Science+Advances
-
 KAIST Develops Customized Tactile Sensor That Can Detect Light Breath, Pressure and Sound
< Photo 1. (From left) Professor Inkyu Park of KAIST Department of Mechanical Engineering (ME), Dr. Jungrak Choi of ETRI, Ph.D. Candidate Donho Lee and M.S. Graduate Chankyu Han of KAIST ME >
When a robot grabs an object or a medical device detects a pulse, the tactile sensor is the technology that senses pressure like a fingertip. Existing sensors had disadvantages, such as slow responses or declining accuracy after repeated use, but Korean researchers have succeeded in developing a sensor that can quickly and accurately detect even light breath, pressure, and sound. This sensor can be used across a broad range — from everyday movements to medical diagnostics.
KAIST (represented by President Kwang Hyung Lee) announced on the 23rd of June that Professor Inkyu Park’s team from the Department of Mechanical Engineering, through a collaborative research project with the Electronics and Telecommunications Research Institute (ETRI, President Seung Chan Bang ) under the National Research Council of Science & Technology (NST, Chairman Young Sik Kim), has developed an innovative technology that overcomes the structural limitations of existing tactile sensors.
The core of this joint research is the implementation of a customized tactile sensor that simultaneously achieves flexibility, precision, and repeatable durability by applying Thermoformed 3D Electronics (T3DE).
< Figure 1. Comparative evaluation of soft elastomer–based 3D structure versus thermoforming-based 3D structure in terms of mechanical properties. >
In particular, soft elastomer-based sensors (rubber, silicone, etc. — materials that stretch and return to their original shape) have structural problems such as slow response times, high hysteresis*, and creep**, but this new platform operates precisely in diverse environments and overcomes these limitations.
*Hysteresis: A phenomenon where the previously applied force or change is retained like a “memory,” so that the same stimulus does not always produce the same result.
**Creep: The phenomenon where a material slowly deforms when a force is continuously applied.
T3DE sensors are manufactured by precisely forming electrodes on a 2D film, then thermoforming them into a 3D structure under heat and pressure. Specifically, the top electrodes and supporting pillar structures of the sensor are designed to allow the fine-tuning of the mechanical properties for different purposes. By adjusting microstructural parameters — such as the thickness, length, and number of support pillars — the sensor’s Young’s modulus* can be tuned across a broad range of 10 Pa to 1 MPa. This matches the stiffness of biological tissues like skin, muscle, and tendons, making them highly suitable as bio-interface sensors.
*Young’s modulus: An index representing a material's stiffness; this research can control this index to match various biological tissues.
The newly developed T3DE sensor uses air as a dielectric material to reduce power consumption and demonstrates outstanding performance in sensitivity, response time, thermal stability, and repeatable accuracy.
Experimental results showed that the sensor achieved △sensitivity of 5,884 kPa⁻¹, △response time of 0.1 ms (less than one-thousandth of a second), △hysteresis of less than 0.5%, and maintained a repeatable precision of 99.9% or higher even after 5,000 repeated measurements.
< Figure 2. Graphic Overview of thermoformed 3D electronics (T3DE) >
The research team also constructed a high-resolution 40×70 array, comprising a total of 2,800 densely packed sensors, to visualize the pressure distribution on the sole of the foot in real time during exercise and confirmed the possibility of using the sensor for wrist pulse measurement to assess vascular health. Furthermore, successful results were also achieved in sound-detection experiments at a level comparable to commercial acoustic sensors. In short, the sensor can precisely and quickly measure foot pressure, pulse, and sound, allowing it to be applied in areas such as sports, health, and sound sensing.
The T3DE technology was also applied to an augmented-reality(AR)-based surgical training system. By adjusting the stiffness of each sensor element to match that of biological tissues, the system provided real-time visual and tactile feedback according to the pressure applied during surgical incisions. It also offered real-time warnings if an incision was too deep or approached a risky area, making it a promising technology for enhancing immersion and accuracy in medical training.
KAIST Professor Inkyu Park stated, “Because this sensor can be precisely tuned from the design stage and operates reliably across diverse environments, it can be used not only in everyday life, but also in a variety of fields such as healthcare, rehabilitation, and virtual reality.”
The research was co-led as first authors by Dr. Jungrak Choi of ETRI, KAIST master’s student Chankyu Han, and Ph.D. candidate Donho Lee, under the overall guidance of Professor Inkyu Park. The research results were published in the May 2025 issue of ‘Science Advances’ and introduced to the global research community through the journal’s official SNS channels (Facebook, Twitter).
※ Thesis Title: Thermoforming 2D films into 3D electronics for high-performance, customizable tactile sensing
※ DOI: 10.1126/sciadv.adv0057
< Figure 3. The introduction of the study on the official SNS posting by Science Advances >
This research was supported by the Ministry of Trade, Industry and Energy, the National Research Foundation of Korea, and the Korea Institute for Advancement of Technology.
2025.06.23 View 570
KAIST Develops Customized Tactile Sensor That Can Detect Light Breath, Pressure and Sound
< Photo 1. (From left) Professor Inkyu Park of KAIST Department of Mechanical Engineering (ME), Dr. Jungrak Choi of ETRI, Ph.D. Candidate Donho Lee and M.S. Graduate Chankyu Han of KAIST ME >
When a robot grabs an object or a medical device detects a pulse, the tactile sensor is the technology that senses pressure like a fingertip. Existing sensors had disadvantages, such as slow responses or declining accuracy after repeated use, but Korean researchers have succeeded in developing a sensor that can quickly and accurately detect even light breath, pressure, and sound. This sensor can be used across a broad range — from everyday movements to medical diagnostics.
KAIST (represented by President Kwang Hyung Lee) announced on the 23rd of June that Professor Inkyu Park’s team from the Department of Mechanical Engineering, through a collaborative research project with the Electronics and Telecommunications Research Institute (ETRI, President Seung Chan Bang ) under the National Research Council of Science & Technology (NST, Chairman Young Sik Kim), has developed an innovative technology that overcomes the structural limitations of existing tactile sensors.
The core of this joint research is the implementation of a customized tactile sensor that simultaneously achieves flexibility, precision, and repeatable durability by applying Thermoformed 3D Electronics (T3DE).
< Figure 1. Comparative evaluation of soft elastomer–based 3D structure versus thermoforming-based 3D structure in terms of mechanical properties. >
In particular, soft elastomer-based sensors (rubber, silicone, etc. — materials that stretch and return to their original shape) have structural problems such as slow response times, high hysteresis*, and creep**, but this new platform operates precisely in diverse environments and overcomes these limitations.
*Hysteresis: A phenomenon where the previously applied force or change is retained like a “memory,” so that the same stimulus does not always produce the same result.
**Creep: The phenomenon where a material slowly deforms when a force is continuously applied.
T3DE sensors are manufactured by precisely forming electrodes on a 2D film, then thermoforming them into a 3D structure under heat and pressure. Specifically, the top electrodes and supporting pillar structures of the sensor are designed to allow the fine-tuning of the mechanical properties for different purposes. By adjusting microstructural parameters — such as the thickness, length, and number of support pillars — the sensor’s Young’s modulus* can be tuned across a broad range of 10 Pa to 1 MPa. This matches the stiffness of biological tissues like skin, muscle, and tendons, making them highly suitable as bio-interface sensors.
*Young’s modulus: An index representing a material's stiffness; this research can control this index to match various biological tissues.
The newly developed T3DE sensor uses air as a dielectric material to reduce power consumption and demonstrates outstanding performance in sensitivity, response time, thermal stability, and repeatable accuracy.
Experimental results showed that the sensor achieved △sensitivity of 5,884 kPa⁻¹, △response time of 0.1 ms (less than one-thousandth of a second), △hysteresis of less than 0.5%, and maintained a repeatable precision of 99.9% or higher even after 5,000 repeated measurements.
< Figure 2. Graphic Overview of thermoformed 3D electronics (T3DE) >
The research team also constructed a high-resolution 40×70 array, comprising a total of 2,800 densely packed sensors, to visualize the pressure distribution on the sole of the foot in real time during exercise and confirmed the possibility of using the sensor for wrist pulse measurement to assess vascular health. Furthermore, successful results were also achieved in sound-detection experiments at a level comparable to commercial acoustic sensors. In short, the sensor can precisely and quickly measure foot pressure, pulse, and sound, allowing it to be applied in areas such as sports, health, and sound sensing.
The T3DE technology was also applied to an augmented-reality(AR)-based surgical training system. By adjusting the stiffness of each sensor element to match that of biological tissues, the system provided real-time visual and tactile feedback according to the pressure applied during surgical incisions. It also offered real-time warnings if an incision was too deep or approached a risky area, making it a promising technology for enhancing immersion and accuracy in medical training.
KAIST Professor Inkyu Park stated, “Because this sensor can be precisely tuned from the design stage and operates reliably across diverse environments, it can be used not only in everyday life, but also in a variety of fields such as healthcare, rehabilitation, and virtual reality.”
