Cell
-
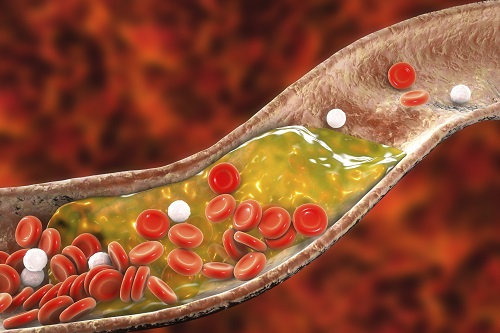 New Nanoparticle Drug Combination For Atherosclerosis
Physicochemical cargo-switching nanoparticles (CSNP) designed by KAIST can help significantly reduce cholesterol and macrophage foam cells in arteries, which are the two main triggers for atherosclerotic plaque and inflammation.
The CSNP-based combination drug delivery therapy was proved to exert cholesterol-lowering, anti-inflammatory, and anti-proliferative functions of two common medications for treating and preventing atherosclerosis that are cyclodextrin and statin. Professor Ji-Ho Park and Dr. Heegon Kim from KAIST’s Department of Bio and Brain Engineering said their study has shown great potential for future applications with reduced side effects.
Atherosclerosis is a chronic inflammatory vascular disease that is characterized by the accumulation of cholesterol and cholesterol-loaded macrophage foam cells in the intima. When this atherosclerotic plaque clogs and narrows the artery walls, they restrict blood flow and cause various cardiovascular conditions such as heart attacks and strokes. Heart attacks and strokes are the world’s first and fifth causes of death respectively.
Oral statin administration has been used in clinics as a standard care for atherosclerosis, which is prescribed to lower blood cholesterol and inhibit its accumulation within the plaque. Although statins can effectively prevent the progression of plaque growth, they have only shown modest efficacy in eliminating the already-established plaque. Therefore, patients are required to take statin drugs for the rest of their lives and will always carry the risk of plaque ruptures that can trigger a blood clot.
To address these issues, Professor Park and Dr. Kim exploited another antiatherogenic agent called cyclodextrin. In their paper published in the Journal of Controlled Release on March 10, Professor Park and Dr. Kim reported that the polymeric formulation of cyclodextrin with a diameter of approximately 10 nanometers(nm) can accumulate within the atherosclerotic plaque 14 times more and effectively reduce the plaque even at lower doses, compared to cyclodextrin in a non-polymer structure.
Moreover, although cyclodextrin is known to have a cytotoxic effect on hair cells in the cochlea, which can lead to hearing loss, cyclodextrin polymers developed by Professor Park’s research group exhibited a varying biodistribution profile and did not have this side effect.
In the follow-up study reported in ACS Nano on April 28, the researchers exploited both cyclodextrin and statin and form the cyclodextrin-statin self-assembly drug complex, based on previous findings that each drug can exert local anti-atherosclerosis effect within the plaque. The complex formation processes were optimized to obtain homogeneous and stable nanoparticles with a diameter of about 100 nm for systematic injection.
The therapeutic synergy of cyclodextrin and statin could reportedly enhance plaque-targeted drug delivery and anti-inflammation. Cyclodextrin led to the regression of cholesterol in the established plaque, and the statins were shown to inhibit the proliferation of macrophage foam cells. The study suggested that combination therapy is required to resolve the complex inflammatory cholesterol-rich microenvironment within the plaque.
Professor Park said, “While nanomedicine has been mainly developed for the treatment of cancers, our studies show that nanomedicine can also play a significant role in treating and preventing atherosclerosis, which causes various cardiovascular diseases that are the leading causes of death worldwide.”
This work was supported by KAIST and the National Research Foundation (NRF) of Korea.
Publications:
1. Heegon Kim, Junhee Han, and Ji-Ho Park. (2020) ‘Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity’ Journal of Controlled Release. Volume 319. Page 77-86. Available online at https://doi.org/10.1016/j.jconrel.2019.12.021
2. Kim, H., et al. (2020) ‘Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy’ ACS Nano. Available online at https://doi.org/10.1021/acsnano.9b08216
Profile: Ji-Ho Park, Ph.D.
Associate Professor
jihopark@kaist.ac.kr
http://openwetware.org/wiki/Park_Lab
Biomaterials Engineering Laboratory (BEL)
Department of Bio and Brain Engineering (BIOENG)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Heegon Kim, Ph.D.
Postdoctoral Researcher
heegon@kaist.ac.kr
BEL, BIOENG, KAIST
(END)
2020.06.16 View 16089
New Nanoparticle Drug Combination For Atherosclerosis
Physicochemical cargo-switching nanoparticles (CSNP) designed by KAIST can help significantly reduce cholesterol and macrophage foam cells in arteries, which are the two main triggers for atherosclerotic plaque and inflammation.
The CSNP-based combination drug delivery therapy was proved to exert cholesterol-lowering, anti-inflammatory, and anti-proliferative functions of two common medications for treating and preventing atherosclerosis that are cyclodextrin and statin. Professor Ji-Ho Park and Dr. Heegon Kim from KAIST’s Department of Bio and Brain Engineering said their study has shown great potential for future applications with reduced side effects.
Atherosclerosis is a chronic inflammatory vascular disease that is characterized by the accumulation of cholesterol and cholesterol-loaded macrophage foam cells in the intima. When this atherosclerotic plaque clogs and narrows the artery walls, they restrict blood flow and cause various cardiovascular conditions such as heart attacks and strokes. Heart attacks and strokes are the world’s first and fifth causes of death respectively.
Oral statin administration has been used in clinics as a standard care for atherosclerosis, which is prescribed to lower blood cholesterol and inhibit its accumulation within the plaque. Although statins can effectively prevent the progression of plaque growth, they have only shown modest efficacy in eliminating the already-established plaque. Therefore, patients are required to take statin drugs for the rest of their lives and will always carry the risk of plaque ruptures that can trigger a blood clot.
To address these issues, Professor Park and Dr. Kim exploited another antiatherogenic agent called cyclodextrin. In their paper published in the Journal of Controlled Release on March 10, Professor Park and Dr. Kim reported that the polymeric formulation of cyclodextrin with a diameter of approximately 10 nanometers(nm) can accumulate within the atherosclerotic plaque 14 times more and effectively reduce the plaque even at lower doses, compared to cyclodextrin in a non-polymer structure.
Moreover, although cyclodextrin is known to have a cytotoxic effect on hair cells in the cochlea, which can lead to hearing loss, cyclodextrin polymers developed by Professor Park’s research group exhibited a varying biodistribution profile and did not have this side effect.
