and+Sustainability
-
 Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 25580
Enhanced Natural Gas Storage to Help Reduce Global Warming
< Professor Atilhan (left) and Professor Yavuz (right) >
Researchers have designed plastic-based materials that can store natural gas more effectively. These new materials can not only make large-scale, cost-effective, and safe natural gas storage possible, but further hold a strong promise for combating global warming.
Natural gas (predominantly methane) is a clean energy alternative. It is stored by compression, liquefaction, or adsorption. Among these, adsorbed natural gas (ANG) storage is a more efficient, cheaper, and safer alternative to conventional compressed natural gas (CNG) and liquefied natural gas (LNG) storage approaches that have drawbacks such as low storage efficiency, high costs, and safety concerns. However, developing adsorptive materials that can more fully exploit the advantages of ANG storage has remained a challenging task.
A KAIST research team led by Professor Cafer T. Yavuz from the Graduate School of Energy, Environment, Water, and Sustainability (EEWS), in collaboration with Professor Mert Atilhan’s group from Texas A&M University, synthesized 29 unique porous polymeric structures with inherent flexibility, and tested their methane gas uptake capacity at high pressures. These porous polymers had varying synthetic complexities, porosities, and morphologies, and the researchers subjected each porous polymer to pure methane gas under various conditions to study the ANG performances.
Of these 29 distinct chemical structures, COP-150 was particularly noteworthy as it achieved a high deliverable gravimetric methane working capacity when cycled between 5 and 100 bar at 273 K, which is 98% of the total uptake capacity. This result surpassed the target set by the United States Department of Energy (US DOE).
COP-150 is the first ever structure to fulfil both the gravimetric and volumetric requirements of the US DOE for successful vehicular use, and the total cost to produce the COP-150 adsorbent was only 1 USD per kilogram.
COP-150 can be produced using freely available and easily accessible plastic materials, and moreover, its synthesis takes place at room temperature, open to the air, and no previous purification of the chemicals is required. The pressure-triggered flexible structure of COP-150 is also advantageous in terms of the total working capacity of deliverable methane for real applications.
The research team believed that the increased pressure flexes the network structure of COP-150 showing “swelling” behavior, and suggested that the flexibility provides rapid desorption and thermal management, while the hydrophobicity and the nature of the covalently bonded framework allow these promising materials to tolerate harsh conditions.
This swelling mechanism of expansion-contraction solves two other major issues, the team noted. Firstly, when using adsorbents based on such a mechanism, unsafe pressure spikes that may occur due to temperature swings can be eliminated. In addition, contamination can also be minimized, since the adsorbent remains contracted when no gas is stored.
Professor Yavuz said, “We envision a whole host of new designs and mechanisms to be developed based on our concept. Since natural gas is a much cleaner fuel than coal and petroleum, new developments in this realm will help switching to the use of less polluting fuels.”
Professor Atilhan agreed the most important impact of their research is on the environment. “Using natural gas more than coal and petroleum will significantly reduce greenhouse gas emissions. We believe, one day, we might see vehicles equipped with our materials that are run by a cleaner natural gas fuel,” he added.
This study, reported in Nature Energy on July 8, was supported by National Research Foundation of Korea (NRF) grants ( NRF-2016R1A2B4011027, NRF-2017M3A7B4042140, and NRF-2017M3A7B4042235).
< Suggested chemical structure of COP-150 >
< Initial ingredients (left) and final product (right) of COP-150 synthesis >
< Comparison of highest reported volumetric working capacities >
(END)
2019.08.09 View 25580 -
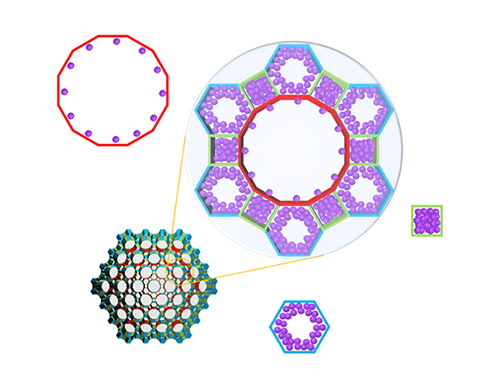 Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 38145
Real-Time Analysis of MOF Adsorption Behavior
Researchers have developed a technology to analyze the adsorption behavior of molecules in each individual pore of a metal organic framework (MOF). This system has large specific surface areas, allowing for the real-time observation of the adsorption process of an MOF, a new material effective for sorting carbon dioxide, hydrogen, and methane.
Accurate measurements and assessments of gas adsorption isotherms are important for characterizing porous materials and developing their applications. The existing technology is only able to measure the amount of gas molecules adsorbed to the material, without directly observing the adsorption behavior.
The research team led by Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability (EEWS) prescribed a real time gas adsorption crystallography system by integrating an existing X-ray diffraction (XRD) measurement device that can provide structural information and a gas adsorption measurement device.