The research was co-led as first authors by Dr. Jungrak Choi of ETRI, KAIST master’s student Chankyu Han, and Ph.D. candidate Donho Lee, under the overall guidance of Professor Inkyu Park. The research results were published in the May 2025 issue of ‘Science Advances’ and introduced to the global research community through the journal’s official SNS channels (Facebook, Twitter).
※ Thesis Title: Thermoforming 2D films into 3D electronics for high-performance, customizable tactile sensing
※ DOI: 10.1126/sciadv.adv0057
< Figure 3. The introduction of the study on the official SNS posting by Science Advances >
This research was supported by the Ministry of Trade, Industry and Energy, the National Research Foundation of Korea, and the Korea Institute for Advancement of Technology.
2025.06.23 View 570 -
 High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 1316
High-Resolution Spectrometer that Fits into Smartphones Developed by KAIST Researchers
- Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering develops an ultra-compact, high-resolution spectrometer using 'double-layer disordered metasurfaces' that generate unique random patterns depending on light's color.
- Unlike conventional dispersion-based spectrometers that were difficult to apply to portable devices, this new concept spectrometer technology achieves 1nm-level high resolution in a device smaller than 1cm, comparable in size to a fingernail.
- It can be utilized as a built-in spectrometer in smartphones and wearable devices in the future, and can be expanded to advanced optical technologies such as hyperspectral imaging and ultrafast imaging.
< Photo 1. (From left) Professor Mooseok Jang, Dong-gu Lee (Ph.D. candidate), Gookho Song (Ph.D. candidate) >
Color, as the way light's wavelength is perceived by the human eye, goes beyond a simple aesthetic element, containing important scientific information like a substance's composition or state. Spectrometers are optical devices that analyze material properties by decomposing light into its constituent wavelengths, and they are widely used in various scientific and industrial fields, including material analysis, chemical component detection, and life science research. Existing high-resolution spectrometers were large and complex, making them difficult for widespread daily use. However, thanks to the ultra-compact, high-resolution spectrometer developed by KAIST researchers, it is now expected that light's color information can be utilized even within smartphones or wearable devices.
KAIST (President Kwang Hyung Lee) announced on the 13th that Professor Mooseok Jang's research team at the Department of Bio and Brain Engineering has successfully developed a reconstruction-based spectrometer technology using double-layer disordered metasurfaces*.
*Double-layer disordered metasurface: An innovative optical device that complexly scatters light through two layers of disordered nanostructures, creating unique and predictable speckle patterns for each wavelength.
Existing high-resolution spectrometers have a large form factor, on the order of tens of centimeters, and require complex calibration processes to maintain accuracy. This fundamentally stems from the operating principle of traditional dispersive elements, such as gratings and prisms, which separate light wavelengths along the propagation direction, much like a rainbow separates colors. Consequently, despite the potential for light's color information to be widely useful in daily life, spectroscopic technology has been limited to laboratory or industrial manufacturing environments.
< Figure 1. Through a simple structure consisting of a double layer of disordered metasurfaces and an image sensor, it was shown that speckles of predictable spectral channels with high spectral resolution can be generated in a compact form factor. The high similarity between the measured and calculated speckles was used to solve the inverse problem and verify the ability to reconstruct the spectrum. >
The research team devised a method that departs from the conventional spectroscopic paradigm of using diffraction gratings or prisms, which establish a one-to-one correspondence between light's color information and its propagation direction, by utilizing designed disordered structures as optical components. In this process, they employed metasurfaces, which can freely control the light propagation process using structures tens to hundreds of nanometers in size, to accurately implement 'complex random patterns (speckle*)'.
*Speckle: An irregular pattern of light intensity created by the interference of multiple wavefronts of light.
Specifically, they developed a method that involves implementing a double-layer disordered metasurface to generate wavelength-specific speckle patterns and then reconstructing precise color information (wavelength) of the light from the random patterns measured by a camera.
As a result, they successfully developed a new concept spectrometer technology that can accurately measure light across a broad range of visible to infrared (440-1,300nm) with a high resolution of 1 nanometer (nm) in a device smaller than a fingernail (less than 1cm) using only a single image capture.
< Figure 2. A disordered metasurface is a metasurface with irregularly arranged structures ranging from tens to hundreds of nanometers in size. In a double-layer structure, a propagation space is placed between the two metasurfaces to control the output speckle with high degrees of freedom, thereby achieving a spectral resolution of 1 nm even in a form factor smaller than 1 cm. >
Dong-gu Lee, a lead author of this study, stated, "This technology is implemented in a way that is directly integrated with commercial image sensors, and we expect that it will enable easy acquisition and utilization of light's wavelength information in daily life when built into mobile devices in the future."
Professor Mooseok Jang said, "This technology overcomes the limitations of existing RGB three-color based machine vision fields, which only distinguish and recognize three color components (red, green, blue), and has diverse applications. We anticipate various applied research for this technology, which expands the horizon of laboratory-level technology to daily-level machine vision technology for applications such as food component analysis, crop health diagnosis, skin health measurement, environmental pollution detection, and bio/medical diagnostics." He added, "Furthermore, it can be extended to various advanced optical technologies such as hyperspectral imaging, which records wavelength and spatial information simultaneously with high resolution, 3D optical trapping technology, which precisely controls light of multiple wavelengths into desired forms, and ultrafast imaging technology, which captures phenomena occurring in very short periods."
This research was collaboratively led by Dong-gu Lee (Ph.D. candidate) and Gookho Song (Ph.D. candidate) from the KAIST Department of Bio and Brain Engineering as co-first authors, with Professor Mooseok Jang as the corresponding author. The findings were published online in the international journal Science Advances on May 28, 2025.* Paper Title: Reconstructive spectrometer using double-layer disordered metasurfaces* DOI: 10.1126/sciadv.adv2376
This research was supported by the Samsung Research Funding and Incubation Center of Samsung Electronics grant, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT).
2025.06.13 View 1316 -
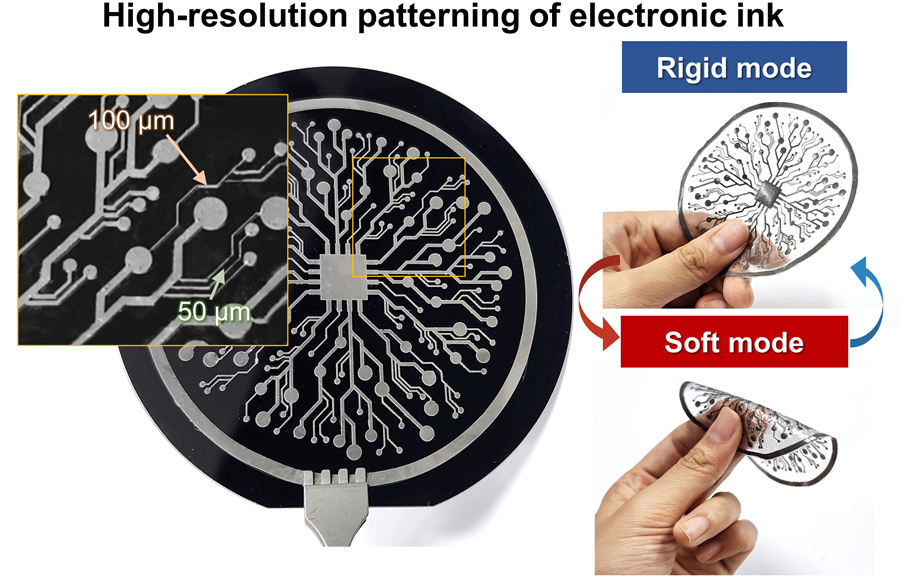 KAIST Research Team Develops Electronic Ink for Room-Temperature Printing of High-Resolution, Variable-Stiffness Electronics
A team of researchers from KAIST and Seoul National University has developed a groundbreaking electronic ink that enables room-temperature printing of variable-stiffness circuits capable of switching between rigid and soft modes. This advancement marks a significant leap toward next-generation wearable, implantable, and robotic devices.
< Photo 1. (From left) Professor Jae-Woong Jeong and PhD candidate Simok Lee of the School of Electrical Engineering, (in separate bubbles, from left) Professor Gun-Hee Lee of Pusan National University, Professor Seongjun Park of Seoul National University, Professor Steve Park of the Department of Materials Science and Engineering>
Variable-stiffness electronics are at the forefront of adaptive technology, offering the ability for a single device to transition between rigid and soft modes depending on its use case. Gallium, a metal known for its high rigidity contrast between solid and liquid states, is a promising candidate for such applications. However, its use has been hindered by challenges including high surface tension, low viscosity, and undesirable phase transitions during manufacturing.
