research
This catalyst capability allowing stable hydrogen production from commercial diesel is expected to be applied in mobile fuel cell systems in the future hydrogen economy
On August 16, a joint research team led by Professors Joongmyeon Bae and Kang Taek Lee of KAIST’s Department of Mechanical Engineering and Dr. Chan-Woo Lee of Korea Institute of Energy Research (KIER) announced the successful development of a highly active and durable reforming catalyst allowing hydrogen production from commercial diesel.
Fuel reforming is a hydrogen production technique that extracts hydrogen from hydrocarbons through catalytic reactions. Diesel, being a liquid fuel, has a high storage density for hydrogen and is easy to transport and store. There have therefore been continuous research efforts to apply hydrogel supply systems using diesel reformation in mobile fuel cells, such as for auxiliary power in heavy trucks or air-independent propulsion (AIP) systems in submarines.
However, diesel is a mixture of high hydrocarbons including long-chained paraffin, double-bonded olefin, and aromatic hydrocarbons with benzene groups, and it requires a highly active catalyst to effectively break them down. In addition, the catalyst must be extremely durable against caulking and sintering, as they are often the main causes of catalyst degradation. Such challenges have limited the use of diesel reformation technologies to date.
The joint research team successfully developed a highly active and durable diesel reforming catalyst through elution (a heat treatment method used to uniformly grow active metals retained in an oxide support as ions in the form of metal nanoparticles), forming alloy nanoparticles. The design was based on the fact that eluted nanoparticles strongly interact with the support, allowing a high degree of dispersion at high temperatures, and that producing an alloy from dissimilar metals can increase the performance of catalysts through a synergistic effect.
The research team introduced a solution combustion synthesis method to produce a multi-component catalyst with a trace amount of platinum (Pt) and ruthenium (Ru) penetrated into a ceria (CeO2) lattice, which is a structure commonly used as a support for catalysts in redox reactions. When exposed to a diesel reforming reaction environment, the catalyst induces Pt-Ru alloy nanoparticle formation upon Pt and Ru elution onto the support surface.
In addition to the catalyst analysis, the research team also succeeded in characterizing the behaviour of active metal elution and alloy formation from an energetic perspective using a density functional theory-based calculation. In a performance comparison test between the Pt-Ru alloy catalyst against existing single-metal catalysts, the reforming activity was shown to have improved, as it showed a 100% fuel conversion rate even at a low temperature (600oC, compared to the original 800oC). In a long-term durability test (800oC, 200 hours), the catalyst showed commercial stability by successfully producing hydrogen from commercial diesel without performance degradation.
The study was conducted by Ph.D. candidate Jaemyung Lee of KAIST’s Department of Mechanical Engineering as the first author. Ph.D. candidate Changho Yeon of KIER, Dr. Jiwoo Oh of KAIST’s Department of Mechanical Engineering, Dr. Gwangwoo Han of KIER, Ph.D. candidate Jeong Do Yoo of KAIST’s Department of Mechanical Engineering, and Dr. Hyung Joong Yun of the Korea Basic Science Institute contributed as co-authors. Dr. Chan-Woo Lee of KIER and Professors Kang Taek Lee and Joongmyeon Bae of KAIST’s Department of Mechanical Engineering contributed as corresponding authors. The research was published in the online version of Applied Catalysis B: Environmental (IF 24.319, JCR 0.93%) on June 17, under the title “Highly Active and Stable Catalyst with Exsolved PtRu Alloy Nanoparticles for Hydrogen Production via Commercial Diesel Reforming”.
Professor Joongmyeon Bae said, “The fact that hydrogen can be stably produced from commercial diesel makes this a very meaningful achievement, and we look forward to this technology contributing to the active introduction of mobile fuel cell systems in the early hydrogen economy.” He added, “Our approach to catalyst design may be applied not only to reforming reactions, but also in various other fields.”
This research was supported by the National Research Foundation of Korea through funding from the Ministry of Science, ICT and Future Planning.
.png)
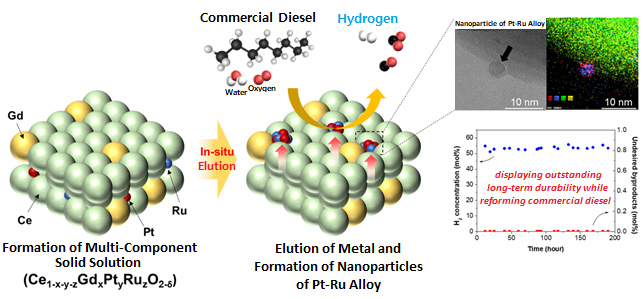
Figure. Schematic diagram of high-performance diesel reforming catalyst with eluted platinum-ruthenium alloy nanoparticles and long-term durability verification experiment results for commercial diesel reforming reaction
-
policy KAIST Entrepreneurial Partnership to Accelerate Startups and Venture Ecosystem
KAIST will launch the KAIST Entrepreneurial Partnership (KEP) program, which connects faculty members who own technology with those who want to launch startup. The program encourages open innovation startups using strategies tailored to market-client demand requirements. This is also one of efforts to help realize ‘one startup per lab,’ initiated by President Kwang Hyung Lee’s new innovation strategy. KEP also aims to introduce the best technologies developing at KAIST to st
2021-10-14 -
policy KAIST Technology Value Tops in Commercialization Market
KAIST became the first Korean university to achieve 10.183 billion KRW in annual technology royalties, and was also selected as an ‘Institution of Outstanding Patent Quality Management’ and an ‘Institution of Outstanding Public Patent Technology Transfer’ for 2020. KAIST earns its technology royalties through 56 technology transfer contracts. Following KAIST in the rankings were Seoul National University (SNU) in second place with 8.8 billion KRW from 87 contracts and
2020-08-18 -
policy Cyber MOU Signing with Zhejiang University
KAIST signed an MOU with Zhejiang University (ZJU) in China on March 25. This MOU signing ceremony took place via video conference due to the outbreak of COVID-19. The collaboration with ZJU had already started with the signing of an MOU for cooperation in technology commercialization last December. Possible cooperation initiatives included facilitating joint start-up businesses, patent portfolios, and technology marketing. With this general agreement signing, it is expected that the two ins
2020-03-30 -
research New Catalyst Recycles Greenhouse Gases into Fuel and Hydrogen Gas
< Professor Cafer T. Yavuz (left), PhD Candidate Youngdong Song (center), and Researcher Sreerangappa Ramesh (right) > Scientists have taken a major step toward a circular carbon economy by developing a long-lasting, economical catalyst that recycles greenhouse gases into ingredients that can be used in fuel, hydrogen gas, and other chemicals. The results could be revolutionary in the effort to reverse global warming, according to the researchers. The study was published on February 1
2020-02-17 -
people Professor Suh Chosen for IT Young Engineer Award
(The ceremony photo of Professor Changho Suh) Professor Changho Suh from the School of Electrical Engineering received the IT Young Engineer Award on June 28. This award is hosted by the Institute of Electrical and Electronics Engineers (IEEE) and the Institute of Electrical and Information Engineers (IEIE) and funded by the Haedong Science Foundation. The IT Young Engineer Award is given to researchers under the age of 40 in Korea. The selection criteria include the resea
2018-07-04