Specifically, the system allowed the observation of a mesoporous MOF that has multiple pores rather than a single pore structure. The research team categorized the adsorption behaviors of MOF molecules by pore type, followed by observations and measurements, resulting in the identification of a stepwise adsorption process that was previously not possible to analyze.
Further, the team systematically and quantitatively analyzed how the pore structure and the type of adsorption molecule affect the adsorption behavior to suggest what type of MOF structure is appropriate as a storage material for each type of adsorption behavior.
Professor Kang said, “We quantitatively analyzed each pore molecule in real time to identify the effects of chemical and structural properties of pores on adsorption behavior.” He continued, “By understanding the real-time adsorption behavior of molecules at the level of the pores that form the material, rather than the whole material, we will be able to apply this technology to develop a new high-capacity storage material.”
This research was published in Nature Chemistry online on May 13, 2019 under the title ‘Isotherms of Individual Pores by Gas Adsorption Crystallography’.
(Figure. Schematic illustration of molecules adsorbed on metal organic frameworks with different pores of various structures, where the In-situ X-ray crystallography has been developed to classify each pore structure and analyze the position of the molecule to determine the amount of molecules adsorbed to each pore.)
2019.06.18 View 38145 -
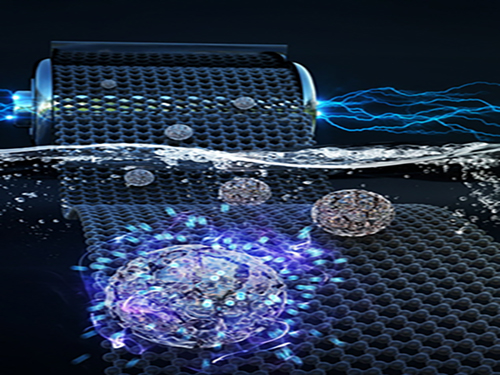 Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 6679
Faster and More Powerful Aqueous Hybrid Capacitor
(Professor Jeung Ku Kang from the Graduate School of EEWS)
A KAIST research team made it one step closer to realizing safe energy storage with high energy density, high power density, and a longer cycle life. This hybrid storage alternative shows power density 100 times faster than conventional batteries, allowing it to be charged within a few seconds. Hence, it is suitable for small portable electronic devices.
Conventional electrochemical energy storage systems, including lithium-ion batteries (LIBs), have a high voltage range and energy density, but are subject to safety issues raised by flammable organic electrolytes, which are used to ensure the beneficial properties. Additionally, they suffer from slow electrochemical reaction rates, which lead to a poor charging rate and low power density with a capacity that fades quickly, resulting in a short cycle life.
On the other hand, capacitors based on aqueous electrolytes are receiving a great deal of attention because they are considered to be safe and environmentally friendly alternatives. However, aqueous electrolytes lag behind energy storage systems based on organic electrolytes in terms of energy density due to their limited voltage range and low capacitance.
Hence, developing aqueous energy storage with high energy density and a long cycle life in addition to the high power density that enables fast charging is the most challenging task for advancing next-generation electrochemical energy storage devices.
Here, Professor Jeung Ku Kang from the Graduate School of Energy, Environment, Water and Sustainability and his team developed an aqueous hybrid capacitor (AHC) that boasts high energy density, high power, and excellent cycle stability by synthesizing two types of porous metal oxide nanoclusters on graphene to create positive and negative electrodes for AHCs.
The porous metal oxide nanoparticles are composed of nanoclusters as small as two to three nanometers and have mesopores that are smaller than five nanometers. In these porous structures, ions can be rapidly transferred to the material surfaces and a large number of ions can be stored inside the metal oxide particles very quickly due to their small particle size and large surface area.
The team applied porous manganese oxide on graphene for positive electrodes and porous iron oxide on graphene for negative electrodes to design an aqueous hybrid capacitor that can operate at an extended voltage range of 2V.
Professor Kang said, “This newly developed AHC with high capacity and power density driven from porous metal oxide electrodes will contribute to commercializing a new type of energy storage system. This technology allows ultra-fast charging within several seconds, making it suitable as a power source for mobile devices or electric vehicles where solar energy is directly stored as electricity.”
This research, co-led by Professor Hyung Mo Jeong from Kangwon National University, was published in Advanced Functional Materials on August 15, 2018.
Figure 1. Image that shows properties of porous metal oxide nanoparticles formed on graphene in the aqueous hybrid capacitor
2018.11.09 View 6679 -
 Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 7503
Permanent, Wireless Self-charging System Using NIR Band
(Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability)
As wearable devices are emerging, there are numerous studies on wireless charging systems. Here, a KAIST research team has developed a permanent, wireless self-charging platform for low-power wearable electronics by converting near-infrared (NIR) band irradiation to electrical energy. This novel technology can be applied to flexible, wearable charging systems without needing any attachments.