In the follow-up study reported in ACS Nano on April 28, the researchers exploited both cyclodextrin and statin and form the cyclodextrin-statin self-assembly drug complex, based on previous findings that each drug can exert local anti-atherosclerosis effect within the plaque. The complex formation processes were optimized to obtain homogeneous and stable nanoparticles with a diameter of about 100 nm for systematic injection.
The therapeutic synergy of cyclodextrin and statin could reportedly enhance plaque-targeted drug delivery and anti-inflammation. Cyclodextrin led to the regression of cholesterol in the established plaque, and the statins were shown to inhibit the proliferation of macrophage foam cells. The study suggested that combination therapy is required to resolve the complex inflammatory cholesterol-rich microenvironment within the plaque.
Professor Park said, “While nanomedicine has been mainly developed for the treatment of cancers, our studies show that nanomedicine can also play a significant role in treating and preventing atherosclerosis, which causes various cardiovascular diseases that are the leading causes of death worldwide.”
This work was supported by KAIST and the National Research Foundation (NRF) of Korea.
Publications:
1. Heegon Kim, Junhee Han, and Ji-Ho Park. (2020) ‘Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity’ Journal of Controlled Release. Volume 319. Page 77-86. Available online at https://doi.org/10.1016/j.jconrel.2019.12.021
2. Kim, H., et al. (2020) ‘Affinity-Driven Design of Cargo-Switching Nanoparticles to Leverage a Cholesterol-Rich Microenvironment for Atherosclerosis Therapy’ ACS Nano. Available online at https://doi.org/10.1021/acsnano.9b08216
Profile: Ji-Ho Park, Ph.D.
Associate Professor
jihopark@kaist.ac.kr
http://openwetware.org/wiki/Park_Lab
Biomaterials Engineering Laboratory (BEL)
Department of Bio and Brain Engineering (BIOENG)
Korea Advanced Institute of Science and Technology (KAIST)
https://www.kaist.ac.kr
Daejeon 34141, Korea
Profile: Heegon Kim, Ph.D.
Postdoctoral Researcher
heegon@kaist.ac.kr
BEL, BIOENG, KAIST
(END)
2020.06.16 View 16089 -
 Unravelling Complex Brain Networks with Automated 3-D Neural Mapping
-Automated 3-D brain imaging data analysis technology offers more reliable and standardized analysis of the spatial organization of complex neural circuits.-
KAIST researchers developed a new algorithm for brain imaging data analysis that enables the precise and quantitative mapping of complex neural circuits onto a standardized 3-D reference atlas.
Brain imaging data analysis is indispensable in the studies of neuroscience. However, analysis of obtained brain imaging data has been heavily dependent on manual processing, which cannot guarantee the accuracy, consistency, and reliability of the results.
Conventional brain imaging data analysis typically begins with finding a 2-D brain atlas image that is visually similar to the experimentally obtained brain image. Then, the region-of-interest (ROI) of the atlas image is matched manually with the obtained image, and the number of labeled neurons in the ROI is counted.
Such a visual matching process between experimentally obtained brain images and 2-D brain atlas images has been one of the major sources of error in brain imaging data analysis, as the process is highly subjective, sample-specific, and susceptible to human error. Manual analysis processes for brain images are also laborious, and thus studying the complete 3-D neuronal organization on a whole-brain scale is a formidable task.
To address these issues, a KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering developed new brain imaging data analysis software named 'AMaSiNe (Automated 3-D Mapping of Single Neurons)', and introduced the algorithm in the May 26 issue of Cell Reports.
AMaSiNe automatically detects the positions of single neurons from multiple brain images, and accurately maps all the data onto a common standard 3-D reference space. The algorithm allows the direct comparison of brain data from different animals by automatically matching similar features from the images, and computing the image similarity score.
This feature-based quantitative image-to-image comparison technology improves the accuracy, consistency, and reliability of analysis results using only a small number of brain slice image samples, and helps standardize brain imaging data analyses.
Unlike other existing brain imaging data analysis methods, AMaSiNe can also automatically find the alignment conditions from misaligned and distorted brain images, and draw an accurate ROI, without any cumbersome manual validation process.
AMaSiNe has been further proved to produce consistent results with brain slice images stained utilizing various methods including DAPI, Nissl, and autofluorescence.
The two co-lead authors of this study, Jun Ho Song and Woochul Choi, exploited these benefits of AMaSiNe to investigate the topographic organization of neurons that project to the primary visual area (VISp) in various ROIs, such as the dorsal lateral geniculate nucleus (LGd), which could hardly be addressed without proper calibration and standardization of the brain slice image samples.
In collaboration with Professor Seung-Hee Lee's group of the Department of Biological Science, the researchers successfully observed the 3-D topographic neural projections to the VISp from LGd, and also demonstrated that these projections could not be observed when the slicing angle was not properly corrected by AMaSiNe. The results suggest that the precise correction of a slicing angle is essential for the investigation of complex and important brain structures.
AMaSiNe is widely applicable in the studies of various brain regions and other experimental conditions. For example, in the research team’s previous study jointly conducted with Professor Yang Dan’s group at UC Berkeley, the algorithm enabled the accurate analysis of the neuronal subsets in the substantia nigra and their projections to the whole brain. Their findings were published in Science on January 24.
AMaSiNe is of great interest to many neuroscientists in Korea and abroad, and is being actively used by a number of other research groups at KAIST, MIT, Harvard, Caltech, and UC San Diego.
Professor Paik said, “Our new algorithm allows the spatial organization of complex neural circuits to be found in a standardized 3-D reference atlas on a whole-brain scale. This will bring brain imaging data analysis to a new level.”
He continued, “More in-depth insights for understanding the function of brain circuits can be achieved by facilitating more reliable and standardized analysis of the spatial organization of neural circuits in various regions of the brain.”
This work was supported by KAIST and the National Research Foundation of Korea (NRF).
Figure and Image Credit: Professor Se-Bum Paik, KAIST
Figure and Image Usage Restrictions: News organizations may use or redistribute these figures and images, with proper attribution, as part of news coverage of this paper only.
Publication:
Song, J. H., et al. (2020). Precise Mapping of Single Neurons by Calibrated 3D Reconstruction of Brain Slices Reveals Topographic Projection in Mouse Visual Cortex. Cell Reports. Volume 31, 107682. Available online at https://doi.org/10.1016/j.celrep.2020.107682
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.06.08 View 15771
Unravelling Complex Brain Networks with Automated 3-D Neural Mapping
-Automated 3-D brain imaging data analysis technology offers more reliable and standardized analysis of the spatial organization of complex neural circuits.-
KAIST researchers developed a new algorithm for brain imaging data analysis that enables the precise and quantitative mapping of complex neural circuits onto a standardized 3-D reference atlas.
