graduate+school+of+EEWS
-
 Materials Developed for Sodium Rechargeable Battery by EEWS
The research group of Professor William Goddard III, You-Sung Jung, and Jang-Wook Choi from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) at KAIST has developed a new sodium-ion rechargeable battery which operates at a high voltage, can be charged, and stably discharges over 10,000 cycles. The research results were published in the online version of the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on December 30, 2013.
Since the material costs of sodium rechargeable batteries is 30 to 40 times lower than lithium batteries, it has received attention as an energy saving tool for smart grids and as the next generation of lithium rechargeable batteries. Until now, sodium-ion rechargeable batteries have had issues with stability when charging and discharging. The research group developed a vanadium-based electrode to solve these problems.
The group said follow-up research will be continued to develop advanced technology on sodium rechargeable batteries as it is still currently in the beginning stages.
The research team: From left to right is Professors William Goddard, You-Sung Jung, and Jang-Wook Choi
2014.01.13 View 12493
Materials Developed for Sodium Rechargeable Battery by EEWS
The research group of Professor William Goddard III, You-Sung Jung, and Jang-Wook Choi from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) at KAIST has developed a new sodium-ion rechargeable battery which operates at a high voltage, can be charged, and stably discharges over 10,000 cycles. The research results were published in the online version of the Proceedings of the National Academy of Sciences of the United States of America (PNAS) on December 30, 2013.
Since the material costs of sodium rechargeable batteries is 30 to 40 times lower than lithium batteries, it has received attention as an energy saving tool for smart grids and as the next generation of lithium rechargeable batteries. Until now, sodium-ion rechargeable batteries have had issues with stability when charging and discharging. The research group developed a vanadium-based electrode to solve these problems.
The group said follow-up research will be continued to develop advanced technology on sodium rechargeable batteries as it is still currently in the beginning stages.
The research team: From left to right is Professors William Goddard, You-Sung Jung, and Jang-Wook Choi
2014.01.13 View 12493 -
 2013 EEWS Forum on National Energy Plan and Smart Grid Strategy
The Graduate School of Energy, Environment, Water, and Sustainability (EEWS) at KAIST hosted a forum on national energy planning and smart grid strategies on December 2 in the Jong-Hyun Choi Hall on KAIST’s Seoul campus. EEWS is a research and education program operated by KAIST to deal with the issues of energy, global warming, water, and sustainable growth.About 20 specialists including Jae-Kyu Lee, President of the Graduate School of Green Growth at KAIST; Kwang-Sik Choi, President of the EEWS Forum; Seong-Hoon Lee, Chairman of the Presidential Committee on Green Growth; Yang-Hoon Sohn, President of the Energy Economics Institute; and Jun-Dong Kim, Deputy Minister in the Ministry of Trade, Industry, and Energy, participated in the forum. Presentations and discussions were made in the fields of national energy plans, smart grid strategies, energy policy, as well as gas, electricity and sustainable energy.
2013.12.11 View 8841
2013 EEWS Forum on National Energy Plan and Smart Grid Strategy
The Graduate School of Energy, Environment, Water, and Sustainability (EEWS) at KAIST hosted a forum on national energy planning and smart grid strategies on December 2 in the Jong-Hyun Choi Hall on KAIST’s Seoul campus. EEWS is a research and education program operated by KAIST to deal with the issues of energy, global warming, water, and sustainable growth.About 20 specialists including Jae-Kyu Lee, President of the Graduate School of Green Growth at KAIST; Kwang-Sik Choi, President of the EEWS Forum; Seong-Hoon Lee, Chairman of the Presidential Committee on Green Growth; Yang-Hoon Sohn, President of the Energy Economics Institute; and Jun-Dong Kim, Deputy Minister in the Ministry of Trade, Industry, and Energy, participated in the forum. Presentations and discussions were made in the fields of national energy plans, smart grid strategies, energy policy, as well as gas, electricity and sustainable energy.
2013.12.11 View 8841 -
 Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12008
Secondary, High Capacity Battery developed from Rice Husks
Rice husks, a waste product from rice polishing, has been successfully utilized as the silicon anode for use in high capacity lithium ion secondary batteries. The new silicon anode derived from rice husks exhibit superior output and lifespan.
Professor Choi Jang Wook (The Graduate School of Energy, Environment, Water and Sustainability (EEWS)) and Professor Park Seung Min (Department of Biochemistry) and their respective research teams separated naturally occurring, highly porous silica material within the rice husks and developed a 3-dimensional, highly porous silicon anode material.
