schematic of the proposed method. A designed diffuser is used instead of a zone plate. The image resolution is finer than the hole size of the diffuser (dx << D).
Fig. 2. The left panel is a surface electron microscopy (SEM) image of the X-ray diffuser used in the experiment. The middle panel shows the design of the X-ray diffuser, and there is an inset in the middle of the panel that shows a corresponding part of the SEM image. The right panel shows an experimental random X-ray diffraction pattern, also known as a speckle pattern, obtained from the X-ray diffuser.
Fig. 3. Images taken from the proposed randomness-based X-ray imaging (bottom) and the corresponding surface electron microscope (SEM) images (top).
2023.04.12 View 7330 -
 X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 15650
X-ray Scattering Shines Light on Protein Folding
- Multiple forms of a non-functional, unfolded protein follow different pathways and timelines to reach its folded, functional state, a study reveals. -
KAIST researchers have used an X-ray method to track how proteins fold, which could improve computer simulations of this process, with implications for understanding diseases and improving drug discovery. Their findings were reported in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on June 30.
When proteins are translated from their DNA codes, they quickly transform from a non-functional, unfolded state into their folded, functional state. Problems in folding can lead to diseases like Alzheimer’s and Parkinson’s.
“Protein folding is one of the most important biological processes, as it forms the functioning 3D protein structure,” explained the physical chemist Hyotcherl Ihee of the Department of Chemistry at KAIST. Dr. Tae Wu Kim, the lead author of this research from Ihee’s group, added, “Understanding the mechanisms of protein folding is important, and could pave the way for disease study and drug development.”
Ihee’s team developed an approach using an X-ray scattering technique to uncover how the protein cytochrome c folds from its initial unfolded state. This protein is composed of a chain of 104 amino acids with an iron-containing heme molecule. It is often used for protein folding studies.
The researchers placed the protein in a solution and shined ultraviolet light on it. This process provides electrons to cytochrome c, reducing the iron within it from the ferric to the ferrous form, which initiates folding. As this was happening, the researchers beamed X-rays at very short intervals onto the sample. The X-rays scattered off all the atomic pairs in the sample and a detector continuously recorded the X-ray scattering patterns. The X-ray scattering patterns provided direct information regarding the 3D protein structure and the changes made in these patterns over time showed real-time motion of the protein during the folding process.
The team found cytochrome c proteins initially exist in a wide variety of unfolded states. Once the folding process is triggered, they stop by a group of intermediates within 31.6 microseconds, and then those intermediates follow different pathways with different folding times to reach an energetically stable folded state.
“We don’t know if this diversity in folding paths can be generalized to other proteins,” Ihee confessed. He continued, “However, we believe that our approach can be used to study other protein folding systems.”
Ihee hopes this approach can improve the accuracy of models that simulate protein interactions by including information on their unstructured states. These simulations are important as they can help identify barriers to proper folding and predict a protein’s folded state given its amino acid sequence. Ultimately, the models could help clarify how some diseases develop and how drugs interact with various protein structures.
Ihee’s group collaborated with Professor Young Min Rhee at the KAIST Department of Chemistry, and this work was supported by the National Research Foundation of Korea (NRF) and the Institute for Basic Science (IBS).
Figure. The scientists found that non-functional unfolded forms of the protein cytochrome c follow different pathways and timelines to reach a stable functional folded state.
Publications:
Kim, T. W., et al. (2020) ‘Protein folding from heterogeneous unfolded state revealed by time-resolved X-ray solution scattering’. PNAS. Volume 117. Issue 26. Page 14996-15005. Available online at https://doi.org/10.1073/pnas.1913442117
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Young Min Rhee, Ph.D.
Professor
ymrhee@kaist.ac.kr
http://singlet.kaist.ac.kr
Rhee Research Group
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.07.09 View 15650 -
 Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 18187
Every Moment of Ultrafast Chemical Bonding Now Captured on Film
- The emerging moment of bond formation, two separate bonding steps, and subsequent vibrational motions were visualized. -
< Emergence of molecular vibrations and the evolution to covalent bonds observed in the research. Video Credit: KEK IMSS >
A team of South Korean researchers led by Professor Hyotcherl Ihee from the Department of Chemistry at KAIST reported the direct observation of the birthing moment of chemical bonds by tracking real-time atomic positions in the molecule. Professor Ihee, who also serves as Associate Director of the Center for Nanomaterials and Chemical Reactions at the Institute for Basic Science (IBS), conducted this study in collaboration with scientists at the Institute of Materials Structure Science of High Energy Accelerator Research Organization (KEK IMSS, Japan), RIKEN (Japan), and Pohang Accelerator Laboratory (PAL, South Korea). This work was published in Nature on June 24.