Colloidal-quantum-dots (CQDs) are promising materials for manufacturing semiconductors; in particular, PbS-based CQDs have facile optical tunability from the visible to infrared wavelength region. Hence, they can be applied to various devices, such as lighting, photovoltaics (PVs), and photodetectors.
Continuous research on CQD-based optoelectronic devices has increased their power conversion efficiency (PCE) to 12%; however, applicable fields have not yet been found for them. Meanwhile, wearable electronic devices commonly face the problem of inconvenient charging systems because users have to constantly charge batteries attached to an energy source.
A joint team led by Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and Jang Wok Choi from Seoul National University decided to apply CQD PVs, which have high quantum efficiency in NIR band to self-charging systems on wearable devices.
They employed a stable and efficient NIR energy conversion strategy. The system was comprised of a PbS CQD-based PV module, a flexible interdigitated lithium-ion battery, and various types of NIR-transparent films.
The team removed the existing battery from the already commercialized wearable healthcare bracelet and replaced it with the proposed self-charging system. They confirmed that the system can be applied to a low power wearable device via the NIR band.
There have been numerous platforms using solar irradiation, but the newly developed platform has more advantages because it allows conventional devices to be much more comfortable to wear and charged easily in everyday life using various irradiation sources for constant charging.
With this aspect, the proposed platform facilitates more flexible designs, which are the important component for actual commercialization. It also secures higher photostability and efficient than existing structures.
Professor Lee said, “By using the NIR band, we proposed a new approach to solve charging system issues of wearable devices. I believe that this platform will be a novel platform for energy conversion and that its application can be further extended to various fields, including mobiles, IoTs, and drones.”
This research, led by PhD Se-Woong Baek and M.S. candidate Jungmin Cho, was published in Advanced Materials on May 11.
Figure 1. a) Conceptual NIR-driven self-charging system including a flexible CQD PVs module and an interdigitatedly structured LIB. b) Photographic images of a conventional wearable healthcare bracelet and a self-charging system-integrated wearable device.
Figure 2. Illustration of the CQD PVs structure and performance of the wireless self-charging platform.
2018.10.08 View 7503 -
 Improved Efficiency of CQD Solar Cells Using an Organic Thin Film
(from left: Professor Jung-Yong Lee and Dr. Se-Woong Baek)
Recently, the power conversion efficiency (PCE) of colloidal quantum dot (CQD)-based solar cells has been enhanced, paving the way for their commercialization in various fields; nevertheless, they are still a long way from being commercialized due to their efficiency not matching their stability. In this research, a KAIST team achieved highly stable and efficient CQD-based solar cells by using an amorphous organic layer to block oxygen and water permeation.
CQD-based solar cells are light-weight, flexible, and they boost light harvesting by absorbing near-infrared lights. Especially, they draw special attention for their optical properties controlled efficiently by changing the quantum dot sizes. However, they are still incompatible with existing solar cells in terms of efficiency, stability, and cost. Therefore, there is great demand for a novel technology that can simultaneously improve both PCE and stability while using an inexpensive electrode material.
Responding to this demand, Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and his team introduced a technology to improve the efficiency and stability of CQD-based solar cells.
The team found that an amorphous organic thin film has a strong resistance to oxygen and water. Using these properties, they employed this doped organic layer as a top-hole selective layer (HSL) for the PbS CQD solar cells, and confirmed that the hydro/oxo-phobic properties of the layer efficiently protected the PbS layer. According to the molecular dynamics simulations, the layer significantly postponed the oxygen and water permeation into the PbS layer. Moreover, the efficient injection of the holes in the layer reduced interfacial resistance and improved performance.
With this technology, the team finally developed CQD-based solar cells with excellent stability. The PCE of their device stood at 11.7% and maintained over 90% of its initial performance when stored for one year under ambient conditions.
Professor Lee said, “This technology can be also applied to QD LEDs and Perovskite devices. I hope this technology can hasten the commercialization of CQD-based solar cells.”
This research, led by Dr. Se-Woong Baek and a Ph.D. student, Sang-Hoon Lee, was published in Energy & Environmental Science on May 10.
Figure 1. The schematic of the equilibrated structure of the amorphous organic film
Figure 2. Schematic illustration of CQD-based solar cells and graphs showing their performance
2018.08.27 View 7278
Improved Efficiency of CQD Solar Cells Using an Organic Thin Film
(from left: Professor Jung-Yong Lee and Dr. Se-Woong Baek)
Recently, the power conversion efficiency (PCE) of colloidal quantum dot (CQD)-based solar cells has been enhanced, paving the way for their commercialization in various fields; nevertheless, they are still a long way from being commercialized due to their efficiency not matching their stability. In this research, a KAIST team achieved highly stable and efficient CQD-based solar cells by using an amorphous organic layer to block oxygen and water permeation.