The result of the research effort was published in the online edition of the Proceedings of the National Academy of Sciences (PNAS) journal, a world renowned journal in the field of natural sciences.
Silicon has attracted much attention as anode material for next generation lithium ion secondary batteries because it exhibits 3~5 times higher capacity than conventional graphene. The high capacity will pave the way to lithium secondary batteries with higher energy densities than conventional batteries. It is anticipated that the application of silicon batteries will yield electronic devices with a longer duration for use in addition to electronic vehicles boasting longer mileage.
The silicon anode is based on the 3-dimensional, highly porous structure of rice husks which remedies the problematic extreme volume expansion of conventional silicon anodes.
Utilization of inexpensive rice husks to create high value silicon anodes will cause a ripple effect on the industry and academia.
2013.08.23 View 12008 -
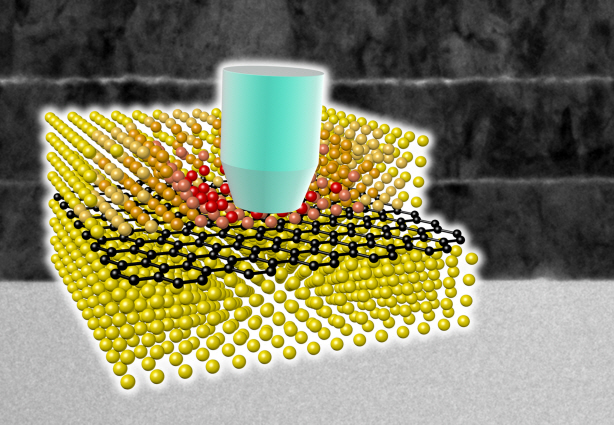 Ultra-High Strength Metamaterial Developed Using Graphene
New metamaterial has been developed, exhibiting hundreds of times greater strength than pure metals.
Professor Seung Min, Han and Yoo Sung, Jeong (Graduate School of Energy, Environment, Water, and Sustainability (EEWS)) and Professor Seok Woo, Jeon (Department of Material Science and Engineering) have developed a composite nanomaterial. The nanomaterial consists of graphene inserted in copper and nickel and exhibits strengths 500 times and 180 times, respectively, greater than that of pure metals. The result of the research was published on the July 2nd online edition in Nature Communications journal.
Graphene displays strengths 200 times greater than that of steel, is stretchable, and is flexible. The U.S. Army Armaments Research, Development and Engineering Center developed a graphene-metal nanomaterial but failed to drastically improve the strength of the material.
To maximize the strength increased by the addition of graphene, the KAIST research team created a layered structure of metal and graphene. Using CVD (Chemical Vapor Deposition), the team grew a single layer of graphene on a metal deposited substrate and then deposited another metal layer. They repeated this process to produce a metal-graphene multilayer composite material, utilizing a single layer of graphene. Micro-compression tests within Transmission Electronic Microscope and Molecular Dynamics simulations effectively showed the strength enhancing effect and the dislocation movement in grain boundaries of graphene on an atomic level.
The mechanical characteristics of the graphene layer within the metal-graphene composite material successfully blocked the dislocations and cracks from external damage from traveling inwards. Therefore the composite material displayed strength beyond conventional metal-metal multilayer materials. The copper-graphene multilayer material with an interplanar distance of 70nm exhibited 500 times greater (1.5GPa) strength than pure copper. Nickel-graphene multilayer material with an interplanar distance of 100nm showed 180 times greater (4.0GPa) strength than pure nickel.
It was found that there is a clear relationship between the interplanar distance and the strength of the multilayer material. A smaller interplanar distance made the dislocation movement more difficult and therefore increased the strength of the material. Professor Han, who led the research, commented, “the result is astounding as 0.00004% in weight of graphene increased the strength of the materials by hundreds of times” and “improvements based on this success, especially mass production with roll-to-roll process or metal sintering process in the production of ultra-high strength, lightweight parts for automobile and spacecraft, may become possible.” In addition, Professor Han mentioned that “the new material can be applied to coating materials for nuclear reactor construction or other structural materials requiring high reliability.”
The research project received support from National Research Foundation, Global Frontier Program, KAIST EEWS-KINC Program and KISTI Supercomputer and was a collaborative effort with KISTI (Korea Institute of Science and Technology Information), KBSI (Korea Basic Science Institute), Stanford University, and Columbia University.