On June 4th, a research team led by Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST, Professor Seongjun Park from the Digital Healthcare Major at Seoul National University, and Professor Steve Park from the Department of Materials Science and Engineering at KAIST introduced a novel liquid metal electronic ink. This ink allows for micro-scale circuit printing – thinner than a human hair – at room temperature, with the ability to reversibly switch between rigid and soft modes depending on temperature.
The new ink combines printable viscosity with excellent electrical conductivity, enabling the creation of complex, high-resolution multilayer circuits comparable to commercial printed circuit boards (PCBs). These circuits can dynamically change stiffness in response to temperature, presenting new opportunities for multifunctional electronics, medical technologies, and robotics.
Conventional electronics typically have fixed form factors – either rigid for durability or soft for wearability. Rigid devices like smartphones and laptops offer robust performance but are uncomfortable when worn, while soft electronics are more comfortable but lack precise handling. As demand grows for devices that can adapt their stiffness to context, variable-stiffness electronics are becoming increasingly important.
< Figure 1. Fabrication process of stable, high-viscosity electronic ink by dispersing micro-sized gallium particles in a polymer matrix (left). High-resolution large-area circuit printing process through pH-controlled chemical sintering (right). >
To address this challenge, the researchers focused on gallium, which melts just below body temperature. Solid gallium is quite stiff, while its liquid form is fluid and soft. Despite its potential, gallium’s use in electronic printing has been limited by its high surface tension and instability when melted.
To overcome these issues, the team developed a pH-controlled liquid metal ink printing process. By dispersing micro-sized gallium particles into a hydrophilic polyurethane matrix using a neutral solvent (dimethyl sulfoxide, or DMSO), they created a stable, high-viscosity ink suitable for precision printing. During post-print heating, the DMSO decomposes to form an acidic environment, which removes the oxide layer on the gallium particles. This triggers the particles to coalesce into electrically conductive networks with tunable mechanical properties.
The resulting printed circuits exhibit fine feature sizes (~50 μm), high conductivity (2.27 × 10⁶ S/m), and a stiffness modulation ratio of up to 1,465 – allowing the material to shift from plastic-like rigidity to rubber-like softness. Furthermore, the ink is compatible with conventional printing techniques such as screen printing and dip coating, supporting large-area and 3D device fabrication.
< Figure 2. Key features of the electronic ink. (i) High-resolution printing and multilayer integration capability. (ii) Batch fabrication capability through large-area screen printing. (iii) Complex three-dimensional structure printing capability through dip coating. (iv) Excellent electrical conductivity and stiffness control capability.>
The team demonstrated this technology by developing a multi-functional device that operates as a rigid portable electronic under normal conditions but transforms into a soft wearable healthcare device when attached to the body. They also created a neural probe that remains stiff during surgical insertion for accurate positioning but softens once inside brain tissue to reduce inflammation – highlighting its potential for biomedical implants.
< Figure 3. Variable stiffness wearable electronics with high-resolution circuits and multilayer structure comparable to commercial printed circuit boards (PCBs). Functions as a rigid portable electronic device at room temperature, then transforms into a wearable healthcare device by softening at body temperature upon skin contact.>
“The core achievement of this research lies in overcoming the longstanding challenges of liquid metal printing through our innovative technology,” said Professor Jeong. “By controlling the ink’s acidity, we were able to electrically and mechanically connect printed gallium particles, enabling the room-temperature fabrication of high-resolution, large-area circuits with tunable stiffness. This opens up new possibilities for future personal electronics, medical devices, and robotics.”
< Figure 4. Body-temperature softening neural probe implemented by coating electronic ink on an optical waveguide structure. (Left) Remains rigid during surgery for precise manipulation and brain insertion, then softens after implantation to minimize mechanical stress on the brain and greatly enhance biocompatibility. (Right) >
This research was published in Science Advances under the title, “Phase-Change Metal Ink with pH-Controlled Chemical Sintering for Versatile and Scalable Fabrication of Variable Stiffness Electronics.” The work was supported by the National Research Foundation of Korea, the Boston-Korea Project, and the BK21 FOUR Program.
2025.06.04 View 2182
KAIST Research Team Develops Electronic Ink for Room-Temperature Printing of High-Resolution, Variable-Stiffness Electronics
A team of researchers from KAIST and Seoul National University has developed a groundbreaking electronic ink that enables room-temperature printing of variable-stiffness circuits capable of switching between rigid and soft modes. This advancement marks a significant leap toward next-generation wearable, implantable, and robotic devices.
< Photo 1. (From left) Professor Jae-Woong Jeong and PhD candidate Simok Lee of the School of Electrical Engineering, (in separate bubbles, from left) Professor Gun-Hee Lee of Pusan National University, Professor Seongjun Park of Seoul National University, Professor Steve Park of the Department of Materials Science and Engineering>
Variable-stiffness electronics are at the forefront of adaptive technology, offering the ability for a single device to transition between rigid and soft modes depending on its use case. Gallium, a metal known for its high rigidity contrast between solid and liquid states, is a promising candidate for such applications. However, its use has been hindered by challenges including high surface tension, low viscosity, and undesirable phase transitions during manufacturing.
On June 4th, a research team led by Professor Jae-Woong Jeong from the School of Electrical Engineering at KAIST, Professor Seongjun Park from the Digital Healthcare Major at Seoul National University, and Professor Steve Park from the Department of Materials Science and Engineering at KAIST introduced a novel liquid metal electronic ink. This ink allows for micro-scale circuit printing – thinner than a human hair – at room temperature, with the ability to reversibly switch between rigid and soft modes depending on temperature.
The new ink combines printable viscosity with excellent electrical conductivity, enabling the creation of complex, high-resolution multilayer circuits comparable to commercial printed circuit boards (PCBs). These circuits can dynamically change stiffness in response to temperature, presenting new opportunities for multifunctional electronics, medical technologies, and robotics.
Conventional electronics typically have fixed form factors – either rigid for durability or soft for wearability. Rigid devices like smartphones and laptops offer robust performance but are uncomfortable when worn, while soft electronics are more comfortable but lack precise handling. As demand grows for devices that can adapt their stiffness to context, variable-stiffness electronics are becoming increasingly important.
< Figure 1. Fabrication process of stable, high-viscosity electronic ink by dispersing micro-sized gallium particles in a polymer matrix (left). High-resolution large-area circuit printing process through pH-controlled chemical sintering (right). >
To address this challenge, the researchers focused on gallium, which melts just below body temperature. Solid gallium is quite stiff, while its liquid form is fluid and soft. Despite its potential, gallium’s use in electronic printing has been limited by its high surface tension and instability when melted.
To overcome these issues, the team developed a pH-controlled liquid metal ink printing process. By dispersing micro-sized gallium particles into a hydrophilic polyurethane matrix using a neutral solvent (dimethyl sulfoxide, or DMSO), they created a stable, high-viscosity ink suitable for precision printing. During post-print heating, the DMSO decomposes to form an acidic environment, which removes the oxide layer on the gallium particles. This triggers the particles to coalesce into electrically conductive networks with tunable mechanical properties.
The resulting printed circuits exhibit fine feature sizes (~50 μm), high conductivity (2.27 × 10⁶ S/m), and a stiffness modulation ratio of up to 1,465 – allowing the material to shift from plastic-like rigidity to rubber-like softness. Furthermore, the ink is compatible with conventional printing techniques such as screen printing and dip coating, supporting large-area and 3D device fabrication.
< Figure 2. Key features of the electronic ink. (i) High-resolution printing and multilayer integration capability. (ii) Batch fabrication capability through large-area screen printing. (iii) Complex three-dimensional structure printing capability through dip coating. (iv) Excellent electrical conductivity and stiffness control capability.>
The team demonstrated this technology by developing a multi-functional device that operates as a rigid portable electronic under normal conditions but transforms into a soft wearable healthcare device when attached to the body. They also created a neural probe that remains stiff during surgical insertion for accurate positioning but softens once inside brain tissue to reduce inflammation – highlighting its potential for biomedical implants.
< Figure 3. Variable stiffness wearable electronics with high-resolution circuits and multilayer structure comparable to commercial printed circuit boards (PCBs). Functions as a rigid portable electronic device at room temperature, then transforms into a wearable healthcare device by softening at body temperature upon skin contact.>
“The core achievement of this research lies in overcoming the longstanding challenges of liquid metal printing through our innovative technology,” said Professor Jeong. “By controlling the ink’s acidity, we were able to electrically and mechanically connect printed gallium particles, enabling the room-temperature fabrication of high-resolution, large-area circuits with tunable stiffness. This opens up new possibilities for future personal electronics, medical devices, and robotics.”
< Figure 4. Body-temperature softening neural probe implemented by coating electronic ink on an optical waveguide structure. (Left) Remains rigid during surgery for precise manipulation and brain insertion, then softens after implantation to minimize mechanical stress on the brain and greatly enhance biocompatibility. (Right) >
This research was published in Science Advances under the title, “Phase-Change Metal Ink with pH-Controlled Chemical Sintering for Versatile and Scalable Fabrication of Variable Stiffness Electronics.” The work was supported by the National Research Foundation of Korea, the Boston-Korea Project, and the BK21 FOUR Program.