CQD-based solar cells are light-weight, flexible, and they boost light harvesting by absorbing near-infrared lights. Especially, they draw special attention for their optical properties controlled efficiently by changing the quantum dot sizes. However, they are still incompatible with existing solar cells in terms of efficiency, stability, and cost. Therefore, there is great demand for a novel technology that can simultaneously improve both PCE and stability while using an inexpensive electrode material.
Responding to this demand, Professor Jung-Yong Lee from the Graduate School of Energy, Environment, Water and Sustainability and his team introduced a technology to improve the efficiency and stability of CQD-based solar cells.
The team found that an amorphous organic thin film has a strong resistance to oxygen and water. Using these properties, they employed this doped organic layer as a top-hole selective layer (HSL) for the PbS CQD solar cells, and confirmed that the hydro/oxo-phobic properties of the layer efficiently protected the PbS layer. According to the molecular dynamics simulations, the layer significantly postponed the oxygen and water permeation into the PbS layer. Moreover, the efficient injection of the holes in the layer reduced interfacial resistance and improved performance.
With this technology, the team finally developed CQD-based solar cells with excellent stability. The PCE of their device stood at 11.7% and maintained over 90% of its initial performance when stored for one year under ambient conditions.
Professor Lee said, “This technology can be also applied to QD LEDs and Perovskite devices. I hope this technology can hasten the commercialization of CQD-based solar cells.”
This research, led by Dr. Se-Woong Baek and a Ph.D. student, Sang-Hoon Lee, was published in Energy & Environmental Science on May 10.
Figure 1. The schematic of the equilibrated structure of the amorphous organic film
Figure 2. Schematic illustration of CQD-based solar cells and graphs showing their performance
2018.08.27 View 7278 -
 Computer Simulation Identifies a Key Principle for Next-generation Carbon Fibers
(from left: Professor Yong-Hoon Kim and PhD candidate Juho Lee)
Performing state-of-the-art computer simulations, a KAIST research team identified an atomistic design principle to produce high-quality, next-generation carbon fibers.
Carbon fibers are light-weight yet excellent in mechanical strength and thermal resistance. Boasting these properties, they can be diversely applied in high-technology sectors, including automotive, aerospace, and nuclear engineering.
They are produced from a polymer precursor through a series of spinning, stabilization, and carbonization processes. However, there is a major obstacle to producing high-quality carbon fibers. That is, when there exist ill-defined regions within the polymer matrixes, they result in disorder and defects within the produced carbon fibers.
As a solution to this problem, it was proposed that the introduction of carbon nanotubes (CNT) could enhance polymer orientation and crystallization. However, although the alignment geometry of the CNT-polymer interface apparently affects the quality of produced fibers, the atomistic understanding of the CNT-polymer interface has been so far lacking, hindering further developments.
To clarify the nature of CNT-polymer interactions, Professor Yong-Hoon Kim from the Graduate School of Energy, Environment, Water and Sustainability and his team employed a multiscale approach that combines first-principles density functional theory (DFT) calculations and force-fields molecular dynamics (MD) simulations and revealed the unique structural and electronic characteristics of polymer-CNT interfaces.
Here, they studied polyacrylonitrile (PAN)-CNT hybrid structures as a representative case of polymer-CNT composites. PAN is the most common polymer precursor, taking more than 90% of carbon fiber production.
Based on their DFT calculations, the team showed that the lying-down PAN configurations give a larger PAN-CNT binding energy than their standing-up counterparts. Moreover, maximizing the lying-down PAN configuration was shown to allow linear alignments of PANs on CNT, enabling the desirable ordered long-range PAN-PAN packing.
They also identified the CNT curvature as another significant factor, giving the largest PAN-CNT binding energy in the zero-curvature graphene limit. Conducting large-scale MD simulations, they then demonstrated that graphene nanoribbons are a promising carbon nano-reinforcement candidate by explicitly showing its strong propensity to induce linear alignments of PANs adsorbed on them.
Professor Kim said, “This research can be an exemplary case where the quantum mechanical simulations identify basic principles for developing advanced materials. Computer simulation studies will play an increasingly greater role thanks to the advances in the simulation theory and computer performance.”
This research, carried out by the PhD candidate Juho Lee, was published in the inside back cover of Advanced Functional Materials on April 11.
Figure 1. Inside back cover of Advanced Functional Materials
Figure 2. Research outline
2018.08.03 View 7885
Computer Simulation Identifies a Key Principle for Next-generation Carbon Fibers
(from left: Professor Yong-Hoon Kim and PhD candidate Juho Lee)
Performing state-of-the-art computer simulations, a KAIST research team identified an atomistic design principle to produce high-quality, next-generation carbon fibers.
