Angewandte+Chemie
-
 Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 21401
Tungsten Suboxide Improves the Efficiency of Platinum in Hydrogen Production
< PhD Candidate Jinkyu Park and Professor Jinwoo Lee >
Researchers presented a new strategy for enhancing catalytic activity using tungsten suboxide as a single-atom catalyst (SAC). This strategy, which significantly improves hydrogen evolution reaction (HER) in metal platinum (pt) by 16.3 times, sheds light on the development of new electrochemical catalyst technologies.
Hydrogen has been touted as a promising alternative to fossil fuels. However, most of the conventional industrial hydrogen production methods come with environmental issues, releasing significant amounts of carbon dioxide and greenhouse gases.
Electrochemical water splitting is considered a potential approach for clean hydrogen production. Pt is one of the most commonly used catalysts to improve HER performance in electrochemical water splitting, but the high cost and scarcity of Pt remain key obstacles to mass commercial applications.
SACs, where all metal species are individually dispersed on a desired support material, have been identified as one way to reduce the amount of Pt usage, as they offer the maximum number of surface exposed Pt atoms.
Inspired by earlier studies, which mainly focused on SACs supported by carbon-based materials, a KAIST research team led by Professor Jinwoo Lee from the Department of Chemical and Biomolecular Engineering investigated the influence of support materials on the performance of SACs.
Professor Lee and his researchers suggested mesoporous tungsten suboxide as a new support material for atomically dispersed Pt, as this was expected to provide high electronic conductivity and have a synergetic effect with Pt.
They compared the performance of single-atom Pt supported by carbon and tungsten suboxide respectively. The results revealed that the support effect occurred with tungsten suboxide, in which the mass activity of a single-atom Pt supported by tungsten suboxide was 2.1 times greater than that of single-atom Pt supported by carbon, and 16.3 times higher than that of Pt nanoparticles supported by carbon.
The team indicated a change in the electronic structure of Pt via charge transfer from tungsten suboxide to Pt. This phenomenon was reported as a result of strong metal-support interaction between Pt and tungsten suboxide.
HER performance can be improved not only by changing the electronic structure of the supported metal, but also by inducing another support effect, the spillover effect, the research group reported. Hydrogen spillover is a phenomenon where adsorbed hydrogen migrates from one surface to another, and it occurs more easily as the Pt size becomes smaller.
The researchers compared the performance of single-atom Pt and Pt nanoparticles supported by tungsten suboxide. The single-atom Pt supported by tungsten suboxide exhibited a higher degree of hydrogen spillover phenomenon, which enhanced the Pt mass activity for hydrogen evolution up to 10.7 times compared to Pt nanoparticles supported by tungsten suboxide.
Professor Lee said, “Choosing the right support material is important for improving electrocatalysis in hydrogen production. The tungsten suboxide catalyst we used to support Pt in our study implies that interactions between the well-matched metal and support can drastically enhance the efficiency of the process.”
This research was supported by the Ministry of Science and ICT and introduced in the International Edition of the German journal Angewandte Chemie.
Figure. Schematic representation of hydrogen evolution reaction (HER) of pseudo single-atom Pt supported by tungsten suboxide
-Publication
Jinkyu Park, Dr. Seonggyu Lee, Hee-Eun Kim, Ara Cho, Seongbeen Kim, Dr. Youngjin Ye, Prof. Jeong Woo Han, Prof. Hyunjoo Lee, Dr. Jong Hyun Jang, and Prof. Jinwoo Lee. 2019. Investigation of the Support Effect in Atomically Dispersed Pt on WO3−x for Utilization of Pt in the Hydrogen Evolution Reaction. International Edition of Angewandte Chemie. Volume No. 58. Issue No. 45. 6 pages. https://doi.org/10.1002/anie.201908122
-ProfileProfessor Jinwoo LeeConvergence of Energy and Nano Science Laboratoryhttp://cens.kaist.ac.kr
Department of Chemical and Biomolecular EngineeringKAIST
2019.10.28 View 21401 -
 KAIST's Doctoral Student Receives a Hoffman Scholarship Award
Hyo-Sun Lee, a doctoral student at the Graduate School of EEWS (Environment, Energy, Water and Sustainability), KAIST, is a recipient of the 2016 Dorothy M. and Earl S. Hoffman Scholarships presented by the American Vacuum Society (AVS). The award ceremony took place during the Society’s 63rd International Symposium and Exhibition on November 6-11, 2016 in Nashville, Tennessee.