2025.06.04 View 2182 -
 KAIST Captures Hot Holes: A Breakthrough in Light-to-Electricity Energy Conversion
When light interacts with metallic nanostructures, it instantaneously generates plasmonic hot carriers, which serve as key intermediates for converting optical energy into high-value energy sources such as electricity and chemical energy. Among these, hot holes play a crucial role in enhancing photoelectrochemical reactions. However, they thermally dissipate within picoseconds (trillionths of a second), making practical applications challenging. Now, a Korean research team has successfully developed a method for sustaining hot holes longer and amplifying their flow, accelerating the commercialization of next-generation, high-efficiency, light-to-energy conversion technologies.
KAIST (represented by President Kwang Hyung Lee) announced on the 12th of March that a research team led by Distinguished Professor Jeong Young Park from the Department of Chemistry, in collaboration with Professor Moonsang Lee from the Department of Materials Science and Engineering at Inha University, has successfully amplified the flow of hot holes and mapped local current distribution in real time, thereby elucidating the mechanism of photocurrent enhancement.
The team designed a nanodiode structure by placing a metallic nanomesh on a specialized semiconductor substrate (p-type gallium nitride) to facilitate hot hole extraction at the surface. As a result, in gallium nitride substrates aligned with the hot hole extraction direction, the flow of hot holes was amplified by approximately two times compared to substrates aligned in other directions.
To fabricate the Au nanomesh, a polystyrene nano-bead monolayer assembly was first placed on a gallium nitride (p-GaN) substrate, and then the polystyrene nano-beads were etched to form a nanomesh template (Figure 1A). Then, a 20 nm thick gold nano-film was deposited, and the etched polystyrene nano-beads were removed to realize the gold nano-mesh structure on the GaN substrate (Figure 1B). The fabricated Au nanomesh exhibited strong light absorption in the visible range due to the plasmonic resonance effect (Figure 1C). >
Furthermore, using a photoconductive atomic force microscopy (pc-AFM)-based photocurrent mapping system, the researchers analyzed the flow of hot holes in real time at the nanometer scale (one hundred-thousandth the thickness of a human hair). They observed that hot hole activation was strongest at "hot spots," where light was locally concentrated on the gold nanomesh. However, by modifying the growth direction of the gallium nitride substrate, hot hole activation extended beyond the hot spots to other areas as well.
Through this research, the team discovered an efficient method for converting light into electrical and chemical energy. This breakthrough is expected to significantly advance next-generation solar cells, photocatalysts, and hydrogen production technologies.
Professor Jeong Young Park stated, "For the first time, we have successfully controlled the flow of hot holes using a nanodiode technique. This innovation holds great potential for various optoelectronic devices and photocatalytic applications. For example, it could lead to groundbreaking advancements in solar energy conversion technologies, such as solar cells and hydrogen production. Additionally, the real-time analysis technology we developed can be applied to the development of ultra-miniaturized optoelectronic devices, including optical sensors and nanoscale semiconductor components."
The study was led by Hyunhwa Lee (PhD., KAIST Department of Chemistry) and Yujin Park (Postdoc Researcher, University of Texas at Austin Department of Chemical Engineering) as co-first authors and Professors Moonsang Lee (Inha University, Department of Materials Science and Engineering) and Jeong Young Park (KAIST, Department of Chemistry) serving as corresponding authors. The research findings were published online in Science Advances on March 7.
(Paper Title: “Reconfiguring hot-hole flux via polarity modulation of p-GaN in plasmonic Schottky architectures”, DOI: https://www.science.org/doi/10.1126/sciadv.adu0086)
This research was supported by the National Research Foundation of Korea (NRF).
2025.03.17 View 3893
KAIST Captures Hot Holes: A Breakthrough in Light-to-Electricity Energy Conversion
When light interacts with metallic nanostructures, it instantaneously generates plasmonic hot carriers, which serve as key intermediates for converting optical energy into high-value energy sources such as electricity and chemical energy. Among these, hot holes play a crucial role in enhancing photoelectrochemical reactions. However, they thermally dissipate within picoseconds (trillionths of a second), making practical applications challenging. Now, a Korean research team has successfully developed a method for sustaining hot holes longer and amplifying their flow, accelerating the commercialization of next-generation, high-efficiency, light-to-energy conversion technologies.
KAIST (represented by President Kwang Hyung Lee) announced on the 12th of March that a research team led by Distinguished Professor Jeong Young Park from the Department of Chemistry, in collaboration with Professor Moonsang Lee from the Department of Materials Science and Engineering at Inha University, has successfully amplified the flow of hot holes and mapped local current distribution in real time, thereby elucidating the mechanism of photocurrent enhancement.
The team designed a nanodiode structure by placing a metallic nanomesh on a specialized semiconductor substrate (p-type gallium nitride) to facilitate hot hole extraction at the surface. As a result, in gallium nitride substrates aligned with the hot hole extraction direction, the flow of hot holes was amplified by approximately two times compared to substrates aligned in other directions.
To fabricate the Au nanomesh, a polystyrene nano-bead monolayer assembly was first placed on a gallium nitride (p-GaN) substrate, and then the polystyrene nano-beads were etched to form a nanomesh template (Figure 1A). Then, a 20 nm thick gold nano-film was deposited, and the etched polystyrene nano-beads were removed to realize the gold nano-mesh structure on the GaN substrate (Figure 1B). The fabricated Au nanomesh exhibited strong light absorption in the visible range due to the plasmonic resonance effect (Figure 1C). >
Furthermore, using a photoconductive atomic force microscopy (pc-AFM)-based photocurrent mapping system, the researchers analyzed the flow of hot holes in real time at the nanometer scale (one hundred-thousandth the thickness of a human hair). They observed that hot hole activation was strongest at "hot spots," where light was locally concentrated on the gold nanomesh. However, by modifying the growth direction of the gallium nitride substrate, hot hole activation extended beyond the hot spots to other areas as well.
Through this research, the team discovered an efficient method for converting light into electrical and chemical energy. This breakthrough is expected to significantly advance next-generation solar cells, photocatalysts, and hydrogen production technologies.
Professor Jeong Young Park stated, "For the first time, we have successfully controlled the flow of hot holes using a nanodiode technique. This innovation holds great potential for various optoelectronic devices and photocatalytic applications. For example, it could lead to groundbreaking advancements in solar energy conversion technologies, such as solar cells and hydrogen production. Additionally, the real-time analysis technology we developed can be applied to the development of ultra-miniaturized optoelectronic devices, including optical sensors and nanoscale semiconductor components."
The study was led by Hyunhwa Lee (PhD., KAIST Department of Chemistry) and Yujin Park (Postdoc Researcher, University of Texas at Austin Department of Chemical Engineering) as co-first authors and Professors Moonsang Lee (Inha University, Department of Materials Science and Engineering) and Jeong Young Park (KAIST, Department of Chemistry) serving as corresponding authors. The research findings were published online in Science Advances on March 7.
(Paper Title: “Reconfiguring hot-hole flux via polarity modulation of p-GaN in plasmonic Schottky architectures”, DOI: https://www.science.org/doi/10.1126/sciadv.adu0086)
This research was supported by the National Research Foundation of Korea (NRF).
2025.03.17 View 3893 -
 KAIST develops a new, bone-like material that strengthens with use in collaboration with GIT
Materials used in apartment buildings, vehicles, and other structures deteriorate over time under repeated loads, leading to failure and breakage. A joint research team from Korea and the United States has successfully developed a bioinspired material that becomes stronger with use, taking inspiration from the way bones synthesize minerals from bodily fluids under stress, increasing bone density.
< (From left) Professor Sung Hoon Kang of the Department of Materials Science and Engineering, Johns Hopkins University Ph.D. candidates Bohan Sun and Grant Kitchen, Professor Yuhang Hu and Ph.D. candidate Dongjung He of Georgia Institute of Technology >
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of February that a research team led by Professor Sung Hoon Kang from the Department of Materials Science and Engineering, in collaboration with Johns Hopkins University and the Georgia Institute of Technology, had developed a new material that strengthens with repeated use, similar to how bones become stronger with exercise.
Professor Kang’s team sought to address the issue of conventional materials degrading with repeated use. Inspired by the biological process where stress triggers cells to form minerals that strengthen bones, the team developed a material that synthesizes minerals under stress without relying on cellular activity. This innovation is expected to enable applications in a variety of fields.
To replace the function of cells, the research team created a porous piezoelectric substrate that converts mechanical force into electricity and actually generates more charge under greater force. They then synthesized a composite material by infusing it with an electrolyte containing mineral components similar to those in blood.