Brain imaging data analysis is indispensable in the studies of neuroscience. However, analysis of obtained brain imaging data has been heavily dependent on manual processing, which cannot guarantee the accuracy, consistency, and reliability of the results.
Conventional brain imaging data analysis typically begins with finding a 2-D brain atlas image that is visually similar to the experimentally obtained brain image. Then, the region-of-interest (ROI) of the atlas image is matched manually with the obtained image, and the number of labeled neurons in the ROI is counted.
Such a visual matching process between experimentally obtained brain images and 2-D brain atlas images has been one of the major sources of error in brain imaging data analysis, as the process is highly subjective, sample-specific, and susceptible to human error. Manual analysis processes for brain images are also laborious, and thus studying the complete 3-D neuronal organization on a whole-brain scale is a formidable task.
To address these issues, a KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering developed new brain imaging data analysis software named 'AMaSiNe (Automated 3-D Mapping of Single Neurons)', and introduced the algorithm in the May 26 issue of Cell Reports.
AMaSiNe automatically detects the positions of single neurons from multiple brain images, and accurately maps all the data onto a common standard 3-D reference space. The algorithm allows the direct comparison of brain data from different animals by automatically matching similar features from the images, and computing the image similarity score.
This feature-based quantitative image-to-image comparison technology improves the accuracy, consistency, and reliability of analysis results using only a small number of brain slice image samples, and helps standardize brain imaging data analyses.
Unlike other existing brain imaging data analysis methods, AMaSiNe can also automatically find the alignment conditions from misaligned and distorted brain images, and draw an accurate ROI, without any cumbersome manual validation process.
AMaSiNe has been further proved to produce consistent results with brain slice images stained utilizing various methods including DAPI, Nissl, and autofluorescence.
The two co-lead authors of this study, Jun Ho Song and Woochul Choi, exploited these benefits of AMaSiNe to investigate the topographic organization of neurons that project to the primary visual area (VISp) in various ROIs, such as the dorsal lateral geniculate nucleus (LGd), which could hardly be addressed without proper calibration and standardization of the brain slice image samples.
In collaboration with Professor Seung-Hee Lee's group of the Department of Biological Science, the researchers successfully observed the 3-D topographic neural projections to the VISp from LGd, and also demonstrated that these projections could not be observed when the slicing angle was not properly corrected by AMaSiNe. The results suggest that the precise correction of a slicing angle is essential for the investigation of complex and important brain structures.
AMaSiNe is widely applicable in the studies of various brain regions and other experimental conditions. For example, in the research team’s previous study jointly conducted with Professor Yang Dan’s group at UC Berkeley, the algorithm enabled the accurate analysis of the neuronal subsets in the substantia nigra and their projections to the whole brain. Their findings were published in Science on January 24.
AMaSiNe is of great interest to many neuroscientists in Korea and abroad, and is being actively used by a number of other research groups at KAIST, MIT, Harvard, Caltech, and UC San Diego.
Professor Paik said, “Our new algorithm allows the spatial organization of complex neural circuits to be found in a standardized 3-D reference atlas on a whole-brain scale. This will bring brain imaging data analysis to a new level.”
He continued, “More in-depth insights for understanding the function of brain circuits can be achieved by facilitating more reliable and standardized analysis of the spatial organization of neural circuits in various regions of the brain.”
This work was supported by KAIST and the National Research Foundation of Korea (NRF).
Figure and Image Credit: Professor Se-Bum Paik, KAIST
Figure and Image Usage Restrictions: News organizations may use or redistribute these figures and images, with proper attribution, as part of news coverage of this paper only.
Publication:
Song, J. H., et al. (2020). Precise Mapping of Single Neurons by Calibrated 3D Reconstruction of Brain Slices Reveals Topographic Projection in Mouse Visual Cortex. Cell Reports. Volume 31, 107682. Available online at https://doi.org/10.1016/j.celrep.2020.107682
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
(END)
2020.06.08 View 15771 -
 Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24085
Highly Efficient and Stable Double Layer Solar Cell Developed
Solar cells convert light into energy, but they can be inefficient and vulnerable to the environment, degrading with, ironically, too much light or other factors, including moisture and low temperature. An international research team has developed a new type of solar cell that can both withstand environmental hazards and is 26.7% efficient in power conversion.
They published their results on March 26 in Science.
The researchers, led by Byungha Shin, a professor from the Department of Materials Science and Engineering at KAIST, focused on developing a new class of light-absorbing material, called a wide bandgap perovskite. The material has a highly effective crystal structure that can process the power needs, but it can become problematic when exposed to environmental hazards, such as moisture. Researchers have made some progress increasing the efficiency of solar cells based on perovskite, but the material has greater potential than what was previously achieved.
To achieve better performance, Shin and his team built a double layer solar cell, called tandem, in which two or more light absorbers are stacked together to better utilize solar energy. To use perovskite in these tandem devices, the scientists modified the material’s optical property, which allows it to absorb a wider range of solar energy. Without the adjustment, the material is not as useful in achieving high performing tandem solar cells. The modification of the optical property of perovskite, however, comes with a penalty — the material becomes hugely vulnerable to the environment, in particular, to light.
To counteract the wide bandgap perovskite’s delicate nature, the researchers engineered combinations of molecules composing a two-dimensional layer in the perovskite, stabilizing the solar cells.
“We developed a high-quality wide bandgap perovskite material and, in combination with silicon solar cells, achieved world-class perovskite-silicon tandem cells,” Shin said.
The development was only possible due to the engineering method, in which the mixing ratio of the molecules building the two-dimensional layer are carefully controlled. In this case, the perovskite material not only improved efficiency of the resulting solar cell but also gained durability, retaining 80% of its initial power conversion capability even after 1,000 hours of continuous illumination. This is the first time such a high efficiency has been achieved with a wide bandgap perovskite single layer alone, according to Shin.
“Such high-efficiency wide bandgap perovskite is an essential technology for achieving ultra-high efficiency of perovskite-silicon tandem (double layer) solar cells,” Shin said. “The results also show the importance of bandgap matching of upper and lower cells in these tandem solar cells.”
The researchers, having stabilized the wide bandgap perovskite material, are now focused on developing even more efficient tandem solar cells that are expected to have more than 30% of power conversion efficiency, something that no one has achieved yet,
“Our ultimate goal is to develop ultra-high-efficiency tandem solar cells that contribute to the increase of shared solar energy among all energy sources,” Shin said. “We want to contribute to making the planet healthier.”
