Immunity
-
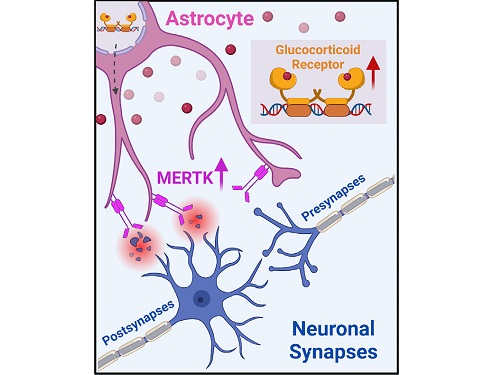 A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 9538
A KAIST research team identifies a cause of mental diseases induced by childhood abuse
Childhood neglect and/or abuse can induce extreme stress that significantly changes neural networks and functions during growth. This can lead to mental illnesses, including depression and schizophrenia, but the exact mechanism and means to control it were yet to be discovered.
On August 1, a KAIST research team led by Professor Won-Suk Chung from the Department of Biological Sciences announced the identification of excessive synapse removal mediated by astrocytes as the cause of mental diseases induced by childhood abuse trauma. Their research was published in Immunity, a top international journal in the field of immunology.
The research team discovered that the excessive astrocyte-mediated removal of excitatory synapses in the brain in response to stress hormones is a cause of mental diseases induced by childhood neglect and abuse. Clinical data have previously shown that high levels of stress can lead to various mental diseases, but the exact mechanism has been unknown. The results of this research therefore are expected to be widely applied to the prevention and treatment of such diseases.
The research team clinically screened an FDA-approved drug to uncover the mechanism that regulates the phagocytotic role of astrocytes, in which they capture external substances and eliminate them. As a result, the team found that synthetic glucocorticoids, namely stress hormones, enhanced astrocyte-mediated phagocytosis to an abnormal level. Glucocorticoids play essential roles in processes that maintain life, such as carbohydrate metabolism and anti-inflammation, but are also secreted in response to external stimuli such as stress, allowing the body to respond appropriately. However, excessive and long-term exposure to glucocorticoids caused by chronic stress can lead to various mental diseases including depression, cognitive disorders, and anxiety.
< Figure 1. Results of screening for compounds that increase astrocyte phagocytosis
(A) Discovered that synthetic glucocorticoid (stress hormone) increases the phagocytosis of astrocytes through screening of FDA-approved clinical compounds. (B-C) When treated with stress hormones, the phagocytosis of astrocytes is greatly increased, but this phenomenon is strongly suppressed by the GR antagonist (Mifepristone). CORT: corticosterone (stress hormone), Eplerenone: mineralocorticoid receptor (MR) antagonist, Mifepristone: glucocorticoid receptor (GR) antagonist >
To understand the changes in astrocyte functions caused by childhood stress, the research team used mice models with early social deprivation, and discovered that stress hormones bind to the glucocorticoid receptors (GRs) of astrocytes. This significantly increased the expression of Mer tyrosine kinase (MERK), which plays an essential role in astrocyte phagocytosis. Surprisingly, out of the various neurons in the cerebral cortex, astrocytes would eliminate only the excitatory synapses of specific neurons. The team found that this builds abnormal neural networks, which can lead to complex behavioral abnormalities such as social deficiencies and depression in adulthood.
The team also observed that microglia, which also play an important role in cerebral immunity, did not contribute to synapse removal in the mice models with early social deprivation. This confirms that the response to stress hormones during childhood is specifically astrocyte-mediated.
To find out whether these results are also applicable in humans, the research team used a brain organoid grown from human-induced pluripotent stem cells to observe human responses to stress hormones. The team observed that the stress hormones induced astrocyte GRs and phagocyte activation in the human brain organoid as well, and confirmed that the astrocytes subsequently eliminated excessive amounts of excitatory synapses. By showing that mice and humans both showed the same synapse control mechanism in response to stress, the team suggested that this discovery is applicable to mental disorders in humans.
< Figure 2. A schematic diagram of the study published in Immunity. Excessive stress hormone secretion in childhood increases the expression of the MERTK phagocytic receptor through the glucocorticoid receptor (GR) of astrocytes, resulting in excessive elimination of excitatory synapses. Excessive synaptic elimination by astrocytes during brain development causes permanent damage to brain circuits, resulting in abnormal neural activity in the adult brain and psychiatric behaviors such as depression and anti-social tendencies. >
Prof. Won-Suk Chung said, “Until now, we did not know the exact mechanism for how childhood stress caused brain diseases. This research was the first to show that the excessive phagocytosis of astrocytes could be an important cause of such diseases.” He added, “In the future, controlling the immune response of astrocytes will be used as a fundamental target for understanding and treating brain diseases.”
This research, written by co-first authors Youkyeong Byun (Ph.D. candidate) and Nam-Shik Kim (post-doctoral associate) from the KAIST Department of Biological Sciences, was published in the internationally renowned journal Immunity, a sister magazine of Cell and one of the best journal in the field of immunology, on July 31 under the title "Stress induces behavioral abnormalities by increasing expression of phagocytic receptor MERTK in astrocytes to promote synapse phagocytosis."