< Figure 1. Schematic diagram of the biomimetic concept based on bone and pitcher plants, the reversible strengthening mechanism, the process of fabricating porous composites, the mechanical property changes with increasing stiffness and energy dissipation after cyclic loading, and the reprogrammable self-folding mechanism and applications >
After subjecting the material to periodic forces and measuring changes in its properties, they observed that its stiffness increased proportionally with the frequency and magnitude of stress and that its energy dissipation capability improved.
The reason for such properties was found to be due to minerals forming inside the porous material under repeated stress, as observed through micro-CT imaging of its internal structure. When subjected to large forces, these minerals fractured and dissipated energy, only to reform under further cyclic stress.
Unlike conventional materials that weaken with repeated use, this new material simultaneously enhances stiffness and impact absorption over time.
< Figure 2. Comparison of the changes in properties of the newly developed new material (LIPPS) with other materials under cyclic loading. (A) Graph showing the relative change rate of energy dissipation after cyclic loading and the relative change rate of elastic modulus upon unloading. LIPPS is in a new area that existing materials have not reached, and shows the characteristics of simultaneous increases in elastic modulus and energy dissipation. (B) Graph comparing the performance of LIPPS with current state-of-the-art mechanically adaptive materials. (Left) The maximum property change rate compared to the baseline after cyclic loading, LIPPS shows much higher changes in elastic modulus, dissipated energy density and ratio, toughness (impact resistance), and stored energy density than the existing adaptive materials. (Right) The absolute value range of the reported properties before and after cyclic loading shows that LIPPS has higher elastic modulus and toughness than the existing adaptive materials. >
Moreover, because its properties improve in proportion to the magnitude and frequency of applied stress, it can self-adjust to achieve mechanical property distributions suitable for different structural applications. It also possesses self-healing capabilities.
Professor Kang stated, "This newly developed material, which strengthens and absorbs impact better with repeated use compared to conventional materials, holds great potential for applications in artificial joints, as well as in aircraft, ships, automobiles, and structural engineering."
This study, with Professor Sung Hoon Kang as the corresponding author, was published in Science Advances (Vol. 11, Issue 6, February).
(Paper title: “A material dynamically enhancing both load-bearing and energy-dissipation capability under cyclic loading”) DOI: 10.1126/sciadv.adt3979
This research was conducted as a joint effort with Johns Hopkins University's Extreme Materials Institute and the Georgia Institute of Technology, supported by the National Research Foundation of Korea’s Brain Pool Plus program.
2025.02.22 View 3361
KAIST develops a new, bone-like material that strengthens with use in collaboration with GIT
Materials used in apartment buildings, vehicles, and other structures deteriorate over time under repeated loads, leading to failure and breakage. A joint research team from Korea and the United States has successfully developed a bioinspired material that becomes stronger with use, taking inspiration from the way bones synthesize minerals from bodily fluids under stress, increasing bone density.
< (From left) Professor Sung Hoon Kang of the Department of Materials Science and Engineering, Johns Hopkins University Ph.D. candidates Bohan Sun and Grant Kitchen, Professor Yuhang Hu and Ph.D. candidate Dongjung He of Georgia Institute of Technology >
KAIST (represented by President Kwang Hyung Lee) announced on the 20th of February that a research team led by Professor Sung Hoon Kang from the Department of Materials Science and Engineering, in collaboration with Johns Hopkins University and the Georgia Institute of Technology, had developed a new material that strengthens with repeated use, similar to how bones become stronger with exercise.
Professor Kang’s team sought to address the issue of conventional materials degrading with repeated use. Inspired by the biological process where stress triggers cells to form minerals that strengthen bones, the team developed a material that synthesizes minerals under stress without relying on cellular activity. This innovation is expected to enable applications in a variety of fields.
To replace the function of cells, the research team created a porous piezoelectric substrate that converts mechanical force into electricity and actually generates more charge under greater force. They then synthesized a composite material by infusing it with an electrolyte containing mineral components similar to those in blood.
< Figure 1. Schematic diagram of the biomimetic concept based on bone and pitcher plants, the reversible strengthening mechanism, the process of fabricating porous composites, the mechanical property changes with increasing stiffness and energy dissipation after cyclic loading, and the reprogrammable self-folding mechanism and applications >
After subjecting the material to periodic forces and measuring changes in its properties, they observed that its stiffness increased proportionally with the frequency and magnitude of stress and that its energy dissipation capability improved.
The reason for such properties was found to be due to minerals forming inside the porous material under repeated stress, as observed through micro-CT imaging of its internal structure. When subjected to large forces, these minerals fractured and dissipated energy, only to reform under further cyclic stress.
Unlike conventional materials that weaken with repeated use, this new material simultaneously enhances stiffness and impact absorption over time.
< Figure 2. Comparison of the changes in properties of the newly developed new material (LIPPS) with other materials under cyclic loading. (A) Graph showing the relative change rate of energy dissipation after cyclic loading and the relative change rate of elastic modulus upon unloading. LIPPS is in a new area that existing materials have not reached, and shows the characteristics of simultaneous increases in elastic modulus and energy dissipation. (B) Graph comparing the performance of LIPPS with current state-of-the-art mechanically adaptive materials. (Left) The maximum property change rate compared to the baseline after cyclic loading, LIPPS shows much higher changes in elastic modulus, dissipated energy density and ratio, toughness (impact resistance), and stored energy density than the existing adaptive materials. (Right) The absolute value range of the reported properties before and after cyclic loading shows that LIPPS has higher elastic modulus and toughness than the existing adaptive materials. >
Moreover, because its properties improve in proportion to the magnitude and frequency of applied stress, it can self-adjust to achieve mechanical property distributions suitable for different structural applications. It also possesses self-healing capabilities.
Professor Kang stated, "This newly developed material, which strengthens and absorbs impact better with repeated use compared to conventional materials, holds great potential for applications in artificial joints, as well as in aircraft, ships, automobiles, and structural engineering."
This study, with Professor Sung Hoon Kang as the corresponding author, was published in Science Advances (Vol. 11, Issue 6, February).
(Paper title: “A material dynamically enhancing both load-bearing and energy-dissipation capability under cyclic loading”) DOI: 10.1126/sciadv.adt3979
This research was conducted as a joint effort with Johns Hopkins University's Extreme Materials Institute and the Georgia Institute of Technology, supported by the National Research Foundation of Korea’s Brain Pool Plus program.
2025.02.22 View 3361 -
 The World’s First Hacking-preventing Cryptographic Semiconductor Chip
With the dramatic increase in the amount of information exchanged between components or devices in the 5G/6G era, such as for the Internet of Things (IoT) and autonomous driving, hacking attacks are becoming more sophisticated. Consequently, enhancing security functions is essential for safely transmitting data between and among devices.
On February 29th, a KAIST research team led by Professors Yang-gyu Choi and Seung-tak Ryu from the School of Electrical Engineering announced the successful development of the world's first security cryptographic semiconductor.
The team has developed the Cryptoristor, a cryptographic transistor based on FinFET technology, produced through a 100% silicon-compatible process, for the first time in the world. Cryptoristor is a random number generator (RNG) with unparalleled characteristics, featuring a unique structure comprising a single transistor and a distinctive mechanism.
In all security environments, including artificial intelligence, the most crucial element is the RNG. In the most commonly used security chip, the Advanced Encryption Standard (AES), the RNG is a core component, occupying approximately 75% of the total chip area and more than 85% of its energy consumption. Hence, there is an urgent need for the development of low-power/ultra-small RNGs suitable for mobile or IoT devices.
Existing RNGs come with limitations as they lack compatibility with silicon CMOS processes and circuit-based RNGs occupy a large surface area.
In contrast, the team’s newly developed Cryptoristor, a cryptographic semiconductor based on a single-component structure, consumes and occupies less than .001 of the power and area compared to the current chips being used. Utilizing the inherent randomness of FinFETs, fabricated on a Silicon-on-Insulator (SOI) substrate with an insulating layer formed beneath the silicon, the team developed an RNG that unpredictably produces zeroes and ones.
< Figure 1. Conceptual diagram of the security cryptographic transistor device. >
Generally speaking, preventing hackers from predicting the encrypted algorithms during data exchanges through mobile devices is pivotal. Therefore, this method ensures unpredictability by generating random sequences of zeroes and ones that change every time.
Moreover, while the Cryptoristor-based RNG research is the world's first of its kind without any international implementation cases, it shares the same transistor structure as existing logic or memory components. This enables 100% production through rapid mass production processes using existing semiconductor facilities at a low cost.
Seung-il Kim, a PhD student who led the research, explained the significance of the study, stating, "As a cryptographic semiconductor, the ultra-small/low-power random number generator enhances security through its distinctive unpredictability, supporting safe hyperconnectivity with secure transmissions between chips or devices. Particularly, compared to previous research, it offers excellent advantages in terms of energy consumption, integration density, and cost, making it suitable for IoT device environments."