Lee is the first Korean and foreign student to receive this scholarship.
The Hoffman Scholarships were established in 2002 to recognize and encourage excellence in graduate studies in the sciences and technologies of interest to AVS. The scholarships are funded by a bequest from Dorothy M. Hoffman, who was a pioneering member of the Society of Women Engineers and served as the president of AVS in 1974.
Lee received the scholarship for her research that detects hot electrons from chemical reactions on catalytic surface using nanodevices. Nano Letters, an academic journal published by the American Chemical Society, described her work in its February 2016 issue as a technology that allows quantitative analysis of hot electrons by employing a new nanodevice and therefore helps researchers understand better the mechanism of chemical reactions on nanocatalytic surface. She also published her work to detect the flow of hot electrons that occur on metal nanocatalytic surface during hydrogen oxidation reactions in Angewandte Chemie.
Lee said, “I am pleased to receive this honor from such a world-renowned academic society. Certainly, this will be a great support for my future study and research.”
Founded in 1953, AVS is an interdisciplinary, professional society composed of approximately 4,500 members worldwide. It supports networking among academic, industrial, government, and consulting professionals involved in a range of established and emerging science and technology areas such as chemistry, physics, engineering, business, and technology development.
2016.11.17 View 10122
KAIST's Doctoral Student Receives a Hoffman Scholarship Award
Hyo-Sun Lee, a doctoral student at the Graduate School of EEWS (Environment, Energy, Water and Sustainability), KAIST, is a recipient of the 2016 Dorothy M. and Earl S. Hoffman Scholarships presented by the American Vacuum Society (AVS). The award ceremony took place during the Society’s 63rd International Symposium and Exhibition on November 6-11, 2016 in Nashville, Tennessee.
Lee is the first Korean and foreign student to receive this scholarship.
The Hoffman Scholarships were established in 2002 to recognize and encourage excellence in graduate studies in the sciences and technologies of interest to AVS. The scholarships are funded by a bequest from Dorothy M. Hoffman, who was a pioneering member of the Society of Women Engineers and served as the president of AVS in 1974.
Lee received the scholarship for her research that detects hot electrons from chemical reactions on catalytic surface using nanodevices. Nano Letters, an academic journal published by the American Chemical Society, described her work in its February 2016 issue as a technology that allows quantitative analysis of hot electrons by employing a new nanodevice and therefore helps researchers understand better the mechanism of chemical reactions on nanocatalytic surface. She also published her work to detect the flow of hot electrons that occur on metal nanocatalytic surface during hydrogen oxidation reactions in Angewandte Chemie.
Lee said, “I am pleased to receive this honor from such a world-renowned academic society. Certainly, this will be a great support for my future study and research.”
Founded in 1953, AVS is an interdisciplinary, professional society composed of approximately 4,500 members worldwide. It supports networking among academic, industrial, government, and consulting professionals involved in a range of established and emerging science and technology areas such as chemistry, physics, engineering, business, and technology development.
2016.11.17 View 10122 -
 Using Light to Treat Alzheimer's Disease
Medical application of photoactive chemicals offers a promising therapeutic strategy for neurodegenerative diseases.
A research team jointly led by Professor Chan Beum Park of the Materials Science and Engineering Department at KAIST and Dr. Kwon Yu from the Bionano Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) conducted research to suppress an abnormal assembly of beta-amyloids, a protein commonly found in the brain, by using photo-excited porphyrins.
Beta-amyloid plaques are known to cause Alzheimer's disease. This research finding suggests new ways to treat neurodegenerative illnesses including Alzheimer's disease. It was published online as the lead article in the September 21th issue of Angewandte Chemie. The title of the article is “Photo-excited Porphyrins as a Strong Suppressor of ß-Amyloid Aggregation and Synaptic Toxicity.”