A schematic diagram shows the structure of metal-graphene multi-layers. The metal-graphene multi-layered composite materials, containing a single-layered graphene, block the dislocation movement of graphene layers, resulting in a greater strength in the materials.
2013.08.23 View 18146
Ultra-High Strength Metamaterial Developed Using Graphene
New metamaterial has been developed, exhibiting hundreds of times greater strength than pure metals.
Professor Seung Min, Han and Yoo Sung, Jeong (Graduate School of Energy, Environment, Water, and Sustainability (EEWS)) and Professor Seok Woo, Jeon (Department of Material Science and Engineering) have developed a composite nanomaterial. The nanomaterial consists of graphene inserted in copper and nickel and exhibits strengths 500 times and 180 times, respectively, greater than that of pure metals. The result of the research was published on the July 2nd online edition in Nature Communications journal.
Graphene displays strengths 200 times greater than that of steel, is stretchable, and is flexible. The U.S. Army Armaments Research, Development and Engineering Center developed a graphene-metal nanomaterial but failed to drastically improve the strength of the material.
To maximize the strength increased by the addition of graphene, the KAIST research team created a layered structure of metal and graphene. Using CVD (Chemical Vapor Deposition), the team grew a single layer of graphene on a metal deposited substrate and then deposited another metal layer. They repeated this process to produce a metal-graphene multilayer composite material, utilizing a single layer of graphene. Micro-compression tests within Transmission Electronic Microscope and Molecular Dynamics simulations effectively showed the strength enhancing effect and the dislocation movement in grain boundaries of graphene on an atomic level.
The mechanical characteristics of the graphene layer within the metal-graphene composite material successfully blocked the dislocations and cracks from external damage from traveling inwards. Therefore the composite material displayed strength beyond conventional metal-metal multilayer materials. The copper-graphene multilayer material with an interplanar distance of 70nm exhibited 500 times greater (1.5GPa) strength than pure copper. Nickel-graphene multilayer material with an interplanar distance of 100nm showed 180 times greater (4.0GPa) strength than pure nickel.
It was found that there is a clear relationship between the interplanar distance and the strength of the multilayer material. A smaller interplanar distance made the dislocation movement more difficult and therefore increased the strength of the material. Professor Han, who led the research, commented, “the result is astounding as 0.00004% in weight of graphene increased the strength of the materials by hundreds of times” and “improvements based on this success, especially mass production with roll-to-roll process or metal sintering process in the production of ultra-high strength, lightweight parts for automobile and spacecraft, may become possible.” In addition, Professor Han mentioned that “the new material can be applied to coating materials for nuclear reactor construction or other structural materials requiring high reliability.”
The research project received support from National Research Foundation, Global Frontier Program, KAIST EEWS-KINC Program and KISTI Supercomputer and was a collaborative effort with KISTI (Korea Institute of Science and Technology Information), KBSI (Korea Basic Science Institute), Stanford University, and Columbia University.
A schematic diagram shows the structure of metal-graphene multi-layers. The metal-graphene multi-layered composite materials, containing a single-layered graphene, block the dislocation movement of graphene layers, resulting in a greater strength in the materials.
2013.08.23 View 18146 -
 Joint Research Center on EEWS with Hyundai Heavy Industries Plans to Open
The research center will conduct collaborative R&D projects on energy, environment, water, and sustainability for the next five years.Hyundai Heavy Industries (HHI), the world’s largest shipbuilding company, signed an MOU with KAIST for future business development and joint research collaboration.
KAIST and HHI signed an MOU as an agreement to establish the “HHI-KAIST EEWS Research Center (HK Research Center) on June 21st.”
The major mission of the HK Research Center is to build a strong base for creating future businesses through developing fundamental, core technology in the field of EEWS and designing business models based on the new technology.
Toward this goal, HHI will sponsor the R&D budget and operation expenses of the research center for the next five years.
Prior to the signing of the MOU, a delegation from HHI, led by the Vice President, Mr. Si-Young Hwang, visited the Office of EEWS Initiative at KAIST and held a workshop. During the workshop, HHI and KAIST agreed to collaborate in fields such as LNG-propelled ships, solar power generation, energy storage, fuel cells, and CO2 capture.