This research, with master’s student Hyung-jin Yoo as the co-author, was officially published in the online edition of Science Advances, a sister journal of Science, in February 2024 (research paper title: Cryptographic transistor for true random number generator with low power consumption).
This research received support from the Next-Generation Intelligent Semiconductor Technology Development Project and the Core Technology Development Project for the National Semiconductor Research Laboratory.
2024.03.07 View 8961
The World’s First Hacking-preventing Cryptographic Semiconductor Chip
With the dramatic increase in the amount of information exchanged between components or devices in the 5G/6G era, such as for the Internet of Things (IoT) and autonomous driving, hacking attacks are becoming more sophisticated. Consequently, enhancing security functions is essential for safely transmitting data between and among devices.
On February 29th, a KAIST research team led by Professors Yang-gyu Choi and Seung-tak Ryu from the School of Electrical Engineering announced the successful development of the world's first security cryptographic semiconductor.
The team has developed the Cryptoristor, a cryptographic transistor based on FinFET technology, produced through a 100% silicon-compatible process, for the first time in the world. Cryptoristor is a random number generator (RNG) with unparalleled characteristics, featuring a unique structure comprising a single transistor and a distinctive mechanism.
In all security environments, including artificial intelligence, the most crucial element is the RNG. In the most commonly used security chip, the Advanced Encryption Standard (AES), the RNG is a core component, occupying approximately 75% of the total chip area and more than 85% of its energy consumption. Hence, there is an urgent need for the development of low-power/ultra-small RNGs suitable for mobile or IoT devices.
Existing RNGs come with limitations as they lack compatibility with silicon CMOS processes and circuit-based RNGs occupy a large surface area.
In contrast, the team’s newly developed Cryptoristor, a cryptographic semiconductor based on a single-component structure, consumes and occupies less than .001 of the power and area compared to the current chips being used. Utilizing the inherent randomness of FinFETs, fabricated on a Silicon-on-Insulator (SOI) substrate with an insulating layer formed beneath the silicon, the team developed an RNG that unpredictably produces zeroes and ones.
< Figure 1. Conceptual diagram of the security cryptographic transistor device. >
Generally speaking, preventing hackers from predicting the encrypted algorithms during data exchanges through mobile devices is pivotal. Therefore, this method ensures unpredictability by generating random sequences of zeroes and ones that change every time.
Moreover, while the Cryptoristor-based RNG research is the world's first of its kind without any international implementation cases, it shares the same transistor structure as existing logic or memory components. This enables 100% production through rapid mass production processes using existing semiconductor facilities at a low cost.
Seung-il Kim, a PhD student who led the research, explained the significance of the study, stating, "As a cryptographic semiconductor, the ultra-small/low-power random number generator enhances security through its distinctive unpredictability, supporting safe hyperconnectivity with secure transmissions between chips or devices. Particularly, compared to previous research, it offers excellent advantages in terms of energy consumption, integration density, and cost, making it suitable for IoT device environments."
This research, with master’s student Hyung-jin Yoo as the co-author, was officially published in the online edition of Science Advances, a sister journal of Science, in February 2024 (research paper title: Cryptographic transistor for true random number generator with low power consumption).
This research received support from the Next-Generation Intelligent Semiconductor Technology Development Project and the Core Technology Development Project for the National Semiconductor Research Laboratory.
2024.03.07 View 8961 -
 KAIST Research Team Develops Sweat-Resistant Wearable Robot Sensor
New electromyography (EMG) sensor technology that allows the long-term stable control of wearable robots and is not affected by the wearer’s sweat and dead skin has gained attention recently. Wearable robots are devices used across a variety of rehabilitation treatments for the elderly and patients recovering from stroke or trauma.
A joint research team led by Professor Jae-Woong Jung from the KAIST School of Electrical Engineering (EE) and Professor Jung Kim from the KAIST Department of Mechanical Engineering (ME) announced on January 23rd that they have successfully developed a stretchable and adhesive microneedle sensor that can electrically sense physiological signals at a high level without being affected by the state of the user’s skin.
For wearable robots to recognize the intentions behind human movement for their use in rehabilitation treatment, they require a wearable electrophysiological sensor that gives precise EMG measurements. However, existing sensors often show deteriorating signal quality over time and are greatly affected by the user’s skin conditions. Furthermore, the sensor’s higher mechanical hardness causes noise since the contact surface is unable to keep up with the deformation of the skin. These shortcomings limit the reliable, long-term control of wearable robots.
< Figure 1. Design and working concept of the Stretchable microNeedle Adhesive Patch (SNAP). (A) Schematic illustration showing the overall system configuration and application of SNAP. (B) Exploded view schematic diagram of a SNAP, consisting of stretchable serpentine interconnects, Au-coated Si microneedle, and ECA made of Ag flakes–silicone composite. (C) Optical images showing high mechanical compliance of SNAP. >
However, the recently developed technology is expected to allow long-term and high-quality EMG measurements as it uses a stretchable and adhesive conducting substrate integrated with microneedle arrays that can easily penetrate the stratum corneum without causing discomfort. Through its excellent performance, the sensor is anticipated to be able to stably control wearable robots over a long period of time regardless of the wearer’s changing skin conditions and without the need for a preparation step that removes sweat and dead cells from the surface of their skin.
The research team created a stretchable and adhesive microneedle sensor by integrating microneedles into a soft silicon polymer substrate. The hard microneedles penetrate through the stratum corneum, which has high electrical resistance. As a result, the sensor can effectively lower contact resistance with the skin and obtain high-quality electrophysiological signals regardless of contamination. At the same time, the soft and adhesive conducting substrate can adapt to the skin’s surface that stretches with the wearer’s movement, providing a comfortable fit and minimizing noise caused by movement.
< Figure 2. Demonstration of the wireless Stretchable microNeedle Adhesive Patch (SNAP) system as an Human-machine interfaces (HMI) for closed-loop control of an exoskeleton robot. (A) Illustration depicting the system architecture and control strategy of an exoskeleton robot. (B) The hardware configuration of the pneumatic back support exoskeleton system. (C) Comparison of root mean square (RMS) of electromyography (EMG) with and without robotic assistance of pretreated skin and non-pretreated skin. >
To verify the usability of the new patch, the research team conducted a motion assistance experiment using a wearable robot. They attached the microneedle patch on a user’s leg, where it could sense the electrical signals generated by the muscle. The sensor then sent the detected intention to a wearable robot, allowing the robot to help the wearer lift a heavy object more easily.
Professor Jae-Woong Jung, who led the research, said, “The developed stretchable and adhesive microneedle sensor can stability detect EMG signals without being affected by the state of a user’s skin. Through this, we will be able to control wearable robots with higher precision and stability, which will help the rehabilitation of patients who use robots.”
The results of this research, written by co-first authors Heesoo Kim and Juhyun Lee, who are both Ph.D. candidates in the KAIST School of EE, were published in Science Advances on January 17th under the title “Skin-preparation-free, stretchable microneedle adhesive patches for reliable electrophysiological sensing and exoskeleton robot control”.
This research was supported by the Bio-signal Sensor Integrated Technology Development Project by the National Research Foundation of Korea, the Electronic Medicinal Technology Development Project, and the Step 4 BK21 Project.
2024.01.30 View 8811
KAIST Research Team Develops Sweat-Resistant Wearable Robot Sensor
New electromyography (EMG) sensor technology that allows the long-term stable control of wearable robots and is not affected by the wearer’s sweat and dead skin has gained attention recently. Wearable robots are devices used across a variety of rehabilitation treatments for the elderly and patients recovering from stroke or trauma.
A joint research team led by Professor Jae-Woong Jung from the KAIST School of Electrical Engineering (EE) and Professor Jung Kim from the KAIST Department of Mechanical Engineering (ME) announced on January 23rd that they have successfully developed a stretchable and adhesive microneedle sensor that can electrically sense physiological signals at a high level without being affected by the state of the user’s skin.
For wearable robots to recognize the intentions behind human movement for their use in rehabilitation treatment, they require a wearable electrophysiological sensor that gives precise EMG measurements. However, existing sensors often show deteriorating signal quality over time and are greatly affected by the user’s skin conditions. Furthermore, the sensor’s higher mechanical hardness causes noise since the contact surface is unable to keep up with the deformation of the skin. These shortcomings limit the reliable, long-term control of wearable robots.
< Figure 1. Design and working concept of the Stretchable microNeedle Adhesive Patch (SNAP). (A) Schematic illustration showing the overall system configuration and application of SNAP. (B) Exploded view schematic diagram of a SNAP, consisting of stretchable serpentine interconnects, Au-coated Si microneedle, and ECA made of Ag flakes–silicone composite. (C) Optical images showing high mechanical compliance of SNAP. >
However, the recently developed technology is expected to allow long-term and high-quality EMG measurements as it uses a stretchable and adhesive conducting substrate integrated with microneedle arrays that can easily penetrate the stratum corneum without causing discomfort. Through its excellent performance, the sensor is anticipated to be able to stably control wearable robots over a long period of time regardless of the wearer’s changing skin conditions and without the need for a preparation step that removes sweat and dead cells from the surface of their skin.