Light-induced treatments using organic photosensitizers have advantages to managing the treatment in time and area. In the case of cancer treatments, doctors use photodynamic therapies where a patient is injected with an organic photosensitizer, and a light is shed on the patient’s lesion. However, such therapies had never been employed to treat neurodegenerative diseases.
Alzheimer's starts when a protein called beta-amyloid is created and deposited in a patient’s brain. The abnormally folded protein created this way harms the brain cells by inducing the degradation of brain functions, for example, dementia. If beta-amyloid creation can be suppressed at an early stage, the formation of amyloid deposits will stop. This could prevent Alzheimer’s disease or halt its progress.
The research team effectively prevented the buildup of beta-amyloids by using blue LED lights and a porphyrin inducer, which is a biocompatible organic compound. By absorbing light energy, a photosensitizer such as porphyrin reaches the excitation state. Active oxygen is created as the porphyrin returns to its ground state. The active oxygen oxidizes a beta-amyloid monomer, and by combining with it, disturbs its assembly.
The technique was tested on drosophilae or fruit flies, which were produced to model Alzheimer on invertebrates. The research showed that symptoms of Alzheimer's disease in the fruit flies such as damage on synapse and muscle, neuronal apoptosis, degradation in motility, and decreased longevity were alleviated. Treatments with light provide additional benefits: less medication is needed than other drug treatments, and there are fewer side effects. When developed, photodynamic therapy will be used widely for this reason.
Professor Park said, “This work has significance as it was the first case to use light and photosensitizers to stop deposits of beta-amyloids. We plan to carry the research further by testing compatibility with other organic and inorganic photosensitizers and by changing the subject of photodynamic therapy to vertebrate such as mice.”
Picture 1 – Deposits of Beta-Amyloid in Fruit Flies Stopped by Using Porphyrin and Blue LED Lights
Picture 2 – The Research Finding Published as the Lead Article in Angewandte Chemie (September 2015)
2015.11.11 View 11586
Using Light to Treat Alzheimer's Disease
Medical application of photoactive chemicals offers a promising therapeutic strategy for neurodegenerative diseases.
A research team jointly led by Professor Chan Beum Park of the Materials Science and Engineering Department at KAIST and Dr. Kwon Yu from the Bionano Center at the Korea Research Institute of Bioscience and Biotechnology (KRIBB) conducted research to suppress an abnormal assembly of beta-amyloids, a protein commonly found in the brain, by using photo-excited porphyrins.
Beta-amyloid plaques are known to cause Alzheimer's disease. This research finding suggests new ways to treat neurodegenerative illnesses including Alzheimer's disease. It was published online as the lead article in the September 21th issue of Angewandte Chemie. The title of the article is “Photo-excited Porphyrins as a Strong Suppressor of ß-Amyloid Aggregation and Synaptic Toxicity.”
Light-induced treatments using organic photosensitizers have advantages to managing the treatment in time and area. In the case of cancer treatments, doctors use photodynamic therapies where a patient is injected with an organic photosensitizer, and a light is shed on the patient’s lesion. However, such therapies had never been employed to treat neurodegenerative diseases.
Alzheimer's starts when a protein called beta-amyloid is created and deposited in a patient’s brain. The abnormally folded protein created this way harms the brain cells by inducing the degradation of brain functions, for example, dementia. If beta-amyloid creation can be suppressed at an early stage, the formation of amyloid deposits will stop. This could prevent Alzheimer’s disease or halt its progress.
The research team effectively prevented the buildup of beta-amyloids by using blue LED lights and a porphyrin inducer, which is a biocompatible organic compound. By absorbing light energy, a photosensitizer such as porphyrin reaches the excitation state. Active oxygen is created as the porphyrin returns to its ground state. The active oxygen oxidizes a beta-amyloid monomer, and by combining with it, disturbs its assembly.
The technique was tested on drosophilae or fruit flies, which were produced to model Alzheimer on invertebrates. The research showed that symptoms of Alzheimer's disease in the fruit flies such as damage on synapse and muscle, neuronal apoptosis, degradation in motility, and decreased longevity were alleviated. Treatments with light provide additional benefits: less medication is needed than other drug treatments, and there are fewer side effects. When developed, photodynamic therapy will be used widely for this reason.