KAIST has run a EEWS graduate program that receives government grants over the last five years, with a research emphasis on energy, environment, water, and sustainability, which are crucial issues to humankind in the 21st century. The EEWS program achieved 24 core technological developments and educates more than 200 masters- and PhD-degree students annually.
The EEWS program also emphasizes commercializing its research outcomes. Through the annual Business Planning Competition and Investment Drive, there have been eight new companies founded by alumni and professors over the last five years of the program. The HK Research Center will be an excellent foundation for future education and research in EEWS.
Professor Jae-Kyu Lee, the head of the HK Research Center and the director of the EEWS Initiative, said, “This event is a benchmarking example of Industry-KAIST collaboration. We hope that the HK Research Center will be a place for disruptive innovations to translate into creative business opportunities.”
MOU signed for Hyundai Heavy Industries-KAIST EEWS Research Center
2013.07.15 View 10174
Joint Research Center on EEWS with Hyundai Heavy Industries Plans to Open
The research center will conduct collaborative R&D projects on energy, environment, water, and sustainability for the next five years.Hyundai Heavy Industries (HHI), the world’s largest shipbuilding company, signed an MOU with KAIST for future business development and joint research collaboration.
KAIST and HHI signed an MOU as an agreement to establish the “HHI-KAIST EEWS Research Center (HK Research Center) on June 21st.”
The major mission of the HK Research Center is to build a strong base for creating future businesses through developing fundamental, core technology in the field of EEWS and designing business models based on the new technology.
Toward this goal, HHI will sponsor the R&D budget and operation expenses of the research center for the next five years.
Prior to the signing of the MOU, a delegation from HHI, led by the Vice President, Mr. Si-Young Hwang, visited the Office of EEWS Initiative at KAIST and held a workshop. During the workshop, HHI and KAIST agreed to collaborate in fields such as LNG-propelled ships, solar power generation, energy storage, fuel cells, and CO2 capture.
KAIST has run a EEWS graduate program that receives government grants over the last five years, with a research emphasis on energy, environment, water, and sustainability, which are crucial issues to humankind in the 21st century. The EEWS program achieved 24 core technological developments and educates more than 200 masters- and PhD-degree students annually.
The EEWS program also emphasizes commercializing its research outcomes. Through the annual Business Planning Competition and Investment Drive, there have been eight new companies founded by alumni and professors over the last five years of the program. The HK Research Center will be an excellent foundation for future education and research in EEWS.
Professor Jae-Kyu Lee, the head of the HK Research Center and the director of the EEWS Initiative, said, “This event is a benchmarking example of Industry-KAIST collaboration. We hope that the HK Research Center will be a place for disruptive innovations to translate into creative business opportunities.”
MOU signed for Hyundai Heavy Industries-KAIST EEWS Research Center
2013.07.15 View 10174 -
 Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12641
Synthesis of a New Organic Supermolecule Succeeded
From left to right: Prof.Stoddart, Prof.Goddard and Prof.Jang Wook Choi
KAIST EEWS graduate school’s research team led by Prof. Stoddart, Prof. Goddard and Prof. Jang Wook Choi has succeeded the synthesis of a new organic supermolecule that is stable in a radical condition under room temperature.
Prof. Stoddart, who mainly led this research, is the world’s great scholar on orgaic molecular structure especially on catenane with an interconnection of several ring structures. Catenane is originated from Latin “catenane” referring to “chain”. The brief structure of the synthesized catenane is as following:
Usually radicals are known to be unstable since they are electronically neutral and have very high reactivity. However, the radicals from this research showed air- and water- stability. It also showed a reversible change in oxidation number from o to +8 through chemical/electrochemical oxidation-reduction reaction. The phenomenon where paramagnetic and diamagnetic characteristics change according to the oxidation number has also been observed.
Thus, the research like this - on the molecules showing various characteristics with stable radical - is expected to give a new direction to the next-generation electromemory system, semiconductor and energy storage system research.
Meanwhile, this research, led by Prof.Stoddart team with Prof.Goddard and Prof. Jang Wook Choi’s team, is conducted under the support of Science and Technology’s World Class University project by Ministry of Education and published in ‘Science’ on 25th of Jan.