Carbon fibers are light-weight yet excellent in mechanical strength and thermal resistance. Boasting these properties, they can be diversely applied in high-technology sectors, including automotive, aerospace, and nuclear engineering.
They are produced from a polymer precursor through a series of spinning, stabilization, and carbonization processes. However, there is a major obstacle to producing high-quality carbon fibers. That is, when there exist ill-defined regions within the polymer matrixes, they result in disorder and defects within the produced carbon fibers.
As a solution to this problem, it was proposed that the introduction of carbon nanotubes (CNT) could enhance polymer orientation and crystallization. However, although the alignment geometry of the CNT-polymer interface apparently affects the quality of produced fibers, the atomistic understanding of the CNT-polymer interface has been so far lacking, hindering further developments.
To clarify the nature of CNT-polymer interactions, Professor Yong-Hoon Kim from the Graduate School of Energy, Environment, Water and Sustainability and his team employed a multiscale approach that combines first-principles density functional theory (DFT) calculations and force-fields molecular dynamics (MD) simulations and revealed the unique structural and electronic characteristics of polymer-CNT interfaces.
Here, they studied polyacrylonitrile (PAN)-CNT hybrid structures as a representative case of polymer-CNT composites. PAN is the most common polymer precursor, taking more than 90% of carbon fiber production.
Based on their DFT calculations, the team showed that the lying-down PAN configurations give a larger PAN-CNT binding energy than their standing-up counterparts. Moreover, maximizing the lying-down PAN configuration was shown to allow linear alignments of PANs on CNT, enabling the desirable ordered long-range PAN-PAN packing.
They also identified the CNT curvature as another significant factor, giving the largest PAN-CNT binding energy in the zero-curvature graphene limit. Conducting large-scale MD simulations, they then demonstrated that graphene nanoribbons are a promising carbon nano-reinforcement candidate by explicitly showing its strong propensity to induce linear alignments of PANs adsorbed on them.
Professor Kim said, “This research can be an exemplary case where the quantum mechanical simulations identify basic principles for developing advanced materials. Computer simulation studies will play an increasingly greater role thanks to the advances in the simulation theory and computer performance.”
This research, carried out by the PhD candidate Juho Lee, was published in the inside back cover of Advanced Functional Materials on April 11.
Figure 1. Inside back cover of Advanced Functional Materials
Figure 2. Research outline
2018.08.03 View 7885 -
 Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 12156
Aqueous Storage Device Needs Only 20 Seconds to Go
(from left: PhD candidate Il Woo Ock and Professor Jeung Ku Kang)
A KAIST research team developed a new hybrid energy storage device that can be charged in less than half a minute. It employs aqueous electrolytes instead of flammable organic solvents, so it is both environmentally friendly and safe. It also facilitates a boosting charge with high energy density, which makes it suitable for portable electronic devices.
Professor Jeung Ku Kang and his team from the Graduate School of Energy, Environment, Water, and Sustainability developed this hybrid energy storage with high energy and power densities along over a long cycle life by assembling fibre-like polymer chain anodes and sub-nanoscale metal oxide cathodes on graphene.
Conventional aqueous electrolyte-based energy storage devices have a limitation for boosting charges and high energy density due to low driving voltage and a shortage of anode materials.
Energy storage device capacity is determined by the two electrodes, and the balance between cathode and anode leads to high stability. In general, two electrodes show differences in electrical properties and differ in ion storage mechanism processes, resulting in poor storage and stability from the imbalance.
The research team came up with new structures and materials to facilitate rapid speed in energy exchange on the surfaces of the electrodes and minimize the energy loss between the two electrodes.
The team made anodes with graphene-based polymer chain materials. The web-like structure of graphene leads to a high surface area, thereby allowing higher capacitance.
For cathode materials, the team used metal oxide in sub-nanoscale structures to elevate atom-by-ion redox reactions. This method realized higher energy density and faster energy exchange while minimizing energy loss.
The developed device can be charged within 20 to 30 seconds using a low-power charging system, such as a USB switching charger or a flexible photovoltaic cell. The developed aqueous hybrid energy device shows more than 100-fold higher power density compared to conventional aqueous batteries and can be rapidly recharged. Further, the device showed high stability with its capacity maintained at 100% at a high charge/discharge current.
Professor Kang said, “This eco-friendly technology can be easily manufactured and is highly applicable. In particular, its high capacity and high stability, compared to existing technologies, could contribute to the commercialization of aqueous capacitors. The device can be rapidly charged using a low-power charging system, and thus can be applied to portable electronic device.”
This research, led by a PhD candidate Il Woo Ock, was published in Advanced Energy Materials on January 15.
