research

(Professor Hyoungsoo Kim, Department of Mechanical Engineering, KAIST)
A research team led by Hyoungsoo Kim, a professor of Mechanical Engineering at KAIST, succeeded in quantifying the phenomenon called, the Marangoni effect, which occurs at the interface between alcohol and water. It is expected that this finding will be a valuable resource used for effectively removing impurities from a surface fluid without any contamination, and developing materials that can replace surfactants.
This research, co-conducted with a research team led by Professor Howard A. Stone at Princeton University, was published online in Nature Physics on July 31.
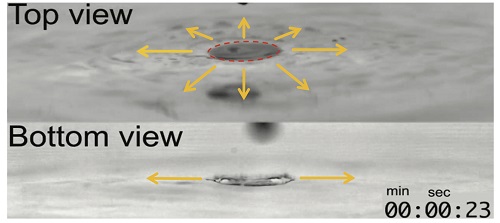
The Marangoni effect, also known as tears of wine, is generated when two fluids having a different surface tension meet, causing finite mixing, spreading time and length scale. Typically, people believe that infinitely miscible liquids immediately mix together; however, it is not always true according to this paper.
The typical surface tension of alcohol is three times lower than that of water, and this different surface tension generates the Marangoni-driven convection flow at the interface of the two liquids. In addition, there is a certain amount of time required for them to mix.
This phenomenon has been discussed many times since it was discovered in early the 20th century, yet there was a limit to quantifying and explaining it.
Professor Kim, considering the mixing and spreading mechanism, used various flow visualization techniques and equipment for capturing high speed images in his experiment.
Through the flow visualization methods, the team succeeded in quantifying and explaining the complex, physicochemical phenomenon generated between water and alcohol. Moreover, they developed a theoretical model to predict the physicochemical hydrodynamic phenomena.
The theoretical model can predict the speed of Marangoni-driven convection flow, the area of a drop of alcohol and the time required to develop the flow field. Hence, this model can map out types of materials (e.g., alcohol) and the volume of a drop of liquid as applicable to target a specific situation.
Moreover, the research team believes that the interfacial flow enables the driving of bulk flows and that it can be a source of technology for effectively delivering drugs and removing impurities from a surface of substance without causing secondary contamination.
Above all, the results show a possibility for replacing surfactant with alcohol as a material used for delivering drugs. In the case of the drug delivery, some drugs are encapsulated with a surfactant in order to be effectively transported in vivo; however, the surfactant accumulates in the body, which can cause various side effects, such as heart disease. Therefore, using new materials like alcohol for drug delivery will contribute to preventing the side effects caused by the surfactant.
“The surfactant is used for delivering drugs, but it is difficult to be expelled from the body. This will cause various side effects, such as heart diseases in asthmatic patients,” said Professor Kim. “I hope that using new materials, like alcohol, will free people from these side effects.”
(Marangoni-driven convection flow generated at the interface between water and alcohol, and the flow visualization results)
- A drop of alcohol on a water surface
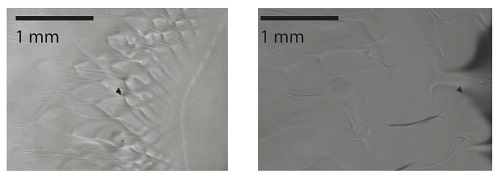
- Comparison of mixing structures on the surface

- Marangoni mixing flow under the free surface
-
research A Hole in One for Holographic Display
(Professor YongKeun Park) Researchers have designed an ultrathin display that can project dynamic, multi-coloured, 3D holographic images, according to a study published in Nature Communications. The system’s critical component is a thin film of titanium filled with tiny holes that precisely correspond with each pixel in a liquid crystal display (LCD) panel. This film acts as a ‘photon sieve’ – each pinhole diffracts light emerging from them widely, resulting in a
2019-04-18 -
research Unravelling Inherent Electrocatalysis to Improve the Performance of Hydrogen Fuel Cells
(Figure 1. Electrode structure for the precise evaluation of the metal nanoparticles’ electrochemical catalytic characteristics at a high temperature.) A KAIST team presented an ideal electrode design to enhance the performance of high-temperature fuel cells. The new analytical platform with advanced nanoscale patterning method quantitatively revealed the electrochemical value of metal nanoparticles dispersed on the oxide electrode, thus leading to electrode design directions that c
2019-03-28 -
research New Catalyst for Synthesizing Chiral Molecules Selectively
(from left: Dr. Yoonsu Park and Professor Sukbok Chang from the Department of Chemistry) Molecules in nature often have “twin” molecules that look identical. In particular, the twin molecules that look like mirror images to each other are called enantiomers. However, even though they have the same type and number of elements, these twin molecules exhibit completely different properties. Professor Sukbok Chang and Dr. Yoonsu Park from the Department of Chemistry
2019-03-05 -
research New LSB with Theoretical Capacity over 90%
(Professor Hee-Tak Kim and Hyunwon Chu) A KAIST research team has developed a lithium sulfur battery (LSB) that realizes 92% of the theoretical capacity and an areal capacity of 4mAh/cm2. LSBs are gaining a great deal of attention as an alternative for lithium ion batteries (LIBs) because they have a theoretical energy density up to six to seven times higher than that of LIBs, and can be manufactured in a more cost-effective way. However, LSBs face the obstacle of
2019-02-11 -
research Noninvasive Light-Sensitive Recombinase for Deep Brain Genetic Manipulation
A KAIST team presented a noninvasive light-sensitive photoactivatable recombinase suitable for genetic manipulation in vivo. The highly light-sensitive property of photoactivatable Flp recombinase will be ideal for controlling genetic manipulation in deep mouse brain regions by illumination with a noninvasive light-emitting diode. This easy-to-use optogenetic module made by Professor Won Do Heo and his team will provide a side-effect free and expandable genetic manipulation tool for neurosci
2019-01-22