2013.02.24 View 12641 -
 A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13635
A Substance with Amazingly Improved Efficiency of Capturing Carbon Dioxides Developed
From left to right: Prof.Ali Coskun, Prof. Cafer T. Yavuz and Prof. Yousung Jung
- Selectivity of CO2 increased by 300 times in comparison to nitrogen, published in Nature Communications-
KAIST EEWS graduate school’s joint research team led by Prof. Cafer T. Yavuz, Prof. Ali Coskun, and Prof. Yousung Jung has developed the world"s most efficient CO2 absorbent that has 300 times higher carbon dioxide selectivity in comparison to nitrogen.
Recently, the importance of CCS* technology, which is about capturing, storing and treating carbon dioxides, has begun to emerge world-widely as a practical alternative for the response to climate change.
* CCS : Carbon Capture and sequestration
Current carbon dioxide capturing technologies are wet capturing using liquid absorbent, dry capturing using solid absorbent and separation-membrane capturing using a thin membrane like a film.
For the places like power plant and forge, where the emission of carbon dioxides is huge, the main task is to maintain the capturing efficiency under extremely hot and humid conditions.
The previously studied dry absorbents, such as MOF or zeolite, had the disadvantages of instability in moist conditions and expensive cost for synthesis.
On the other hand, the research team"s newly discovered dry absorbent, named ‘Azo-COP’, can be synthesized without any expensive catalysts so the production cost is very low. It is also stable under hot and humid conditions.
COP is a structure consisting of simple organic molecules combined into porous polymer and is the first dry carbon dioxide capturing material developed by this research team.
The research team introduced an additional functional group called "Azo" to the substance, so that it can selectively capture carbon dioxides among the mixture of gas.
Azo-COP, which includes ‘Azo’ functional group, is manufactured easily by using common synthesis methods, and impurities are removed simply by using cheap solvents like water and acetone instead of expensive catalysts. As a result, the manufacturing cost has lowered drastically.
Especially, Azo-COP is combined with carbon dioxides by weak attraction force rather than chemical attraction so the recycling energy cost for the absorbent can be reduced innovatively, and it is expected to be used for capturing substances other than carbon dioxides in various areas as it is stable under extreme conditions even under 350 degrees Celsius.
This research is supported by Korea Carbon Capture&Sequestration R&D Center(Head: Sangdo Park) and KAIST EEWS planning group.
Prof. Cafer T. Yavuz and Prof. Ali Coskun said that “when Azo-COP is used for separation of CO2 and N2, the capturing efficiency has increased by hundred times.” He continued “This substance does not need any catalysts and has great chemical characteristics like water stability and structure stability so is expected to be used in various fields including carbon dioxides capturing”
Meanwhile, this research is published in ‘Nature’s stablemate ‘Nature Communications’ on 15th of Jan.
2013.02.24 View 13635 -
 Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10604
Prof. Jang-Uk Choi develops Strong, Long-lasting Lithium-ion Battery
Lithium-ion secondary battery with high power, as well asmuch longer life span, has been developed using nanotechnology. Professor Jang-Uk Choi and his colleagues at KAIST University EEWS graduate school has succeeded in developing a new lithium-ion secondary battery that has more than five times the output and three times the life span of the conventional batteries.
The industry expects the new battery to significantly improve the acceleration performance and solve the drawbacks of slow electric cars, which occurred due to failure of battery performance to keep up with the output of the motors during acceleration.
It is also expected that the new battery could be utilized in various fields that require high power batteries such as Smart Grid, which is the next generation intelligent electrical grid, as well as electric tools and many others.
Currently, the most widely used commercial lithium ion batteries’ lithium-cobalt-based cathode material has the disadvantage of expensive cost, high toxicity, short life expectancy and long-charge/discharge time. Also, it has been difficult to apply in electric cars that require a large current density and are vulnerable to heat generated during charging/discharging.
On the other hand, Professor Choi and his colleagues’ lithium-manganese based cathode material is gaining popularity for having the advantages such as abundant raw materials, cheap prices, eco-friendliness and especially excellent high-temperature stability and high output, which are suitable for use as electrode material in electric cars.
The pure lithium manganese based cathode material has a critical drawback of a very short life expectancy, only lasting about average of 1-2 years, which is due to the elution when the melted manganese flows out into the electrolyte. There have been various studies to solve this problem; however, the unique crystal structure of the material remained as a challenge for many scientists.
Professor Choi’s team analyzed the structure of the crystal at the time shortly before manganese oxides were formed, while controlling the reaction temperature at the step of synthesizing nanomaterial. It has been found that, at 220℃, there are simultaneously existing two crystal faces, one that inhibits the dissolution of manganese ions and the other that enables lithium ions to move smoothly.