Figure 1. Switching wearable LED kit with two AHCs in series charged by a flexible photovoltaic cell
Figure 2. Schematic diagram for aqueous hybrid capacitors
Figure 3. TEM images of anode and cathode
2018.02.28 View 12156 -
 Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 9091
Highly-Efficient Photoelectrochemical CO2 Reduction
Direct CO2 conversion has continuously attracted a great deal of attention as a technology to produce fuels and chemical building blocks from renewable energy resources. Specifically, substances such as carbon feedstocks and fuels can be produced by utilizing sunlight, water, and CO2 as semiconductors and a water interface through photoelectrochemical CO2 reduction.
A KAIST research team demonstrated a novel photoelectrode structure for highly-selective and efficient photoelectrochemical CO2 reduction reactions. The research team led by Professor Jihun Oh of the Graduate School of EEWS (Energy, Environment, Water and Sustainability) presented a Si photoelectrode with a nanoporous Au thin film that is capable of reducing CO2 to CO with 90 percent selectivity in aqueous solution. The research team’s technology will provide a basic framework for designing the semiconductor photoelectrode structure necessary for photoelectrochemical conversion.
In order to achieve steady conversion of CO2, it is necessary to use a high-performance catalyst to lower overpotential. Among the metal catalysts, Au is known to be an electrocatalyst that converts CO2 to CO.
Conventionally, bare Au, as a catalyst, produces a lot of hydrogen gas due to its low CO selectivity. In addition, the high cost of Au remains a challenge in using the catalyst. Professor Oh’s research team addressed the issue by creating a nanoporous Au thin film formed by the electrochemical reduction of an anodized Au thin film.
As a result, the team could demonstrate an efficient, selective photoelectrochemical reduction reaction of CO2 to CO using electrochemically-treated Au thin films on a Si photoelectrode. The electrochemical reduction on anodized Au thin films forms a nanoporous thin layer exhibiting many grain boundaries of nanoparticles on the Au surface. This dramatically improves the selectivity of the reduction reaction with a maximum CO faradaic efficiency of over 90% at low overpotential and durability.
The research team also used an Au thin film of about 200 nanometers, 50,000 times thinner than previously reported nanostructured Au catalysts, resulting in a cost-effective catalyst. When depositing the catalyst on the semiconductor surface in the type of nanoparticles, the substrate of the thin film will be affected in the course of electrochemical reduction.
Thus, the research team designed a new Si photoelectrode with mesh-type co-catalysts that are independently wired at the front and back of the photoelectrode without influencing the photoelectrode, and made it possible for electrochemical reduction.
Due to the superior CO2 reduction reaction activity of the nanoporous Au mesh and high photovoltage from Si, the Si photoelectrode with the nanoporous Au thin film mesh shows conversion of CO2 to CO with 91% Faradaic efficiency at positive potential than CO equilibrium potential.
Professor Oh explained, “This technology will serve as a platform for diverse semiconductors and catalysts. Researchers can further improve the solar-to-CO2 conversion efficiency using this technology.
Dr. Jun Tae Song, the first author continued, “This new approach made it possible to develop a simple but very important type of electrode structure. It is the first time to achieve CO2 conversion at the potential lower than equilibrium potential. We believe that our research will contribute to efficient CO2 conversion.”
This research was published in the inside front cover of Advanced Energy Materials on February 8, 2017. The research was funded and supported by the Korea Carbon Capture & Sequestration R&D Center. Professor Sung-Yoon Chung of the EEWS also participated in this research.
(Figure: Schematic diagram of a Si photoelectrode that patterns with mesh-type nanoporous Au)
2017.03.08 View 9091 -
 Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 9362
Adsorbent That Can Selectively Remove Water Contaminants
Professor Cafer T. Yavuz and his team at the Graduate School of Energy, Environment, Water, and Sustainability (EEWS) have developed an adsorbent that can selectively capture soluble organic contaminants in water.
This water treatment adsorbent is a fluorine-based nanoporous polymer that can selectively remove water-soluble micromolecules. It has the added advantage of being cheap and easily synthesized, while also being renewable.
The results of this research have been published online in Nature Communication on November 10, 2016. The research paper is titled “Charge-specific Size-dependent Separation of Water-soluble Organic Molecules by Fluorinated Nanoporous Networks.” (DOI: 10.1038/ncomms13377)
Water pollution is accelerating as a result of global industrial development and warming. As new materials are produced and applied in the agricultural and industrial sectors, the types of contaminants expelled as sewage and waste water are also becoming diverse.
Chemicals such as dyes and pesticides can be especially harmful because they are made up of small and highly soluble organic particles that cannot be completely removed during the water treatment process, ultimately ending up in our drinking water.
The current conventional water treatment systems utilize processes such as activated carbon, ozonolysis, and reverse osmosis membrane. These processes, however, are designed to remove larger organic molecules with lower solubility, thus removal of very small molecules with high solubility is difficult. In addition, these micromolecules tend to be charged, therefore are less easily separated in aqueous form.