This work was supported by the National Research Foundation of Korea, the Korea Institute of Energy Technology Evaluation and Planning, the Ministry of Trade Industry and Energy of Korea, and the U.S. Department of Energy.
Other contributors include Daehan Kim, Jekyung Kim, Passarut Boonmongkolras, Seong Ryul Pae and Minkyu Kim, all of whom affiliated with the Department of Materials Science and Engineering at KAIST. Other authors include Byron W. Larson, Sean P. Dunfield, Chuanxiao Xiao, Jinhui Tong, Fei Zhang, Joseph J. Berry, Kai Zhu and Dong Hoe Kim, all of who are affiliated with the National Renewable Energy Laboratory in Colorado. Dunfield is also affiliated with the Materials Science and Engineering Program at the University of Colorado; Berry is also affiliated with the Department of Physics and the Renewable and Sustainable Energy Institute at the University of Colorado Boulder; and Kim is also affiliated with the Department of Nanotechnology and Advanced Materials Engineering at Sejong University. Hee Joon Jung and Vinayak Dravid of the Department of Materials Science and Engineering at Northwestern University; Ik Jae Park, Su Geun Ji and Jin Young Kim of the Department of Materials Science and Engineering at Seoul National University; and Seok Beom Kang of the Department of Nanotechnology and Advanced Materials Engineering of Sejong University also contributed.
Image credit: Professor Byungha Shin, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication: Kim et al. (2020) “Efficient, stable silicon tandem cells enabled by anion-engineered wide band gap perovskites”. Science. Available online at https://doi.org/10.1126/science.aba3433
Profile:
Byungha Shin
Professor
byungha@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
Profile:
Daehan Kim
Ph.D. Candidate
zxzx4592@kaist.ac.kr
http://energymatlab.kaist.ac.kr/
Department of Materials Science and Engineering
KAIST
(END)
2020.03.27 View 24085 -
 A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 16385
A Single Biological Factor Predicts Distinct Cortical Organizations across Mammalian Species
-A KAIST team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization.-
Researchers have explained how visual cortexes develop uniquely across the brains of different mammalian species. A KAIST research team led by Professor Se-Bum Paik from the Department of Bio and Brain Engineering has identified a single biological factor, the retino-cortical mapping ratio, that predicts distinct cortical organizations across mammalian species.
This new finding has resolved a long-standing puzzle in understanding visual neuroscience regarding the origin of functional architectures in the visual cortex. The study published in Cell Reports on March 10 demonstrates that the evolutionary variation of biological parameters may induce the development of distinct functional circuits in the visual cortex, even without species-specific developmental mechanisms.
In the primary visual cortex (V1) of mammals, neural tuning to visual stimulus orientation is organized into one of two distinct topographic patterns across species. While primates have columnar orientation maps, a salt-and-pepper type organization is observed in rodents.
For decades, this sharp contrast between cortical organizations has spawned fundamental questions about the origin of functional architectures in the V1. However, it remained unknown whether these patterns reflect disparate developmental mechanisms across mammalian taxa, or simply originate from variations in biological parameters under a universal development process.
To identify a determinant predicting distinct cortical organizations, Professor Paik and his researchers Jaeson Jang and Min Song examined the exact condition that generates columnar and salt-and-pepper organizations, respectively. Next, they applied a mathematical model to investigate how the topographic information of the underlying retinal mosaics pattern could be differently mapped onto a cortical space, depending on the mapping condition.
The research team proved that the retino-cortical feedforwarding mapping ratio appeared to be correlated to the cortical organization of each species. In the model simulations, the team found that distinct cortical circuitries can arise from different V1 areas and retinal ganglion cell (RGC) mosaic sizes. The team’s mathematical sampling model shows that retino-cortical mapping is a prime determinant in the topography of cortical organization, and this prediction was confirmed by neural parameter analysis of the data from eight phylogenetically distinct mammalian species.
Furthermore, the researchers proved that the Nyquist sampling theorem explains this parametric division of cortical organization with high accuracy. They showed that a mathematical model predicts that the organization of cortical orientation tuning makes a sharp transition around the Nyquist sampling frequency, explaining why cortical organizations can be observed in either columnar or salt-and-pepper organizations, but not in intermediates between these two stages.
Professor Paik said, “Our findings make a significant impact for understanding the origin of functional architectures in the visual cortex of the brain, and will provide a broad conceptual advancement as well as advanced insights into the mechanism underlying neural development in evolutionarily divergent species.”
He continued, “We believe that our findings will be of great interest to scientists working in a wide range of fields such as neuroscience, vision science, and developmental biology.”
This work was supported by the National Research Foundation of Korea (NRF).
Image credit: Professor Se-Bum Paik, KAIST
Image usage restrictions: News organizations may use or redistribute this image, with proper attribution, as part of news coverage of this paper only.
Publication:
Jaeson Jang, Min Song, and Se-Bum Paik. (2020). Retino-cortical mapping ratio predicts columnar and salt-and-pepper organization in mammalian visual cortex. Cell Reports. Volume 30. Issue 10. pp. 3270-3279. Available online at https://doi.org/10.1016/j.celrep.2020.02.038
Profile:
Se-Bum Paik
Assistant Professor
sbpaik@kaist.ac.kr
http://vs.kaist.ac.kr/
VSNN Laboratory
Department of Bio and Brain Engineering
Program of Brain and Cognitive Engineering
http://kaist.ac.kr
Korea Advanced Institute of Science and Technology (KAIST)
Daejeon, Republic of Korea
Profile:
Jaeson Jang
Ph.D. Candidate
jaesonjang@kaist.ac.kr
Department of Bio and Brain Engineering, KAIST
Profile:
Min Song
Ph.D. Candidate
night@kaist.ac.kr
Program of Brain and Cognitive Engineering, KAIST
(END)
2020.03.11 View 16385 -
 A Mathematical Model Reveals Long-Distance Cell Communication Mechanism
How can tens of thousands of people in a large football stadium all clap together with the same beat even though they can only hear the people near them clapping?
A combination of a partial differential equation and a synthetic circuit in microbes answers this question. An interdisciplinary collaborative team of Professor Jae Kyoung Kim at KAIST, Professor Krešimir Josić at the University of Houston, and Professor Matt Bennett at Rice University has identified how a large community can communicate with each other almost simultaneously even with very short distance signaling. The research was reported at Nature Chemical Biology.
Cells often communicate using signaling molecules, which can travel only a short distance. Nevertheless, the cells can also communicate over large distances to spur collective action. The team revealed a cell communication mechanism that quickly forms a network of local interactions to spur collective action, even in large communities.