Professor Park said, “This work has significance as it was the first case to use light and photosensitizers to stop deposits of beta-amyloids. We plan to carry the research further by testing compatibility with other organic and inorganic photosensitizers and by changing the subject of photodynamic therapy to vertebrate such as mice.”
Picture 1 – Deposits of Beta-Amyloid in Fruit Flies Stopped by Using Porphyrin and Blue LED Lights
Picture 2 – The Research Finding Published as the Lead Article in Angewandte Chemie (September 2015)
2015.11.11 View 11586 -
 Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 10897
Light Driven Drug-Enzyme Reaction Catalytic Platform Developed
Low Cost Dye Used, Hope for Future Development of High Value Medicinal Products to Treat Cardiovascular Disease and Gastric Ulcers
A KAIST research team from the Departments of Materials Science and Engineering and of Chemical and Biomolecular Engineering, led respectively by Professors Chan Beum Park and Ki Jun Jeong, has developed a new reaction platform to induce drug-enzyme reaction using light.
The research results were published in the journal Angewandte Chemie, International Edition, as the back cover on 12 January 2015.
Applications of this technology may enable production of high value products such as medicine for cardiovascular disease and gastric ulcers, for example Omeprazole, using an inexpensive dye.
Cytochrome P450 is an enzyme involved in oxidative response which has an important role in drug and hormone metabolism in organisms. It is known to be responsible for metabolism of 75% of drugs in humans and is considered a fundamental factor in new drug development.
To activate cytochrome P450, the enzyme must receive an electron by reducing the enzyme. In addition, NADPH (a coenzyme) needs to be present. However, since NADPH is expensive, the use of cytochrome P450 was limited to the laboratory and has not yet been commercialized.
The research team used photosensitizer eosin Y instead of NADPH to develop “Whole Cell Photo-Biocatalysis” in bacteria E. coli. By exposing inexpensive eosin Y to light, cytochrome P450 reaction was catalyzed to produce the expensive metabolic material.
Professor Park said, “This research enabled industrial application of cytochrome P450 enzyme, which was previous limited.” He continued, “This technology will help greatly in producing high value medical products using cytochrome P450 enzyme.”
The research was funded by the National Research Foundation of Korea and KAIST's High Risk High Return Project (HRHRP).
Figure 1: Mimetic Diagram of Electron Transfer from Light to Cytochrome P450 Enzyme via Eosin Y, EY
Figure 2: The back cover of Angewandte Chemie published on 12 January 2015, showing the research results
2015.01.26 View 10897 -
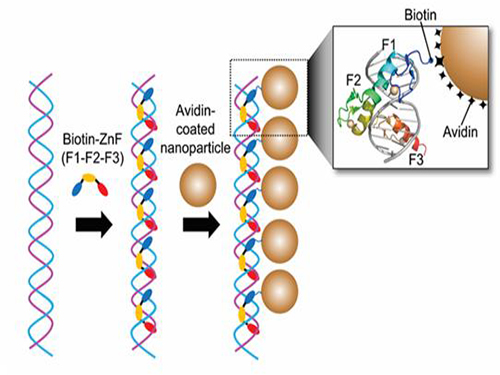 Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 13565
Nanoparticle Cluster Manufacturing Technique Using DNA Binding Protein Developed
Professor Hak-Sung Kim of the Department of Biological Sciences at KAIST and Yiseul Ryu, a doctoral candidate, used the Zinc Finger protein that specifically binds to target DNA sequence to develop a new manufacturing technique for size-controllable magnetic Nanoparticle Clusters (NPCs). Their research results were published in Angewandte Chemie International Edition online on 25 November 2014.
NPCs are structures consisting of magnetic nanoparticles, gold nanoparticles, and quantum dots, each of which are smaller than 100 nm (10-9m). NPCs have a distinctive property of collectivity not seen in single nanoparticles.
Specifically NPCS differ in physical and optical properties such as Plasmon coupling absorbance, energy transfers between particles, electron transfers, and conductivity. Therefore, NPCs can be employed in biological and medical research as well as the development of nanoelectric and nanoplasmon devices.