The research team aimed to remove these small molecules using a new adsorbent technology.
In order to remove aqueous organic molecular contaminants, the team needed an adsorbent that can adsorb micro-sized molecules. It also needed to introduce a chemical function that would allow it to selectively adsorb molecules, and lastly, the adsorbent needed to be structurally stable as it would be used underwater.
The team subsequently developed an adsorbent of fluorine-based porous organic polymer that met all the conditions listed above. By controlling the size of the pores, this adsorbent is able to selectively adsorb aqueous micromolecules of less than 1-2 nm in size.
In addition, in order to separate specific contaminants, there should be a chemical functionality, such as the ability to strongly interact with the target material. Fluorine, the most electronegative atom, interacts strongly with charged soluble organic molecules.
The research team incorporated fluorine into an adsorbent, enabling it to separate charged organic molecules up to 8 times faster than neutral molecules.
The adsorbent developed by Professor Yavuz’s team has wide industrial applications. It can be used in batch-adsorption tests, as well as in column separation for size- and charge-specific adsorption.
Professor Yavuz stated that “the charge-selective properties displayed by fluorine has the potential to be applied in desalination or water treatment processes using membranes."
This paper was first-authored by Dr. Jeehye Byun, and the research was funded by KAIST’s High Risk High Return Program and the Ministry of Science, ICT and Future Planning of Korea’s Mid-Career Researcher Program, as well as its Technology Development Program to Solve Climate Change.
Figure 1. Diagram conceptualizing the process of charge- and size-specific separation by the fluorine-based porous polymer adsorbent
Figure 2. Difference in absorbance before and after using a porous fluorine polymer column to separate organic molecules
Figure 3. Adsorption properties of a fluorine polymer according to the charge and size of organic molecules
2017.01.17 View 9362 -
 Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
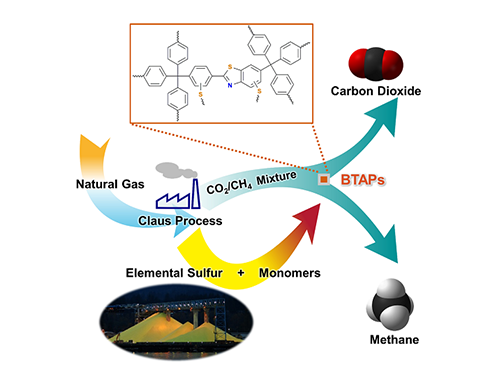
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 8341
Direct Utilization of Elemental Sulfur for Microporous Polymer Synthesis
Using elemental sulfur as an alternative chemical feedstock, KAIST researchers have produced novel microporous polymers to sift CO2 from methane in natural-gas processing.
Methane, a primary component of natural gas, has emerged recently as an important energy source, largely owing to its abundance and relatively clean nature compared with other fossil fuels. In order to use natural gas as a fuel, however, it must undergo a procedure called “hydrodesulfurization” or “natural gas sweetening” to reduce sulfur-dioxide emissions from combustion of fossil fuels. This process leads to excessive and involuntary production of elemental sulfur. Although sulfur is one of the world’s most versatile and common elements, it has relatively few large-scale applications, mostly for gunpowder and sulfuric acid production.
Thus, the development of synthetic and processing methods to convert sulfur into useful chemicals remains a challenge. A research team led by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) has recently introduced a new approach to resolving this problem by employing elemental sulfur directly in the synthesis of microporous polymers for the process of natural-gas sweetening.
Natural gas, containing varying amounts of carbon dioxide (CO2) and hydrogen sulfide (H2S), is generally treated with amine solutions, followed by the regeneration of these solutions at increased temperatures to release captured CO2 and H2S. A two-step separation is involved in removing these gases. The amine solutions first remove H2S, and then CO2 is separated from methane (CH4) with either amine solutions or porous sorbents such as microporous polymers.
Using elemental sulfur and organic linkers, the research team developed a solvent and catalyst-free strategy for the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were found to be highly porous and showed exceptional physiochemical stability. In-situ chemical impregnation of sulfur within the micropores increased CO2 affinity of the sorbent, while limiting diffusion of CH4. BTAPs, as low-cost, scalable solid-sorbents, showed outstanding CO2 separation ability for flue gas, as well as for natural and landfill gas conditions.
The team noted that: “Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues.”
This work was published online in Chem on September 8, 2016 and also highlighted in C&EN (Chemical & Engineering News) by the American Chemical Society (ACS) on September 19, 2016. The research paper was entitled “Direct Utilization of Elemental Sulfur in the Synthesis of Microporous Polymers for Natural Gas Sweetening.” (DOI: 10.1016/j.chempr.2016.08.003)
Figure 1. A Schematic Image of Direct Utilization of Elemental Sulfur
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance.