The research team used an engineered transcriptional circuit of combined positive and negative feedback loops in E. coli, which can periodically release two types of signaling molecules: activator and repressor. As the signaling molecules travel over a short distance, cells can only talk to their nearest neighbors. However, cell communities synchronize oscillatory gene expression in spatially extended systems as long as the transcriptional circuit contains a positive feedback loop for the activator.
Professor Kim said that analyzing and understanding such high-dimensional dynamics was extremely difficult. He explained, “That’s why we used high-dimensional partial differential equation to describe the system based on the interactions among various types of molecules.” Surprisingly, the mathematical model accurately simulates the synthesis of the signaling molecules in the cell and their spatial diffusion throughout the chamber and their effect on neighboring cells.
The team simplified the high-dimensional system into a one-dimensional orbit, noting that the system repeats periodically. This allowed them to discover that cells can make one voice when they lowered their own voice and listened to the others. “It turns out the positive feedback loop reduces the distance between moving points and finally makes them move all together. That’s why you clap louder when you hear applause from nearby neighbors and everyone eventually claps together at almost the same time,” said Professor Kim.
Professor Kim added, “Math is a powerful as it simplifies complex thing so that we can find an essential underlying property. This finding would not have been possible without the simplification of complex systems using mathematics."
The National Institutes of Health, the National Science Foundation, the Robert A. Welch Foundation, the Hamill Foundation, the National Research Foundation of Korea, and the T.J. Park Science Fellowship of POSCO supported the research.
(Figure: Complex molecular interactions among microbial consortia is simplified as interactions among points on a limit cycle (right).)
2019.10.15 View 30777
A Mathematical Model Reveals Long-Distance Cell Communication Mechanism
How can tens of thousands of people in a large football stadium all clap together with the same beat even though they can only hear the people near them clapping?
A combination of a partial differential equation and a synthetic circuit in microbes answers this question. An interdisciplinary collaborative team of Professor Jae Kyoung Kim at KAIST, Professor Krešimir Josić at the University of Houston, and Professor Matt Bennett at Rice University has identified how a large community can communicate with each other almost simultaneously even with very short distance signaling. The research was reported at Nature Chemical Biology.
Cells often communicate using signaling molecules, which can travel only a short distance. Nevertheless, the cells can also communicate over large distances to spur collective action. The team revealed a cell communication mechanism that quickly forms a network of local interactions to spur collective action, even in large communities.
The research team used an engineered transcriptional circuit of combined positive and negative feedback loops in E. coli, which can periodically release two types of signaling molecules: activator and repressor. As the signaling molecules travel over a short distance, cells can only talk to their nearest neighbors. However, cell communities synchronize oscillatory gene expression in spatially extended systems as long as the transcriptional circuit contains a positive feedback loop for the activator.
Professor Kim said that analyzing and understanding such high-dimensional dynamics was extremely difficult. He explained, “That’s why we used high-dimensional partial differential equation to describe the system based on the interactions among various types of molecules.” Surprisingly, the mathematical model accurately simulates the synthesis of the signaling molecules in the cell and their spatial diffusion throughout the chamber and their effect on neighboring cells.
The team simplified the high-dimensional system into a one-dimensional orbit, noting that the system repeats periodically. This allowed them to discover that cells can make one voice when they lowered their own voice and listened to the others. “It turns out the positive feedback loop reduces the distance between moving points and finally makes them move all together. That’s why you clap louder when you hear applause from nearby neighbors and everyone eventually claps together at almost the same time,” said Professor Kim.
Professor Kim added, “Math is a powerful as it simplifies complex thing so that we can find an essential underlying property. This finding would not have been possible without the simplification of complex systems using mathematics."
The National Institutes of Health, the National Science Foundation, the Robert A. Welch Foundation, the Hamill Foundation, the National Research Foundation of Korea, and the T.J. Park Science Fellowship of POSCO supported the research.
(Figure: Complex molecular interactions among microbial consortia is simplified as interactions among points on a limit cycle (right).)
2019.10.15 View 30777 -
 Two Professors Recognized for the National R&D Excellence 100
< Professor Haeng-Ki Lee (left) and Professor Jeong-Ho Lee (right) >
Two KAIST professors were listed among the 2019 National R&D Excellence 100 announced by the Ministry of Science and ICT and the Korea Institute of S&T Evaluation and Planning.
Professor Haeng-Ki Lee from the Department of Civil and Environmental Engineering was recognized in the field of mechanics and materials for his research on developing new construction materials through the convergence of nano- and biotechnologies.
In the field of life and marine science, Professor Jeong-Ho Lee from the Graduate School of Medical Science and Engineering was lauded for his research of diagnostic tools and therapies for glioblastoma and pediatric brain tumors.
A certificate from the Minister of Ministry of Science and ICT will be conferred to these two professors, and their names will be inscribed on a special 2019 National R&D Excellence 100 plaque to celebrate their achievements. The professors will also be given privileges during the process of new R&D project selection.
(END)
2019.10.15 View 14660
Two Professors Recognized for the National R&D Excellence 100
< Professor Haeng-Ki Lee (left) and Professor Jeong-Ho Lee (right) >
Two KAIST professors were listed among the 2019 National R&D Excellence 100 announced by the Ministry of Science and ICT and the Korea Institute of S&T Evaluation and Planning.
Professor Haeng-Ki Lee from the Department of Civil and Environmental Engineering was recognized in the field of mechanics and materials for his research on developing new construction materials through the convergence of nano- and biotechnologies.
In the field of life and marine science, Professor Jeong-Ho Lee from the Graduate School of Medical Science and Engineering was lauded for his research of diagnostic tools and therapies for glioblastoma and pediatric brain tumors.
A certificate from the Minister of Ministry of Science and ICT will be conferred to these two professors, and their names will be inscribed on a special 2019 National R&D Excellence 100 plaque to celebrate their achievements. The professors will also be given privileges during the process of new R&D project selection.
(END)
2019.10.15 View 14660 -
 Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 22694
Researchers Describe a Mechanism Inducing Self-Killing of Cancer Cells
(Professor Kim (left) and lead author Lee)
Researchers have described a new mechanism which induces the self-killing of cancer cells by perturbing ion homeostasis. A research team from the Department of Biochemical Engineering has developed helical polypeptide potassium ionophores that lead to the onset of programmed cell death. The ionophores increase the active oxygen concentration to stress endoplasmic reticulum to the point of cellular death.