The research team created a stretchable and adhesive microneedle sensor by integrating microneedles into a soft silicon polymer substrate. The hard microneedles penetrate through the stratum corneum, which has high electrical resistance. As a result, the sensor can effectively lower contact resistance with the skin and obtain high-quality electrophysiological signals regardless of contamination. At the same time, the soft and adhesive conducting substrate can adapt to the skin’s surface that stretches with the wearer’s movement, providing a comfortable fit and minimizing noise caused by movement.
< Figure 2. Demonstration of the wireless Stretchable microNeedle Adhesive Patch (SNAP) system as an Human-machine interfaces (HMI) for closed-loop control of an exoskeleton robot. (A) Illustration depicting the system architecture and control strategy of an exoskeleton robot. (B) The hardware configuration of the pneumatic back support exoskeleton system. (C) Comparison of root mean square (RMS) of electromyography (EMG) with and without robotic assistance of pretreated skin and non-pretreated skin. >
To verify the usability of the new patch, the research team conducted a motion assistance experiment using a wearable robot. They attached the microneedle patch on a user’s leg, where it could sense the electrical signals generated by the muscle. The sensor then sent the detected intention to a wearable robot, allowing the robot to help the wearer lift a heavy object more easily.
Professor Jae-Woong Jung, who led the research, said, “The developed stretchable and adhesive microneedle sensor can stability detect EMG signals without being affected by the state of a user’s skin. Through this, we will be able to control wearable robots with higher precision and stability, which will help the rehabilitation of patients who use robots.”
The results of this research, written by co-first authors Heesoo Kim and Juhyun Lee, who are both Ph.D. candidates in the KAIST School of EE, were published in Science Advances on January 17th under the title “Skin-preparation-free, stretchable microneedle adhesive patches for reliable electrophysiological sensing and exoskeleton robot control”.
This research was supported by the Bio-signal Sensor Integrated Technology Development Project by the National Research Foundation of Korea, the Electronic Medicinal Technology Development Project, and the Step 4 BK21 Project.
2024.01.30 View 8811 -
 KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 11137
KAIST develops an artificial muscle device that produces force 34 times its weight
- Professor IlKwon Oh’s research team in KAIST’s Department of Mechanical Engineering developed a soft fluidic switch using an ionic polymer artificial muscle that runs with ultra-low power to lift objects 34 times greater than its weight.
- Its light weight and small size make it applicable to various industrial fields such as soft electronics, smart textiles, and biomedical devices by controlling fluid flow with high precision, even in narrow spaces.
Soft robots, medical devices, and wearable devices have permeated our daily lives. KAIST researchers have developed a fluid switch using ionic polymer artificial muscles that operates at ultra-low power and produces a force 34 times greater than its weight. Fluid switches control fluid flow, causing the fluid to flow in a specific direction to invoke various movements.
KAIST (President Kwang-Hyung Lee) announced on the 4th of January that a research team under Professor IlKwon Oh from the Department of Mechanical Engineering has developed a soft fluidic switch that operates at ultra-low voltage and can be used in narrow spaces.
Artificial muscles imitate human muscles and provide flexible and natural movements compared to traditional motors, making them one of the basic elements used in soft robots, medical devices, and wearable devices. These artificial muscles create movements in response to external stimuli such as electricity, air pressure, and temperature changes, and in order to utilize artificial muscles, it is important to control these movements precisely.
Switches based on existing motors were difficult to use within limited spaces due to their rigidity and large size. In order to address these issues, the research team developed an electro-ionic soft actuator that can control fluid flow while producing large amounts of force, even in a narrow pipe, and used it as a soft fluidic switch.
< Figure 1. The separation of fluid droplets using a soft fluid switch at ultra-low voltage. >
The ionic polymer artificial muscle developed by the research team is composed of metal electrodes and ionic polymers, and it generates force and movement in response to electricity. A polysulfonated covalent organic framework (pS-COF) made by combining organic molecules on the surface of the artificial muscle electrode was used to generate an impressive amount of force relative to its weight with ultra-low power (~0.01V).
As a result, the artificial muscle, which was manufactured to be as thin as a hair with a thickness of 180 µm, produced a force more than 34 times greater than its light weight of 10 mg to initiate smooth movement. Through this, the research team was able to precisely control the direction of fluid flow with low power.
< Figure 2. The synthesis and use of pS-COF as a common electrode-electrolyte host for electroactive soft fluid switches. A) The synthesis schematic of pS-COF. B) The schematic diagram of the operating principle of the electrochemical soft switch. C) The schematic diagram of using a pS-COF-based electrochemical soft switch to control fluid flow in dynamic operation. >
Professor IlKwon Oh, who led this research, said, “The electrochemical soft fluidic switch that operate at ultra-low power can open up many possibilities in the fields of soft robots, soft electronics, and microfluidics based on fluid control.” He added, “From smart fibers to biomedical devices, this technology has the potential to be immediately put to use in a variety of industrial settings as it can be easily applied to ultra-small electronic systems in our daily lives.”
The results of this study, in which Dr. Manmatha Mahato, a research professor in the Department of Mechanical Engineering at KAIST, participated as the first author, were published in the international academic journal Science Advances on December 13, 2023. (Paper title: Polysulfonated Covalent Organic Framework as Active Electrode Host for Mobile Cation Guests in Electrochemical Soft Actuator)
This research was conducted with support from the National Research Foundation of Korea's Leader Scientist Support Project (Creative Research Group) and Future Convergence Pioneer Project.
* Paper DOI: https://www.science.org/doi/abs/10.1126/sciadv.adk9752
2024.01.11 View 11137 -
 Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13379
Tinkering with Roundworm Proteins Offers Hope for Anti-aging Drugs
- The somatic nuclear protein kinase VRK-1 increases the worm’s lifespan through AMPK activation, and this mechanism can be applied to promoting human longevity, the study reveals. -
KAIST researchers have been able to dial up and down creatures’ lifespans by altering the activity of proteins found in roundworm cells that tell them to convert sugar into energy when their cellular energy is running low. Humans also have these proteins, offering up the intriguing possibilities for developing longevity-promoting drugs. These new findings were published on July 1 in Science Advances.
The roundworm Caenorhabditis elegans (C. elegans), a millimeter-long nematode commonly used in lab testing, enjoyed a boost in its lifespan when researchers tinkered with a couple of proteins involved in monitoring the energy use by its cells.
The proteins VRK-1 and AMPK work in tandem in roundworm cells, with the former telling the latter to get to work by sticking a phosphate molecule, composed of one phosphorus and four oxygen atoms, on it. In turn, AMPK’s role is to monitor energy levels in cells, when cellular energy is running low. In essence, VRK-1 regulates AMPK, and AMPK regulates the cellular energy status.
Using a range of different biological research tools, including introducing foreign genes into the worm, a group of researchers led by Professor Seung-Jae V. Lee from the Department of Biological Sciences at KAIST were able to dial up and down the activity of the gene that tells cells to produce the VRK-1 protein. This gene has remained pretty much unchanged throughout evolution. Most complex organisms have this same gene, including humans.
Lead author of the study Sangsoon Park and his colleagues confirmed that the overexpression, or increased production, of the VRK-1 protein boosted the lifespan of the C. elegans, which normally lives just two to three weeks, and the inhibition of VRK-1 production reduced its lifespan.
The research team found that the activity of the VRK-1-to-AMPK cellular-energy monitoring process is increased in low cellular energy status by reduced mitochondrial respiration, the set of metabolic chemical reactions that make use of the oxygen the worm breathes to convert macronutrients from food into the energy “currency” that cells spend to do everything they need to do.
It is already known that mitochondria, the energy-producing engine rooms in cells, play a crucial role in aging, and declines in the functioning of mitochondria are associated with age-related diseases. At the same time, the mild inhibition of mitochondrial respiration has been shown to promote longevity in a range of species, including flies and mammals.
When the research team performed similar tinkering with cultured human cells, they found they could also replicate this ramping up and down of the VRK-1-to-AMPK process that occurs in roundworms.
“This raises the intriguing possibility that VRK-1 also functions as a factor in governing human longevity, and so perhaps we can start developing longevity-promoting drugs that alter the activity of VRK-1,” explained Professor Lee.
At the very least, the research points us in an interesting direction for investigating new therapeutic strategies to combat metabolic disorders by targeting the modulation of VRK-1. Metabolic disorders involve the disruption of chemical reactions in the body, including diseases of the mitochondria.
But before metabolic disorder therapeutics or longevity drugs can be contemplated by scientists, further research still needs to be carried out to better understand how VRK-1 works to activate AMPK, as well as figure out the precise mechanics of how AMPK controls cellular energy.