Each of these crystal faces improves both the life span and output, increasing the output more than five times and life expectancy over three times. In addition, the existing high temperature life span, that was known to be especially vulnerable, has improved ten-fold.
“By controlling the crystal face of lithium manganese anode material, which has previously existed in the battery as chunks of about 10 micro-meter particles, both output and life span has significantly improved,” said Professor Choi, “Domestic and international patent application for the regarding technology has been finished and we have plans to work with companies in the future for commercialization within 2-3 years.”
Professor Yi Cui of Stanford University, the world’s leading scholar on the secondary battery, has evaluated that “This research exemplifies how nanotechnology can innovatively develop the field of secondary battery.”
Meanwhile, the research led by Professor Jang-Uk Choi and participated by researcher Ju-Seong Kim has been published on the online edition (dated Nov 27th) of Nanoletters, the world’s leading authority on Nanoscience.
2012.12.21 View 10604 -
 Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9705
Principle behind increasing the catalytic property of nanocatalysts proven
The technology that allows full control of the catalytic property of nanocatalysts using oxide formation on nanocatalysts has been developed by KAIST researchers. The breakthrough opens up the possibility of the development of a new kind of catalysts that maximizes catalytic property and minimizes waste.
*nanocatalyst is a material that catalyzes gas reactions on its surface. It is composed of a high surface area oxide scaffold with nano-sized metal particles dispersed.
The team was led by Professor Park Jeong Young of the KAIST EEWS Graduate School and consists of Kamran Qadir Ph.D. candidate (1st Author), Professor Joo Sang Hoon of UNIST, Professor Moon Bong Jin of Hanyang University, and Professor Gabor Somorajai of UC Berkeley. Support for the research was provided from Ministry of Education Science and Technology, National Research Foundation, and Ministry of Knowledge Economy. The results were published as the online edition of Nano Letters: “Intrinsic Relation between Catalytic Activity of CO Oxidation on Ru Nanoparticles and Ru Oxides Uncovered with Ambient Pressure XPS”.
Catalysts are included in above 80% of all the products used in everyday life and are therefore included in most aspects of our lives.
The focus on nanocatalysts is based on finding solutions to increasing the efficiency for application to energy production and for solving environmental issues.
Most nanocatalysts are composed of nanoparticles and oxides where the nanoparticles increase the surface area of the catalyst to increase its activity.
The efficiency of a nanocatalyst is affected by the surface oxide of the nanoparticles. However the proving of this assumption remained difficult to do as it required in-situ measurement of the oxide state of the nanoparticles in the specific environment. Thus far, the experiments were conducted in a vacuum and therefore did not reflect the actual behavior in real life. The recently developed X-ray Photoelectron Spectroscopy allows for measurement of the oxidization state at standard atmospheric pressure.
Professor Park’s research team successfully measured the oxidization state of the nanoparticle using the atmospheric pressure X-ray Photoelectron Spectroscopy in the specified environment.
They confirmed the effect the oxidization state on the catalytic effect of the nanoparticles and additionally found that a thin layer of oxide can increase the catalytic effect and the effectiveness of the nanoparticle can controlled by the oxidation state.
2012.11.29 View 9705 -
 3rd EEWS CEO Forum Held
KAIST EEWS (Energy Environment Water and Sustainability) held the 3rd EEWS CEO Forum at KAIST Seoul Campus.
EEWS is a research/education project initiated by KAIST to solve the global issues that the world faces including issues such as: energy depletion, global warming, water shortage, and sustainable development.
The 3rd EEWS CEO Forum is dedicated to providing the opportunity to share the vision and experience on technology and policy for green growth. The forum was founded in 2011 with active participation from Woo Ki Jeong (Director of Statistics), Choi Kwang Sik (Korea City Airport, Logistics and Travel, CEO), Kang Young Joong (Daekyo Group, CEO), Yoo Kyung Sun (Eugene Group, CEO), all experts in the field of green growth.
The forum consisted of presentations and debate on topics such as: international outlook on green growth, development projects based on new renewable energy, battery of electric vehicles, and development of solar cells.
Kim Sang Hyup member of the Presidential Committee on Green Growth started off the series of lectures with the topic of ‘International Outlook on Green Growth’. Kim Joong Gyum CEO of KEPCO followed up with ‘the Future of Electricity Generation Industry and Renewable Energy’, Kim Soo Ryung Director of LG Chemicals gave a talk on ‘Electric Vehicles and the Future of the Battery Industry’, and finally Choi Gi Hyuk CEO of SDN Ltd. gave the final lecture on ‘the Inflection Point of Solar Cell Industry’.