To make use of these novel properties, the size and the composition of the cluster must be exquisitely controlled. However, previous techniques relied on chemical binding which required complex steps, making it difficult to control the size and composition of NPCs.
Professor Kim’s team used Zinc Finger, a DNA binding protein, to develop a NPCs manufacturing technique to create clusters of the desired size easily. The Zinc Finger protein contains a zinc ion and specifically recognizes DNA sequence upon binding, which allows the exquisite control of the size and the cluster composition. The technique is also bio-friendly.
Professor Kim’s team created linear structure of different sizes of NPCs using Zinc Finger proteins and three DNA sequences of different lengths. The NPCs they produced confirmed their ability to control the size and structure of the cluster by using different DNA lengths.
The NPCs showed tripled T2 relaxation rates compared to the existing MRI contrast media (Feridex) and effectively transported to targeted cells. The research findings show the potential use of NPCs in biological and medical fields such as MRI contrast media, fluorescence imaging, and drug transport.
The research used the specific binding property of protein and DNA to develop a new method to create an inorganic nanoparticle’s supramolecular assembly. The technique can be used and applied extensively in other nanoparticles for future research in diagnosis, imaging, and drug and gene delivery.
Figure 1. A Mimetic Diagram of NPCs Manufacturing Technique Using DNA Binding Protein Zinc Finger
Figure 2. Transmission Electron Microscopy Images showing different sizes of NPCs depending on the length of the DNA
2014.12.04 View 13565 -
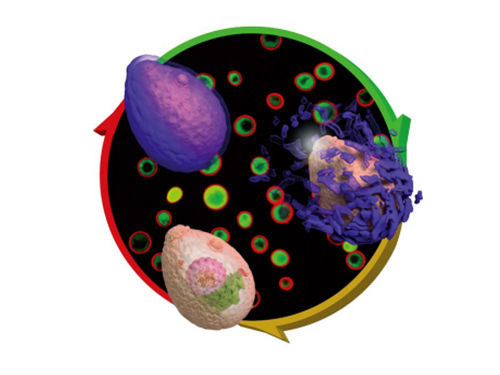 Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 10790
Eggshell-like Cell Encapsulation and Degradation Technology Developed
Some bacteria form endospores on cell walls to protect their DNA in case of nutrient deficiency. When an endospore meets a suitable environment for survival, the cell can revert to the original state from which it can reproduce.
The technique that can artificially control such phenomenon was developed by an international team of researchers. At first, a cell is wrapped and preserved like an egg. When the cell is needed, the technique allows the endospore to decompose while it is alive. Future applications for this technique include cell-based biosensor, cell therapy, and biocatalyst processes.
Professors Insung Choi and Younghoon Lee from the Department of Chemistry at KAIST as well as and Professor Frank Caruso from the University of Melbourne developed this technique which permits a cell to stay alive by coating it with film on a nanometer scale and then to be decomposed while it is alive.
The research finding was published in the November 10th issue of Angewandte Chemie International Edition as the lead article.
Cell encapsulation allows researchers to capture a cell in a tight capsule while it is alive. It is highly recognized in cell-based applications where the control of cell stability and cell-division is the biggest issue.
Traditional cell encapsulation methods utilized organic film or inorganic capsules that are made of organic film moldings. Although these films tightly closed around the cell, because they were not easily decomposable, it was difficult to apply the method.
The research team succeeded in encapsulating each cell in a metal-polyphenol film by mixing tannic acid and iron ion solution with yeast cells.
Usually extracted from oak barks or grape peels, tannic acid is a natural substance. It forms a metal-polyphenol film within ten seconds when it meets iron ions due to its high affinity with cells. Cells encapsulated with this film presented high survival rates. Since the film forms quickly in a simple manner, it was possible to obtain large amount of encapsulated cells.
The research team also found that the metal-polyphenol film was stable in neutral pH, but is easily degradable under a weak acidic condition. Using this property, they were able to control cell division by restoring the cell to its pre-encapsulated state at a desired moment.