Figure 2. BTAP’s Breakthrough Experiment under Pre-mixed Gas Conditions
This data presents the breakthrough measurements for CO2-containing binary gas-mixture streams with different feed-gas compositions to investigate the CO2 capture capacity of ultramicroporous benzothiazole polymers (BTAPs) for large-scale applications under simulated conditions of natural and landfill gases.
2016.10.05 View 8341 -
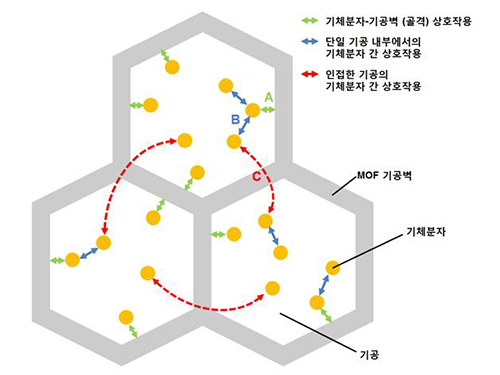 A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 7772
A New Way to Look at MOFs
An international research team composed of researchers from KAIST (led by Professors Osamu Terasaki and Jeung Ku Kang at the Graduate School of Energy, Environment, Water and Sustainability) and other universities, including UC Berkeley, has recently published research results on the adsorption process of metal-organic frameworks (MOFs) in Nature (November 9, 2015).
MOFs are porous three-dimensional crystals with a high internal surface area, which have a wide range of applications involving adsorption such as hydrogen, methane, or carbon dioxide storage. In the paper entitled “Extra Adsorption and Adsorbate Superlattice Formation in Metal-organic Frameworks,” the research team described their observation of a very specific interpore interaction process in MOFs.
For additional information, please see:
A New Way to Look at MOFs
International study challenges prevailing view on how metal organic frameworks store gases
EurekAlert, November 9, 2015
http://www.eurekalert.org/pub_releases/2015-11/dbnl-anw110915.php
(Courtesy of the US Department of Energy and Lawrence Berkeley National Laboratory news release)
2015.11.13 View 7772 -
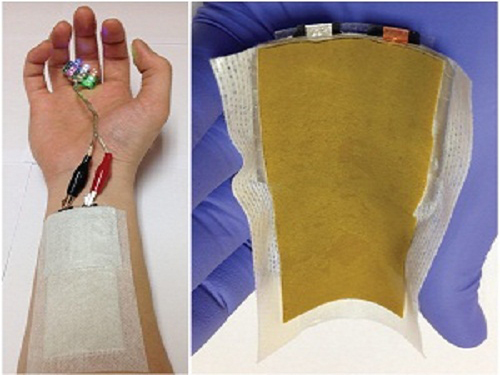 KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 10705
KAIST Develops a Credit-Card-Thick Flexible Lithium Ion Battery
Since the battery can be charged wirelessly, useful applications are expected including medical patches and smart cards.
Professor Jang Wook Choi at KAIST’s Graduate School of Energy, Environment, Water, and Sustainability (EEWS) and Dr. Jae Yong Song at the Korea Research Institute of Standards and Science jointly led research to invent a flexible lithium ion battery that is thinner than a credit card and can be charged wirelessly.
Their research findings were published online in Nano Letters on March 6, 2015. Lithium ion batteries are widely used today in various electronics including mobile devices and electronic cars. Researchers said that their work could help accelerate the development of flexible and wearable electronics.
Conventional lithium ion batteries are manufactured based on a layering technology, stacking up anodes, separating films, and cathodes like a sandwich, which makes it difficult to reduce their thickness. In addition, friction arises between layers, making the batteries impossible to bend. The coating films of electrodes easily come off, which contributes to the batteries’ poor performance.
The research team abandoned the existing production technology. Instead, they removed the separating films, layered the cathodes and anodes collinearly on a plane, and created a partition between electrodes to eliminate potential problems, such as short circuits and voltage dips, commonly present in lithium ion batteries.
After more than five thousand consecutive flexing experiments, the research team confirmed the possibility of a more flexible electrode structure while maintaining the battery performance comparable to the level of current lithium ion batteries.
Flexible batteries can be applied to integrated smart cards, cosmetic and medical patches, and skin adhesive sensors that can control a computer with voice commands or gesture as seen in the movie “Iron Man.”
Moreover, the team has successfully developed wireless-charging technology using electromagnetic induction and solar batteries.
They are currently developing a mass production process to combine this planar battery technology and printing, to ultimately create a new paradigm to print semiconductors and batteries using 3D printers.
Professor Choi said, “This new technology will contribute to diversifying patch functions as it is applicable to power various adhesive medical patches.”
Picture 1: Medical patch (left) and flexible secondary battery (right)
Picture 2: Diagram of flexible battery
Picture 3: Smart card embedding flexible battery
2015.03.24 View 10705