Targeted cancer drugs work by striking a tight bond between cancer cell and specific molecular targets that are involved in the growth and spread of cancer. Detailed images of such chemical bonding sites or pathways can provide key information necessary for maximizing the efficacy of oncogene treatments. However, atomic movements in a molecule have never been captured in the middle of the action, not even for an extremely simple molecule such as a triatomic molecule, made of only three atoms.
Professor Ihee's group and their international collaborators finally succeeded in capturing the ongoing reaction process of the chemical bond formation in the gold trimer. "The femtosecond-resolution images revealed that such molecular events took place in two separate stages, not simultaneously as previously assumed," says Professor Ihee, the corresponding author of the study. "The atoms in the gold trimer complex atoms remain in motion even after the chemical bonding is complete. The distance between the atoms increased and decreased periodically, exhibiting the molecular vibration. These visualized molecular vibrations allowed us to name the characteristic motion of each observed vibrational mode." adds Professor Ihee.
Atoms move extremely fast at a scale of femtosecond (fs) ― quadrillionths (or millionths of a billionth) of a second. Its movement is minute in the level of angstrom equal to one ten-billionth of a meter. They are especially elusive during the transition state where reaction intermediates are transitioning from reactants to products in a flash. The KAIST-IBS research team made this experimentally challenging task possible by using femtosecond x-ray liquidography (solution scattering). This experimental technique combines laser photolysis and x-ray scattering techniques. When a laser pulse strikes the sample, X-rays scatter and initiate the chemical bond formation reaction in the gold trimer complex. Femtosecond x-ray pulses obtained from a special light source called an x-ray free-electron laser (XFEL) were used to interrogate the bond-forming process. The experiments were performed at two XFEL facilities (4th generation linear accelerator) that are PAL-XFEL in South Korea and SACLA in Japan, and this study was conducted in collaboration with researchers from KEK IMSS, PAL, RIKEN, and the Japan Synchrotron Radiation Research Institute (JASRI).
Scattered waves from each atom interfere with each other and thus their x-ray scattering images are characterized by specific travel directions. The KAIST-IBS research team traced real-time positions of the three gold atoms over time by analyzing x-ray scattering images, which are determined by a three-dimensional structure of a molecule. Structural changes in the molecule complex resulted in multiple characteristic scattering images over time. When a molecule is excited by a laser pulse, multiple vibrational quantum states are simultaneously excited. The superposition of several excited vibrational quantum states is called a wave packet. The researchers tracked the wave packet in three-dimensional nuclear coordinates and found that the first half round of chemical bonding was formed within 35 fs after photoexcitation. The second half of the reaction followed within 360 fs to complete the entire reaction dynamics.
They also accurately illustrated molecular vibration motions in both temporal- and spatial-wise. This is quite a remarkable feat considering that such an ultrafast speed and a minute length of motion are quite challenging conditions for acquiring precise experimental data.
In this study, the KAIST-IBS research team improved upon their 2015 study published by Nature. In the previous study in 2015, the speed of the x-ray camera (time resolution) was limited to 500 fs, and the molecular structure had already changed to be linear with two chemical bonds within 500 fs. In this study, the progress of the bond formation and bent-to-linear structural transformation could be observed in real time, thanks to the improvement time resolution down to 100 fs. Thereby, the asynchronous bond formation mechanism in which two chemical bonds are formed in 35 fs and 360 fs, respectively, and the bent-to-linear transformation completed in 335 fs were visualized. In short, in addition to observing the beginning and end of chemical reactions, they reported every moment of the intermediate, ongoing rearrangement of nuclear configurations with dramatically improved experimental and analytical methods.
They will push this method of 'real-time tracking of atomic positions in a molecule and molecular vibration using femtosecond x-ray scattering' to reveal the mechanisms of organic and inorganic catalytic reactions and reactions involving proteins in the human body. "By directly tracking the molecular vibrations and real-time positions of all atoms in a molecule in the middle of reaction, we will be able to uncover mechanisms of various unknown organic and inorganic catalytic reactions and biochemical reactions," notes Dr. Jong Goo Kim, the lead author of the study.