The electrochemical gradient between extracellular and intracellular conditions plays an important role in cell growth and metabolism. When a cell’s ion homeostasis is disturbed, critical functions accelerating the activation of apoptosis are inhibited in the cell.
Although ionophores have been intensively used as an ion homeostasis disturber, the mechanisms of cell death have been unclear and the bio-applicability has been limited. In the study featured at Advanced Science, the team presented an alpha helical peptide-based anticancer agent that is capable of transporting potassium ions with water solubility. The cationic, hydrophilic, and potassium ionic groups were combined at the end of the peptide side chain to provide both ion transport and hydrophilic properties.
These peptide-based ionophores reduce the intracellular potassium concentration and at the same time increase the intracellular calcium concentration. Increased intracellular calcium concentrations produce intracellular reactive oxygen species, causing endoplasmic reticulum stress, and ultimately leading to apoptosis.
Anticancer effects were evaluated using tumor-bearing mice to confirm the therapeutic effect, even in animal models. It was found that tumor growth was strongly inhibited by endoplasmic stress-mediated apoptosis.
Lead author Dr. Dae-Yong Lee said, “A peptide-based ionophore is more effective than conventional chemotherapeutic agents because it induces apoptosis via elevated reactive oxygen species levels. Professor Yeu-Chun Kim said he expects this new mechanism to be widely used as a new chemotherapeutic strategy. This research was funded by the National Research Foundation.
2019.08.28 View 22694 -
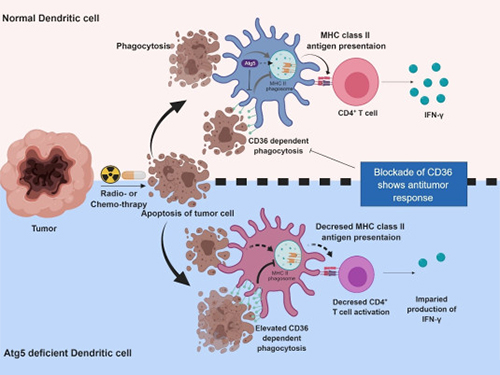 Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 52312
Autophagy in Dendritic Cells Helps Anticancer Activity
Autophagy contributes to the homeostasis of a cell and recently another function of autophagy has been reported. A KAIST research team found that the autophagy of dendritic cells supports T-cell anticancer activity.
Autophagy is a process of maintaining cell homeostasis by removing cellular waste and damaged cellular organelles; nevertheless, its role in the presentation of phagocytized tumor-associated antigens remains vague.
Meanwhile, dendritic cells are the ones that recognize pathogens or cancer antigens, and induce immune responses in T cells. When cancer cells are killed by radiation or an anticancer drug, dendritic cells absorb and remove them and present antigens on their surface to transfer them to T-cells.
Professor Heung Kyu Lee from the Graduate School of Medical Science and Engineering and his team found that the autophagy of dendritic cells plays a key role in T-cell activation and they proposed the principles of enhancing anti-cancer effects.
Their experiments showed that T-cell activation of dendritic cells as well as anticancer immune response dropped when there is a deficiency of Atg5 (autophagy-related) in dendritic cells.
Interestingly, Atg5-deficient dendritic cells significantly elevated receptor CD36 on the surface of the cells, which increased the phagocytosis of apoptotic tumor cells yet restricted the activation of T-cells.
At this time, when introducing antibodies into the system in order to block the receptor CD36, the anti-tumor T-cell response increased substantially while tumor growth declined.
Professor Lee said, “This study allowed us to explore the role of autophagy in the anti-cancer immune response of T-cells. We look forward to developing targeted anti-cancer therapies using the receptor CD36.”
This research was published in Autophagy (10.1080/15548627.2019.1596493) on March 22, 2019.
Figure 1.Mechanism of autophagy in dendritic cells
Figure 2. A role of autophagy in dendritic cells
2019.05.13 View 52312 -
 KAIST Unveils the Hidden Control Architecture of Brain Networks
(Professor Kwang-Hyun Cho and his team)
A KAIST research team identified the intrinsic control architecture of brain networks. The control properties will contribute to providing a fundamental basis for the exogenous control of brain networks and, therefore, has broad implications in cognitive and clinical neuroscience.
Although efficiency and robustness are often regarded as having a trade-off relationship, the human brain usually exhibits both attributes when it performs complex cognitive functions. Such optimality must be rooted in a specific coordinated control of interconnected brain regions, but the understanding of the intrinsic control architecture of brain networks is lacking.
Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering and his team investigated the intrinsic control architecture of brain networks. They employed an interdisciplinary approach that spans connectomics, neuroscience, control engineering, network science, and systems biology to examine the structural brain networks of various species and compared them with the control architecture of other biological networks, as well as man-made ones, such as social, infrastructural and technological networks.
In particular, the team reconstructed the structural brain networks of 100 healthy human adults by performing brain parcellation and tractography with structural and diffusion imaging data obtained from the Human Connectome Project database of the US National Institutes of Health.
The team developed a framework for analyzing the control architecture of brain networks based on the minimum dominating set (MDSet), which refers to a minimal subset of nodes (MD-nodes) that control the remaining nodes with a one-step direct interaction. MD-nodes play a crucial role in various complex networks including biomolecular networks, but they have not been investigated in brain networks.
By exploring and comparing the structural principles underlying the composition of MDSets of various complex networks, the team delineated their distinct control architectures. Interestingly, the team found that the proportion of MDSets in brain networks is remarkably small compared to those of other complex networks. This finding implies that brain networks may have been optimized for minimizing the cost required for controlling networks. Furthermore, the team found that the MDSets of brain networks are not solely determined by the degree of nodes, but rather strategically placed to form a particular control architecture.
Consequently, the team revealed the hidden control architecture of brain networks, namely, the distributed and overlapping control architecture that is distinct from other complex networks. The team found that such a particular control architecture brings about robustness against targeted attacks (i.e., preferential attacks on high-degree nodes) which might be a fundamental basis of robust brain functions against preferential damage of high-degree nodes (i.e., brain regions).
Moreover, the team found that the particular control architecture of brain networks also enables high efficiency in switching from one network state, defined by a set of node activities, to another – a capability that is crucial for traversing diverse cognitive states.
Professor Cho said, “This study is the first attempt to make a quantitative comparison between brain networks and other real-world complex networks. Understanding of intrinsic control architecture underlying brain networks may enable the development of optimal interventions for therapeutic purposes or cognitive enhancement.”
This research, led by Byeongwook Lee, Uiryong Kang and Hongjun Chang, was published in iScience (10.1016/j.isci.2019.02.017) on March 29, 2019.