This work was supported by a National Research Foundation of Korea grant, the Korea Health Industry Development Institute (KHIDI), and the Korea Dementia Research Center (KDRC).
2023.08.04 View 9538 -
 Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 16582
Study of T Cells from COVID-19 Convalescents Guides Vaccine Strategies
Researchers confirm that most COVID-19 patients in their convalescent stage carry stem cell-like memory T cells for months
A KAIST immunology research team found that most convalescent patients of COVID-19 develop and maintain T cell memory for over 10 months regardless of the severity of their symptoms. In addition, memory T cells proliferate rapidly after encountering their cognate antigen and accomplish their multifunctional roles. This study provides new insights for effective vaccine strategies against COVID-19, considering the self-renewal capacity and multipotency of memory T cells.
COVID-19 is a disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection. When patients recover from COVID-19, SARS-CoV-2-specific adaptive immune memory is developed. The adaptive immune system consists of two principal components: B cells that produce antibodies and T cells that eliminate infected cells. The current results suggest that the protective immune function of memory T cells will be implemented upon re-exposure to SARS-CoV-2.
Recently, the role of memory T cells against SARS-CoV-2 has been gaining attention as neutralizing antibodies wane after recovery. Although memory T cells cannot prevent the infection itself, they play a central role in preventing the severe progression of COVID-19. However, the longevity and functional maintenance of SARS-CoV-2-specific memory T cells remain unknown.
Professor Eui-Cheol Shin and his collaborators investigated the characteristics and functions of stem cell-like memory T cells, which are expected to play a crucial role in long-term immunity. Researchers analyzed the generation of stem cell-like memory T cells and multi-cytokine producing polyfunctional memory T cells, using cutting-edge immunological techniques.
This research is significant in that revealing the long-term immunity of COVID-19 convalescent patients provides an indicator regarding the long-term persistence of T cell immunity, one of the main goals of future vaccine development, as well as evaluating the long-term efficacy of currently available COVID-19 vaccines.
The research team is presently conducting a follow-up study to identify the memory T cell formation and functional characteristics of those who received COVID-19 vaccines, and to understand the immunological effect of COVID-19 vaccines by comparing the characteristics of memory T cells from vaccinated individuals with those of COVID-19 convalescent patients.
PhD candidate Jae Hyung Jung and Dr. Min-Seok Rha, a clinical fellow at Yonsei Severance Hospital, who led the study together explained, “Our analysis will enhance the understanding of COVID-19 immunity and establish an index for COVID-19 vaccine-induced memory T cells.”
“This study is the world’s longest longitudinal study on differentiation and functions of memory T cells among COVID-19 convalescent patients. The research on the temporal dynamics of immune responses has laid the groundwork for building a strategy for next-generation vaccine development,” Professor Shin added. This work was supported by the Samsung Science and Technology Foundation and KAIST, and was published in Nature Communications on June 30.
-Publication:
Jung, J.H., Rha, MS., Sa, M. et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Communications 12, 4043 (2021). https://doi.org/10.1038/s41467-021-24377-1
-Profile:
Professor Eui-Cheol Shin
Laboratory of Immunology & Infectious Diseases (http://liid.kaist.ac.kr/)
Graduate School of Medical Science and Engineering
KAIST
2021.07.05 View 16582 -
 Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 13798
Professor Ko Kyu Young Appointed as a Distinguished Professor at KAIST
Professor Ko Kyu Young of the Graduate School of Medical Sciences was appointed as the Distinguished Professor at KAIST.
Professor Ko is famous internationally for his work on the catalyst for blood vessel growth COMP-ANG1, and also for his research on blood vessel growth and lymph duct growth control.
Professor Ko developed the Double Anti-Angiogenic Protein (DAAP) which effectively restricts the blood vessels from growing, opening a new approach to curing caner. The paper was published in ‘Cancer Cell’ as the cover paper (2010 August 17th edition) and is widely recognized as the marker that sums up the new paradigm of cure for cancer.
In addition, his work on explaining how the new antigen interacts with the T-lymphocyte during a vaccination lead to the possibility of the increase of the efficiency of vaccination. The result of the research was published as the cover paper in ‘Immunity’ magazine.
As is obvious to see his work with blood vessel growth and lymph duct growth and control is being published in major scientific journals. In addition he is continuously invited to international conferences as guest speakers and leader, effectively leading the field. As a result, he was appointed as the editor of ‘Blood’ magazine, the world’s best journal in the field of hematology and received ‘2010 KAISTian of the Year’ Award.
The title Distinguished Professor is appointed to those who have made world-class research results and educational results and actively lead their respective field. They are provided with extra incentives and can even continue on with the professorship after retirement.
It is only limited to 3% of the professors at KAIST and has to be someone recommended by the President, Vice-President, and the Deans of department and their worthiness is scrutinized by a foreign expert.
2011.03.25 View 13798