Protecting the cell from the external environment like an egg shell, the metal-polyphenol film protected the cell against foreign conditions such as lytic enzymes, extended exposure to UV radiation, and silver nanoparticles. The research indicated that the encapsulated cells had a high survival rate even under extreme environments.
Professor Lee said that “not only the cells remain alive during the encapsulation stage, but also they can be protected under extreme environment.” He added, “This is an advanced cell encapsulation technology that allows controlling cell-division of those cells through responsive shell degradation on-demand.”
Professor Choi commented, “Although the cell encapsulation technology is still in its infancy, as the technology matures the application of cell-manipulation technology will be actualized.” He highlighted that “it will serve as a breakthrough to problems faced by cell-based applications.”
Sponsored by the Ministry of Science, ICT and Future Planning and the National Research Foundation of Korea, the research was led by two Master’s candidates, Ji Hun Park and Kyung Hwan Kim, under the joint guidance of research professors from KAIST and the University of Melbourne.
Figure 1: Lead article of Angewandte Chemie
Background: Shows a live native yeast (in green) encapsulated in a metal-polyphenol film (in red) illustrating the vitality of the yeast
Front: A native yeast at each encapsulation stage
Pictured on the bottom left is a cell prior to encapsulation. Following the red arrow, the native yeast is in purple to show metal-polyphenol film formed around the cell. The cell after the green arrow is a visualization of the degradation of the film in weak acidic condition.
Figure 2: A mimetic diagram of cell encapsulation with a metal-polyphenol film
Top: A native yeast before encapsulation
Middle: A native yeast encapsulated with Tannic Acid-Fe (III) Nanoshell – cell-division of the encapsulated cell is controlled by pH and the shell is protected against silver nanoparticle, lytic enzyme, and UV-C
Bottom: Shell degradation on-demand depending on pH
2014.11.18 View 10790 -
 Explanation for the polymerized nucleic acid enzyme's abnormal activation found
KAIST’s Professor Park Hyun Kyu of the Department of Bio Chemical Engineering revealed on the 23rd of December 2010 that his team had successfully developed the technology that uses the metal ions to control the abnormal activation of nucleic acids’ enzymes and using this, created a logic gate, which is a core technology in the field of future bio electrons.
The polymerized nucleic acid enzyme works to increase the synthesis of DNA and kicks into action only when the target DNA and primers form complimentary pairs (A and T, C and G).
Professor Park broke the common conception and found that it is possible for none complimentary pairs like T-T and C-C to initiate the activation of the enzyme and thus increase the nucleic acid production, given that there are certain metal ions present.
What Professor Park realized is that the enzymes mistake the uncomplimentary T-T and C-C pairs (with stabilized structures due to the bonding with mercury and silver ions) as being complimentary base pairs. Professor Park described this phenomenon as the “illusionary polymerase activity.”
The research team developed a logic gate based on the “illusionary polymerase activity’ phenomenon.” The logic gate paves the way to the development of future bio electron needed for bio computers and high performance memories.
Professor Park commented, “The research is an advancement of the previous research carried on about metal ions and nucleic acid synthesis. Our research is the first attempt at merging the concepts of the two previously separately carried out researches and can be adapted for testing presence of metal ions and development of a new single nucleotide polymorphic gene analysis technology.”
Professor Park added that, “Our research is a great stride in the field of nano scale electron element research as the results made possible the formation of accurate logic gates through relatively cost efficient and simple system designs.”
On a side note, the research was funded by Korea Research Foundation (Chairman: Park Chan Mo) and was selected as the cover paper for the December issue of ‘Angewandte Chemie International Edition’.
2011.01.18 View 12276
Explanation for the polymerized nucleic acid enzyme's abnormal activation found
KAIST’s Professor Park Hyun Kyu of the Department of Bio Chemical Engineering revealed on the 23rd of December 2010 that his team had successfully developed the technology that uses the metal ions to control the abnormal activation of nucleic acids’ enzymes and using this, created a logic gate, which is a core technology in the field of future bio electrons.
The polymerized nucleic acid enzyme works to increase the synthesis of DNA and kicks into action only when the target DNA and primers form complimentary pairs (A and T, C and G).