Figure 1. Schematic of identification of control architecture of brain networks.
Figure 2. Identified control architectures of brain networks and other real-world complex networks.
2019.04.23 View 39381
KAIST Unveils the Hidden Control Architecture of Brain Networks
(Professor Kwang-Hyun Cho and his team)
A KAIST research team identified the intrinsic control architecture of brain networks. The control properties will contribute to providing a fundamental basis for the exogenous control of brain networks and, therefore, has broad implications in cognitive and clinical neuroscience.
Although efficiency and robustness are often regarded as having a trade-off relationship, the human brain usually exhibits both attributes when it performs complex cognitive functions. Such optimality must be rooted in a specific coordinated control of interconnected brain regions, but the understanding of the intrinsic control architecture of brain networks is lacking.
Professor Kwang-Hyun Cho from the Department of Bio and Brain Engineering and his team investigated the intrinsic control architecture of brain networks. They employed an interdisciplinary approach that spans connectomics, neuroscience, control engineering, network science, and systems biology to examine the structural brain networks of various species and compared them with the control architecture of other biological networks, as well as man-made ones, such as social, infrastructural and technological networks.
In particular, the team reconstructed the structural brain networks of 100 healthy human adults by performing brain parcellation and tractography with structural and diffusion imaging data obtained from the Human Connectome Project database of the US National Institutes of Health.
The team developed a framework for analyzing the control architecture of brain networks based on the minimum dominating set (MDSet), which refers to a minimal subset of nodes (MD-nodes) that control the remaining nodes with a one-step direct interaction. MD-nodes play a crucial role in various complex networks including biomolecular networks, but they have not been investigated in brain networks.
By exploring and comparing the structural principles underlying the composition of MDSets of various complex networks, the team delineated their distinct control architectures. Interestingly, the team found that the proportion of MDSets in brain networks is remarkably small compared to those of other complex networks. This finding implies that brain networks may have been optimized for minimizing the cost required for controlling networks. Furthermore, the team found that the MDSets of brain networks are not solely determined by the degree of nodes, but rather strategically placed to form a particular control architecture.
Consequently, the team revealed the hidden control architecture of brain networks, namely, the distributed and overlapping control architecture that is distinct from other complex networks. The team found that such a particular control architecture brings about robustness against targeted attacks (i.e., preferential attacks on high-degree nodes) which might be a fundamental basis of robust brain functions against preferential damage of high-degree nodes (i.e., brain regions).
Moreover, the team found that the particular control architecture of brain networks also enables high efficiency in switching from one network state, defined by a set of node activities, to another – a capability that is crucial for traversing diverse cognitive states.
Professor Cho said, “This study is the first attempt to make a quantitative comparison between brain networks and other real-world complex networks. Understanding of intrinsic control architecture underlying brain networks may enable the development of optimal interventions for therapeutic purposes or cognitive enhancement.”
This research, led by Byeongwook Lee, Uiryong Kang and Hongjun Chang, was published in iScience (10.1016/j.isci.2019.02.017) on March 29, 2019.
Figure 1. Schematic of identification of control architecture of brain networks.
Figure 2. Identified control architectures of brain networks and other real-world complex networks.
2019.04.23 View 39381 -
 Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 6452
Novel Strategies to Transform a Commercially Available Iboga Alkaloid to Post-Iboga Alkaloids
(PhD candidate HyeonggeunLim, Professor Sunkyu Han, PhD candidate Sikwang Seong)
KAIST chemists have synthesized seven different iboga and post-iboga natural products from commercially available catharanthine by mirroring nature’s biosynthetic post-modification of the iboga skeleton. They devised a novel strategy to biosynthesize the natural products via a series of selective and efficient oxidation and rearrangement reactions. This will serve as a stepping stone for developing therapeutic medications against cancer and narcotics addiction.
The research team, led by Professor Sunkyu Han, conceptualized and coined the term “Post-Iboga” alkaloids to describe the natural products that are biosynthetically derived from iboga-type alkaloids, which are composed of rearranged indole and/or isoquinuclidine backbones.
Iboga alkaloids have attracted significant attention from the scientific community due to their intriguing polycyclic structures and potential therapeutic uses against drug addictions. Nature has evolved to add architectural repertoires to this family of secondary metabolites by diversifying the iboga frameworks.
Notable examples are the FDA-approved anticancer drugs vinblastine and vincristine, both derived by the oxidative dimerization of catharanthine and vindoline subunits. Admittedly, synthetic foci toward the biosynthetic iboga-derivatives have historically been on these aforementioned dimeric natural products.
Recent natural product isolation studies on Tabernaemontana corymbosa and Ervatamia officinalis species have resulted in discoveries of various secondary metabolites that are biosynthetically derived from iboga alkaloids. These recent outbursts of iboga-derived natural product isolation reports have kindled interests toward these family of natural products.
The research team utilized (+)-catharanthine, the starting material for the industrial production of the anticancer drug Navelbine®. Well-orchestrated oxidations at the C19 position and the indole moiety of the catharanthine derivative, followed by differential rearrangements under acidic conditions, provided synthetic samples of voatinggine and tabertinggine respectively.
On the other hand, opportune oxidations at the C19 position and the alpha position of the tertiary amine moiety of the catharantine derivative, followed by a transhemiaminalization, produced the first synthetic sample of chippiine/dippinine-type natural product, dippinine B.
It is important to note that the chippiine and dippinine-type alkaloids have been targeted among synthetic chemists for over 30 years but had not succumbed to synthesis prior to this report.
Professor Han believes that their study will serve as a blueprint for further explorations of the synthesis, biosynthesis, and pharmacology of this emerging family of natural products. This study was published in Chem on November 15, 2018 (DOI: 10.1016/j.chempr.2018.10.009).
2018.11.16 View 6452 -
 Dr. Sejeong Kim Recognized as Excellent Young Scientist
(Dr. Sejeong Kim)
Dr. Sejeong Kim, a postdoctoral research associate in the School of Mathematical and Physical Sciences at the University of Technology Sydney was honored to receive the Excellence Award for a Young Scientist by the Korea Federation of Women’s Science & Technology Association (KOFWST). The award ceremony will be held on October 31 in Seoul.
KOFWST recognizes ten promising young female scientists and engineers every year who show significant potential, passion, and remarkable achievement in their work. The awardees are selected among those who finished their degree within the previous five years. Dr. Kim earned her Ph.D. in physics at KAIST in 2014 and was selected as the winner in the field of physics in recognition of her outstanding research activities in photonics.