2012.10.16 View 10991
3rd EEWS CEO Forum Held
KAIST EEWS (Energy Environment Water and Sustainability) held the 3rd EEWS CEO Forum at KAIST Seoul Campus.
EEWS is a research/education project initiated by KAIST to solve the global issues that the world faces including issues such as: energy depletion, global warming, water shortage, and sustainable development.
The 3rd EEWS CEO Forum is dedicated to providing the opportunity to share the vision and experience on technology and policy for green growth. The forum was founded in 2011 with active participation from Woo Ki Jeong (Director of Statistics), Choi Kwang Sik (Korea City Airport, Logistics and Travel, CEO), Kang Young Joong (Daekyo Group, CEO), Yoo Kyung Sun (Eugene Group, CEO), all experts in the field of green growth.
The forum consisted of presentations and debate on topics such as: international outlook on green growth, development projects based on new renewable energy, battery of electric vehicles, and development of solar cells.
Kim Sang Hyup member of the Presidential Committee on Green Growth started off the series of lectures with the topic of ‘International Outlook on Green Growth’. Kim Joong Gyum CEO of KEPCO followed up with ‘the Future of Electricity Generation Industry and Renewable Energy’, Kim Soo Ryung Director of LG Chemicals gave a talk on ‘Electric Vehicles and the Future of the Battery Industry’, and finally Choi Gi Hyuk CEO of SDN Ltd. gave the final lecture on ‘the Inflection Point of Solar Cell Industry’.
2012.10.16 View 10991 -
 Successful development and analysis of mesoporous quasicrystal structures
Professor Osamu Terasaki’s research team from the EEWS Graduate School at KAIST successfully synthesized mesoporous quasicrystalline silica and developed a new method of analyzing its growth. The theory proposed by the team laid the foundation for the scientific examination of quasicrystal phenomena during the formation of micelles particles, a type of soft matter. The paper was published in the July edition of Nature magazine.
Scientists have faced difficulty in systematically explaining the mesoporous quasicrystal structures that are found in solidified versions of soft matter systems. However, the theoretical foundation from this research is expected to help promote the research and development of new nano-structured materials.
Mesoporous quaicrystals are soft matters that have high symmetry and a larger characteristic length scale than the nanoscale, thereby making it possible to develop materials that have controllable optical properties. This technology can be applied to the sustainable storage, use, and reproduction of energy.
Professor Terasaki’s team succeeded in synthesizing mesoporous quasicrystalline silica and proved the formation of dodecagonal column-shaped crystals as well as dodecagonal, rotationally symmetric electron diffraction patterns near the crystals using Transmission Electron Microscopy.
Quasicrystals are an abbreviation of ‘quasiperiodic crystals’ and have what is called the ‘third solid’ property; they have a structural arrangement that is between arranged crystal structures, such as metals, and non-crystalline structures, such as glass. This crystalline structure was only recently found, and the 2011 Nobel Chemistry Award was given to research in this field.
When porous materials are synthesized into quasicrystals, the crystalline structures of the pores can be designed and controlled in any way, making it possible to create new materials for a wide range of fields.
Professor Terasaki said that ‘The discovery of highly symmetric quasicrystals can lead to the alteration of a material’s optical properties, allowing the development of photonic crystals in the visible spectra.’ He also explained that this control of a material’s optical energy absorption could be the core technology behind energy harvesting.
This research was jointly conducted by Professor Terasaki from the EEWS Graduate School at KAIST and Stockholm University in Sweden.
2012.08.01 View 9661
Successful development and analysis of mesoporous quasicrystal structures
Professor Osamu Terasaki’s research team from the EEWS Graduate School at KAIST successfully synthesized mesoporous quasicrystalline silica and developed a new method of analyzing its growth. The theory proposed by the team laid the foundation for the scientific examination of quasicrystal phenomena during the formation of micelles particles, a type of soft matter. The paper was published in the July edition of Nature magazine.
Scientists have faced difficulty in systematically explaining the mesoporous quasicrystal structures that are found in solidified versions of soft matter systems. However, the theoretical foundation from this research is expected to help promote the research and development of new nano-structured materials.