This work was supported by the National Research Foundation (NRF), and the Ministry of Science and ICT (MSIT) of Korea.
Image credit: Seung-Jae V. LEE, KAIST.
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Park, S., et al. (2020) ‘VRK-1 extends life span by activation of AMPK via phosphorylation’. Science Advances, Volume 6. No. 27, eaaw7824. Available online at https://doi.org/10.1126/sciadv.aaw7824
Profile: Seung-Jae V. Lee, Ph.D.
Professor
seungjaevlee@kaist.ac.kr
https://sites.google.com/view/mgakaist
Molecular Genetics of Aging Laboratory
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.krDaejeon 34141, Korea
(END)
2020.07.31 View 13379 -
 A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 14698
A Study Finds Neuropeptide Somatostatin Enhances Visual Processing
Researchers have confirmed that neuropeptide somatostatin can improve cognitive function in the brain. A research group of Professor Seung-Hee Lee from the Department of Biological Sciences at KAIST found that the application of neuropeptide somatostatin improves visual processing and cognitive behaviors by reducing excitatory inputs to parvalbumin-positive interneurons in the cortex.
This study, reported at Science Advances on April 22nd (EST), sheds a new light on the therapeutics of neurodegenerative diseases. According to a recent study in Korea, one in ten seniors over 65 is experiencing dementia-related symptoms in their daily lives such like memory loss, cognitive decline, and motion function disorders. Professor Lee believes that somatostatin treatment can be directly applied to the recovery of cognitive functions in Alzheimer’s disease patients.
Professor Lee started this study noting the fact that the level of somatostatin expression was dramatically decreased in the cerebral cortex and cerebrospinal fluid of Alzheimer’s disease patients
Somatostatin-expressing neurons in the cortex are known to exert the dendritic inhibition of pyramidal neurons via GABAergic transmission. Previous studies focused on their inhibitory effects on cortical circuits, but somatostatin-expressing neurons can co-release somatostatin upon activation. Despite the abundant expression of somatostatin and its receptors in the cerebral cortex, it was not known if somatostatin could modulate cognitive processing in the cortex.
The research team demonstrated that the somatostatin treatment into the cerebral cortex could enhance visual processing and cognitive behaviors in mice. The research team combined behaviors, in vivo and in vitro electrophysiology, and electron microscopy techniques to reveal how the activation of somatostatin receptors in vivo enhanced the ability of visual recognition in animals. Interestingly, somatostatin release can reduce excitatory synaptic transmission to another subtype of GABAergic interneurons, parvalbumin (PV)-expressing neurons.
As somatostatin is a stable and safe neuropeptide expressed naturally in the mammalian brain, it was safe to be injected into the cortex and cerebrospinal fluid, showing a potential application to drug development for curing cognitive disorders in humans.
Professor Lee said, “Our research confirmed the key role of the neuropeptide SST in modulating cortical function and enhancing cognitive ability in the mammalian brain. I hope new drugs can be developed based on the function of somatostatin to treat cognitive disabilities in many patients suffering from neurological disorders.”
This study was supported by the National Research Foundation of Korea.
Publication:
Song, Y. H et al. (2020) ‘Somatostatin enhances visual processing and perception by suppressing excitatory inputs to parvalbumin-positive interneurons in V1’, Science Advances, 6(17). Available online at https://doi.org/10.1126/sciadv.aaz0517
Profile:
Seung-Hee Lee
Associate Professor
shlee1@kaist.ac.kr
https://sites.google.com/site/leelab2013/
Sensory Processing Lab (SPL)
Department of Biological Sciences (BIO)
Korea Advanced Institute of Science and Technology (KAIST)
Profile:
You-Hyang Song
Researcher (Ph.D.)
dbgidtm17@kaist.ac.kr
SPL, KAIST BIO
Profile:
Yang-Sun Hwang
Researcher (M.S.)
hys940129@kaist.ac.kr
SPL, KAIST BIO
(END)
2020.04.23 View 14698 -
 What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 16876
What Fuels a “Domino Effect” in Cancer Drug Resistance?
KAIST researchers have identified mechanisms that relay prior acquired resistance to the first-line chemotherapy to the second-line targeted therapy, fueling a “domino effect” in cancer drug resistance. Their study featured in the February 7 edition of Science Advances suggests a new strategy for improving the second-line setting of cancer treatment for patients who showed resistance to anti-cancer drugs.
Resistance to cancer drugs is often managed in the clinic by chemotherapy and targeted therapy. Unlike chemotherapy that works by repressing fast-proliferating cells, targeted therapy blocks a single oncogenic pathway to halt tumor growth. In many cases, targeted therapy is engaged as a maintenance therapy or employed in the second-line after front-line chemotherapy.
A team of researchers led by Professor Yoosik Kim from the Department of Chemical and Biomolecular Engineering and the KAIST Institute for Health Science and Technology (KIHST) has discovered an unexpected resistance signature that occurs between chemotherapy and targeted therapy. The team further identified a set of integrated mechanisms that promotes this kind of sequential therapy resistance.
“There have been multiple clinical accounts reflecting that targeted therapies tend to be least successful in patients who have exhausted all standard treatments,” said the first author of the paper Mark Borris D. Aldonza. He continued, “These accounts ignited our hypothesis that failed responses to some chemotherapies might speed up the evolution of resistance to other drugs, particularly those with specific targets.”
Aldonza and his colleagues extracted large amounts of drug-resistance information from the open-source database the Genomics of Drug Sensitivity in Cancer (GDSC), which contains thousands of drug response data entries from various human cancer cell lines. Their big data analysis revealed that cancer cell lines resistant to chemotherapies classified as anti-mitotic drugs (AMDs), toxins that inhibit overacting cell division, are also resistant to a class of targeted therapies called epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs).
In all of the cancer types analyzed, more than 84 percent of those resistant to AMDs, representatively ‘paclitaxel’, were also resistant to at least nine EGFR-TKIs. In lung, pancreatic, and breast cancers where paclitaxel is often used as a first-line, standard-of-care regimen, greater than 92 percent showed resistance to EGFR-TKIs. Professor Kim said, “It is surprising to see that such collateral resistance can occur specifically between two chemically different classes of drugs.”
To figure out how failed responses to paclitaxel leads to resistance to EGFR-TKIs, the team validated co-resistance signatures that they found in the database by generating and analyzing a subset of slow-doubling, paclitaxel-resistant cancer models called ‘persisters’.
The results demonstrated that paclitaxel-resistant cancers remodel their stress response by first becoming more stem cell-like, evolving the ability to self-renew to adapt to more stressful conditions like drug exposures. More surprisingly, when the researchers characterized the metabolic state of the cells, EGFR-TKI persisters derived from paclitaxel-resistant cancer cells showed high dependencies to energy-producing processes such as glycolysis and glutaminolysis.
“We found that, without an energy stimulus like glucose, these cells transform to becoming more senescent, a characteristic of cells that have arrested cell division. However, this senescence is controlled by stem cell factors, which the paclitaxel-resistant cancers use to escape from this arrested state given a favorable condition to re-grow,” said Aldonza.
Professor Kim explained, “Before this research, there was no reason to expect that acquiring the cancer stem cell phenotype that dramatically leads to a cascade of changes in cellular states affecting metabolism and cell death is linked with drug-specific sequential resistance between two classes of therapies.”
He added, “The expansion of our work to other working models of drug resistance in a much more clinically-relevant setting, perhaps in clinical trials, will take on increasing importance, as sequential treatment strategies will continue to be adapted to various forms of anti-cancer therapy regimens.”
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF-2016R1C1B2009886), and the KAIST Future Systems Healthcare Project (KAISTHEALTHCARE42) funded by the Korean Ministry of Science and ICT (MSIT). Undergraduate student Aldonza participated in this research project and presented the findings as the lead author as part of the Undergraduate Research Participation (URP) Program at KAIST.
< Figure 1. Schematic overview of the study. >
< Figure 2. Big data analysis revealing co-resistance signatures between classes of anti-cancer drugs. >
Publication:
Aldonza et al. (2020) Prior acquired resistance to paclitaxel relays diverse EGFR-targeted therapy persistence mechanisms. Science Advances, Vol. 6, No. 6, eaav7416. Available online at http://dx.doi.org/10.1126/sciadv.aav7416
Profile: Prof. Yoosik Kim, MA, PhD
ysyoosik@kaist.ac.kr
https://qcbio.kaist.ac.kr/
Assistant Professor
Bio Network Analysis Laboratory
Department of Chemical and Biomolecular Engineering
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
Profile: Mark Borris D. Aldonza
borris@kaist.ac.kr
Undergraduate Student
Department of Biological Sciences
Korea Advanced Institute of Science and Technology (KAIST)
http://kaist.ac.kr
Daejeon, Republic of Korea
(END)
2020.02.10 View 16876