Dr. Kim conducted various research activities in the field of photonics and was published in high impact journals including Nano Letters and Advanced materials. In July, she developed the first photonic cavity from van der Waals materials and published the study in Nature Communications titled “Photonic Crystal Cavities from Hexagonal Boron Nitride.” At UTS, she carries out research activities supervised by Professor Igor Aharonovich and has engaged in many science outreach activities.
2018.10.18 View 6876
Dr. Sejeong Kim Recognized as Excellent Young Scientist
(Dr. Sejeong Kim)
Dr. Sejeong Kim, a postdoctoral research associate in the School of Mathematical and Physical Sciences at the University of Technology Sydney was honored to receive the Excellence Award for a Young Scientist by the Korea Federation of Women’s Science & Technology Association (KOFWST). The award ceremony will be held on October 31 in Seoul.
KOFWST recognizes ten promising young female scientists and engineers every year who show significant potential, passion, and remarkable achievement in their work. The awardees are selected among those who finished their degree within the previous five years. Dr. Kim earned her Ph.D. in physics at KAIST in 2014 and was selected as the winner in the field of physics in recognition of her outstanding research activities in photonics.
Dr. Kim conducted various research activities in the field of photonics and was published in high impact journals including Nano Letters and Advanced materials. In July, she developed the first photonic cavity from van der Waals materials and published the study in Nature Communications titled “Photonic Crystal Cavities from Hexagonal Boron Nitride.” At UTS, she carries out research activities supervised by Professor Igor Aharonovich and has engaged in many science outreach activities.
2018.10.18 View 6876 -
 Mechanism Leading to Cortical Malformation from Brain-Only Mutations Identified
Focal malformations of cortical development (FMCDs) are a heterogeneous group of brain cortical abnormalities. These conditions are the most common causes of medically refractory epilepsy in children and are highly associated with intellectual disability, developmental delay, and autism-spectrum disorders. Despite a broad spectrum of cortical abnormalities in FMCDs, the defective migration of neuronal cells is considered a key pathological hallmark.
A research team led by Professor Jeong Ho Lee in the Graduate School of Medical Science and Engineering at KAIST has recently investigated the molecular mechanism of defective neuronal migration in FMCDs. Their research results were published online in Neuron on June 21, 2018.
The research team previously demonstrated that brain-only mutations in the mechanistic target of rapamycin (MTOR) gene causes focal cortical dysplasia, one major form of FMCDs leading to intractable epilepsy in children. However, the molecular mechanisms by which brain-only mutations in MTOR lead to cortical dyslamination and defective neuronal migration in FMCDs remain unclear.
To study the molecular mechanism of brain cortical dyslamination, the research team utilized patients’ brain tissues and modeled the MTOR mutation-carrying cell and animal models recapitulating the pathogenesis and symptoms of FMCD patients. By performing comprehensive molecular genetic experiments, they found that the formation of primary cilia, one of cellular organelles, was disrupted in MTOR mutation-carrying neurons and demonstrated that this ciliary disruption was a cause of cortical dyslamination in FMCDs.
MTOR mutations prevented degradation of the OFD1 protein, one of the negative regulators of ciliary formation. As a result, the OFD1 protein was abnormally accumulated in MTOR mutation-carrying neurons, causing focal cortical dyslamination. By suppressing the expression of the OFD1 protein, the research team was able to rescue the defective formation of primary cilia, leading to the restoration of cortical dyslamination and defective neuronal migration considerably.
Based on these results, the research team is carrying out further research to develop novel therapeutics for patients with FMCDs caused by brain-only mutations.
This work was supported by grants from the Suh Kyungbae Foundation and Citizens United for Research in Epilepsy.
The research paper is titled “Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination.” (Digital Object Identifier #: 10.1016/j.neuron.2018.05.039)
Picture 1: The disrupted formation of primary cilia in brain tissues of FMCD mouse models and patients with FMCDs caused by brain somatic mutations in MTOR.
Picture 2: The rescue of defective ciliary formation in FMCD mouse models leading to the restoration of cortical dyslamination and defective neuronal migration.
2018.07.02 View 9085
Mechanism Leading to Cortical Malformation from Brain-Only Mutations Identified
Focal malformations of cortical development (FMCDs) are a heterogeneous group of brain cortical abnormalities. These conditions are the most common causes of medically refractory epilepsy in children and are highly associated with intellectual disability, developmental delay, and autism-spectrum disorders. Despite a broad spectrum of cortical abnormalities in FMCDs, the defective migration of neuronal cells is considered a key pathological hallmark.
A research team led by Professor Jeong Ho Lee in the Graduate School of Medical Science and Engineering at KAIST has recently investigated the molecular mechanism of defective neuronal migration in FMCDs. Their research results were published online in Neuron on June 21, 2018.
The research team previously demonstrated that brain-only mutations in the mechanistic target of rapamycin (MTOR) gene causes focal cortical dysplasia, one major form of FMCDs leading to intractable epilepsy in children. However, the molecular mechanisms by which brain-only mutations in MTOR lead to cortical dyslamination and defective neuronal migration in FMCDs remain unclear.
To study the molecular mechanism of brain cortical dyslamination, the research team utilized patients’ brain tissues and modeled the MTOR mutation-carrying cell and animal models recapitulating the pathogenesis and symptoms of FMCD patients. By performing comprehensive molecular genetic experiments, they found that the formation of primary cilia, one of cellular organelles, was disrupted in MTOR mutation-carrying neurons and demonstrated that this ciliary disruption was a cause of cortical dyslamination in FMCDs.
MTOR mutations prevented degradation of the OFD1 protein, one of the negative regulators of ciliary formation. As a result, the OFD1 protein was abnormally accumulated in MTOR mutation-carrying neurons, causing focal cortical dyslamination. By suppressing the expression of the OFD1 protein, the research team was able to rescue the defective formation of primary cilia, leading to the restoration of cortical dyslamination and defective neuronal migration considerably.
Based on these results, the research team is carrying out further research to develop novel therapeutics for patients with FMCDs caused by brain-only mutations.
This work was supported by grants from the Suh Kyungbae Foundation and Citizens United for Research in Epilepsy.
The research paper is titled “Brain Somatic Mutations in MTOR Disrupt Neuronal Ciliogenesis, Leading to Focal Cortical Dyslamination.” (Digital Object Identifier #: 10.1016/j.neuron.2018.05.039)
Picture 1: The disrupted formation of primary cilia in brain tissues of FMCD mouse models and patients with FMCDs caused by brain somatic mutations in MTOR.
Picture 2: The rescue of defective ciliary formation in FMCD mouse models leading to the restoration of cortical dyslamination and defective neuronal migration.
2018.07.02 View 9085