Professor Park broke the common conception and found that it is possible for none complimentary pairs like T-T and C-C to initiate the activation of the enzyme and thus increase the nucleic acid production, given that there are certain metal ions present.
What Professor Park realized is that the enzymes mistake the uncomplimentary T-T and C-C pairs (with stabilized structures due to the bonding with mercury and silver ions) as being complimentary base pairs. Professor Park described this phenomenon as the “illusionary polymerase activity.”
The research team developed a logic gate based on the “illusionary polymerase activity’ phenomenon.” The logic gate paves the way to the development of future bio electron needed for bio computers and high performance memories.
Professor Park commented, “The research is an advancement of the previous research carried on about metal ions and nucleic acid synthesis. Our research is the first attempt at merging the concepts of the two previously separately carried out researches and can be adapted for testing presence of metal ions and development of a new single nucleotide polymorphic gene analysis technology.”
Professor Park added that, “Our research is a great stride in the field of nano scale electron element research as the results made possible the formation of accurate logic gates through relatively cost efficient and simple system designs.”
On a side note, the research was funded by Korea Research Foundation (Chairman: Park Chan Mo) and was selected as the cover paper for the December issue of ‘Angewandte Chemie International Edition’.
2011.01.18 View 12276 -
 Prof. Woo's Team Discovers Eco-Friendly Solid-Oxide Fuel Cell System
A KAIST research team led by Prof. Seong-Ihl Woo of the Department of Chemical & Biomolecular Engineering has found a method to use glycerol, a byproduct from the production of biodiesel, as fuel for solid oxide fuel cells (SOFC), university authorities said on Tuesday (Oct. 27).
The research finding shows that glycerol can be an environmentally sustainable fuel when it is used for operating SOFCs with internal reforming, instead of hydrogen and methane. The finding was published in the Oct. 14, 2009 online edition of ChemSusChem, a sister journal of Angewandte Chemie, the world"s leading chemistry journal.
Biodiesel is an attractive alternative energy source because of its low sulfur content and demand is growing worldwide as oil price soars. Bio-derived glycerol will not contribute to the greenhouse effect and has the potential to contribute to reducing global warming.
Currently, glycereol is used as a raw material in the cosmetic, pharmacy, food, and tobacco industries. However, its supply exceeds its demand as the volume of biodiesel production increases. The production of 1 ton of biodiesel produces 0.1 ton of glycerol. Many researchers have investigated various routes for the consumption of surplus glycerol.
The research is expected to contribute to sustainable growth by reducing the emissions of carbon dioxide and reusing generated carbon dioxide for the production of biomass. The new method enables manufacturers to use glycerol as a fuel for operating SOFC.
2009.10.28 View 14157
Prof. Woo's Team Discovers Eco-Friendly Solid-Oxide Fuel Cell System
A KAIST research team led by Prof. Seong-Ihl Woo of the Department of Chemical & Biomolecular Engineering has found a method to use glycerol, a byproduct from the production of biodiesel, as fuel for solid oxide fuel cells (SOFC), university authorities said on Tuesday (Oct. 27).
The research finding shows that glycerol can be an environmentally sustainable fuel when it is used for operating SOFCs with internal reforming, instead of hydrogen and methane. The finding was published in the Oct. 14, 2009 online edition of ChemSusChem, a sister journal of Angewandte Chemie, the world"s leading chemistry journal.
Biodiesel is an attractive alternative energy source because of its low sulfur content and demand is growing worldwide as oil price soars. Bio-derived glycerol will not contribute to the greenhouse effect and has the potential to contribute to reducing global warming.
Currently, glycereol is used as a raw material in the cosmetic, pharmacy, food, and tobacco industries. However, its supply exceeds its demand as the volume of biodiesel production increases. The production of 1 ton of biodiesel produces 0.1 ton of glycerol. Many researchers have investigated various routes for the consumption of surplus glycerol.
The research is expected to contribute to sustainable growth by reducing the emissions of carbon dioxide and reusing generated carbon dioxide for the production of biomass. The new method enables manufacturers to use glycerol as a fuel for operating SOFC.
2009.10.28 View 14157