Mesoporous quaicrystals are soft matters that have high symmetry and a larger characteristic length scale than the nanoscale, thereby making it possible to develop materials that have controllable optical properties. This technology can be applied to the sustainable storage, use, and reproduction of energy.
Professor Terasaki’s team succeeded in synthesizing mesoporous quasicrystalline silica and proved the formation of dodecagonal column-shaped crystals as well as dodecagonal, rotationally symmetric electron diffraction patterns near the crystals using Transmission Electron Microscopy.
Quasicrystals are an abbreviation of ‘quasiperiodic crystals’ and have what is called the ‘third solid’ property; they have a structural arrangement that is between arranged crystal structures, such as metals, and non-crystalline structures, such as glass. This crystalline structure was only recently found, and the 2011 Nobel Chemistry Award was given to research in this field.
When porous materials are synthesized into quasicrystals, the crystalline structures of the pores can be designed and controlled in any way, making it possible to create new materials for a wide range of fields.
Professor Terasaki said that ‘The discovery of highly symmetric quasicrystals can lead to the alteration of a material’s optical properties, allowing the development of photonic crystals in the visible spectra.’ He also explained that this control of a material’s optical energy absorption could be the core technology behind energy harvesting.
This research was jointly conducted by Professor Terasaki from the EEWS Graduate School at KAIST and Stockholm University in Sweden.
2012.08.01 View 9661 -
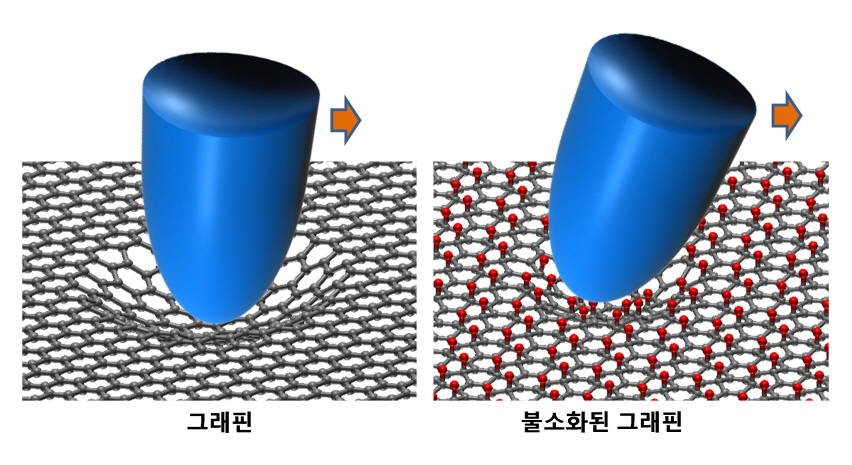 KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 18077
KAIST researchers verify and control the mechanical properties of graphene
KAIST researchers have successfully verified and controlled the mechanical properties of graphene, a next-generation material. Professor Park Jung Yong from the EEWS Graduate School and Professor Kim Yong Hyun from the Graduate School of Nanoscience and Technology have succeeded in fluorinating a single atomic-layered graphene sample and controlling its frictional and adhesive properties. This is the first time the frictional properties of graphene have been examined at the atomic level, and the technology is expected to be applied to nano-sized robots and microscopic joints.
Graphene is often dubbed “the dream material” because of its ability to conduct high amounts of electricity even when bent, making it the next-generation substitute for silicon semiconductors, paving the way for flexible display and wearable computer technologies. Graphene also has high potential applications in mechanical engineering because of its great material strength, but its mechanical properties remained elusive until now.
Professor Park’s research team successfully produced individual graphene samples with fluorine-deficiency at the atomic level by placing the samples in Fluoro-xenon (XeF2) gas and applying heat. The surface of the graphene was scanned using a micro probe and a high vacuum atomic microscope to measure its dynamic properties.
The research team found that the fluorinated graphene sample had 6 times more friction and 0.7 times more adhesiveness than the original graphene. Electrical measurements confirmed the fluorination process, and the analysis of the findings helped setup the theory of frictional changes in graphene.
Professor Park stated that “graphene can be used for the lubrication of joints in nano-sized devices” and that this research has numerous applications such as the coating of graphene-based microdynamic devices.
This research was published in the online June edition of Nano Letters and was supported by the Ministry of Science, Technology, and Education and the National Research Foundation as part of the World Class University (WCU) program.
2012.07.24 View 18077