Publications:
Kim, J. G., et al. (2020) ‘Mapping the emergence of molecular vibrations mediating bond formation’. Nature. Volume 582. Page 520-524. Available online at https://doi.org/10.1038/s41586-020-2417-3
Profile: Hyotcherl Ihee, Ph.D.
Professor
hyotcherl.ihee@kaist.ac.kr
http://time.kaist.ac.kr/
Ihee Laboratory
Department of Chemistry
KAIST
https://www.kaist.ac.kr
Daejeon 34141, Korea
(END)
2020.06.24 View 18187 -
 Two KAIST Professors Elected Fellows of APS
Profs. Sung-Chul Shin and Chang-Hee Nam of the Department of Physics, KAIST, have recently been elected the 2009 fellows of the American Physical Society (APS), university officials said on Tuesday (Dec. 2).
The APS fellowship is a prestigious recognition of the two professors" outstanding academic achievements in the field of physics, the officials said. The selection criteria are known to be extremely stringent and only a small fraction of APS members become fellows.
Prof. Shin was cited for his pioneering contributions to the understanding of magnetization reversal dynamics, in particular critical scaling behavior of Barkhausen avalanches of 2D ferromagnets, and discovery of novel magnetic thin films and multilayers for high-density data storage.
Prof. Nam was recognized for his contributions to the theory and experiments of physical processes of high harmonic generation for the development of attosecond coherent x-ray sources and related femtosecond laser technology.
The American Physical Society, founded in 1899, is the world"s second largest organization of physicists, behind the Deutsche Physikalische Gesellschaft. It has 46,000 members across the world.
2008.12.04 View 15516
Two KAIST Professors Elected Fellows of APS
Profs. Sung-Chul Shin and Chang-Hee Nam of the Department of Physics, KAIST, have recently been elected the 2009 fellows of the American Physical Society (APS), university officials said on Tuesday (Dec. 2).
The APS fellowship is a prestigious recognition of the two professors" outstanding academic achievements in the field of physics, the officials said. The selection criteria are known to be extremely stringent and only a small fraction of APS members become fellows.
Prof. Shin was cited for his pioneering contributions to the understanding of magnetization reversal dynamics, in particular critical scaling behavior of Barkhausen avalanches of 2D ferromagnets, and discovery of novel magnetic thin films and multilayers for high-density data storage.
Prof. Nam was recognized for his contributions to the theory and experiments of physical processes of high harmonic generation for the development of attosecond coherent x-ray sources and related femtosecond laser technology.
The American Physical Society, founded in 1899, is the world"s second largest organization of physicists, behind the Deutsche Physikalische Gesellschaft. It has 46,000 members across the world.
2008.12.04 View 15516 -
 A doctorate of Mechanical Engineering Named Recipient of Best Student Paper Award at International Society
Seung-Min Ryu, a doctorate of Mechanical Engineering under the supervision of Professor Dong-Yul Yang, has been named as a recipient of the best student paper award of the Society for Information Display (SID).
The title of the paper is ‘the study on the fabrication of super-high resolution cathode separators by X-ray lithography processes’. He proposed at this paper the fabrication of 12 micron-thick cathode separators, which can fabricate further delicate separators than the current 50 micron-thick commercial PDP separators, thereby significantly improving the resolution of PDPs in the future.
Ryu will make an oral presentation on this paper and win the award at the SID conference, which will take place in the U.S. for six days from May 20.
2007.04.23 View 16367
A doctorate of Mechanical Engineering Named Recipient of Best Student Paper Award at International Society
Seung-Min Ryu, a doctorate of Mechanical Engineering under the supervision of Professor Dong-Yul Yang, has been named as a recipient of the best student paper award of the Society for Information Display (SID).
The title of the paper is ‘the study on the fabrication of super-high resolution cathode separators by X-ray lithography processes’. He proposed at this paper the fabrication of 12 micron-thick cathode separators, which can fabricate further delicate separators than the current 50 micron-thick commercial PDP separators, thereby significantly improving the resolution of PDPs in the future.
Ryu will make an oral presentation on this paper and win the award at the SID conference, which will take place in the U.S. for six days from May 20.
2007.04.23 View 16367