research
-
 Development of Gold Nanowire Probe Needle with an Increased Sensitivity
Professor Bongsoo Kim
The newly developed nano-probe needle’s thickness estimates only at 0.1 micrometers with an increased 1,000-fold sensitivity and spatial resolution of 1mm.
Professor Bongsoo Kim and his research team from the Department of Chemistry, KASIT, including the first author, Dr. Mijeong Kang, succeeded in measuring nerve signals of a mouse using the world’s thinnest nano-probe needle made of single-crystal gold nanowires.
The newly developed nano-probe needle possesses the thickness of 100 nanometers (nm), which shows 1,000 times more sensitivity than the conventional nerve probe needles, as well as accurately measures nerve signals with an extremely fine resolution of less than 1mm. Unlike the existing probe needles that cause neural tissues to be damaged during insertion, the new nano-probe needle minimizes the damage and thus can detect large nerve signals.
The brain neural probe, which collects and analyzes electrical nerve signals generated in the brain, is the most essential element in brain research. A neural probe should minimize tissue damages, but needs to possess a good electrical sensitivity.
The researchers first applied heat on the gold, which is the necessary material for a probe, until it turned to a vapor phase. Then, the gold evaporation slug was transported to a colder board and left to form single-crystal gold nano structures by condensation. Because the new gold nanowire, produced by using this principle, is a flawless single crystal structure, it shows strong and flexible properties.
Professor Kim and his team applied the nano-probe needle into the brain of a mouse that has been administered a drug to induce epilepsy. They were able to find the exact area in the brain that triggers epilepsy. Furthermore, the researchers also detected neural signal changes in the brain of the mouse when it encountered the intrusion of a stranger mouse.
Professor Bongsoo Kim commented the meaning of his research:
“The new nano-probe needle is able to detect signals from a single nerve cell with high sensitivity while preserving the nerve cells intact. The probe needle will be useful for creating a precise three-dimensional brain map, as well as providing electrical treatment for brain diseases such as dementia and Parkinson's disease.”
This research results were published online in the August 12, 2014 edition of ACS Nano.
2014.09.06 View 6453
Development of Gold Nanowire Probe Needle with an Increased Sensitivity
Professor Bongsoo Kim
The newly developed nano-probe needle’s thickness estimates only at 0.1 micrometers with an increased 1,000-fold sensitivity and spatial resolution of 1mm.
Professor Bongsoo Kim and his research team from the Department of Chemistry, KASIT, including the first author, Dr. Mijeong Kang, succeeded in measuring nerve signals of a mouse using the world’s thinnest nano-probe needle made of single-crystal gold nanowires.
The newly developed nano-probe needle possesses the thickness of 100 nanometers (nm), which shows 1,000 times more sensitivity than the conventional nerve probe needles, as well as accurately measures nerve signals with an extremely fine resolution of less than 1mm. Unlike the existing probe needles that cause neural tissues to be damaged during insertion, the new nano-probe needle minimizes the damage and thus can detect large nerve signals.
The brain neural probe, which collects and analyzes electrical nerve signals generated in the brain, is the most essential element in brain research. A neural probe should minimize tissue damages, but needs to possess a good electrical sensitivity.
The researchers first applied heat on the gold, which is the necessary material for a probe, until it turned to a vapor phase. Then, the gold evaporation slug was transported to a colder board and left to form single-crystal gold nano structures by condensation. Because the new gold nanowire, produced by using this principle, is a flawless single crystal structure, it shows strong and flexible properties.
Professor Kim and his team applied the nano-probe needle into the brain of a mouse that has been administered a drug to induce epilepsy. They were able to find the exact area in the brain that triggers epilepsy. Furthermore, the researchers also detected neural signal changes in the brain of the mouse when it encountered the intrusion of a stranger mouse.
Professor Bongsoo Kim commented the meaning of his research:
“The new nano-probe needle is able to detect signals from a single nerve cell with high sensitivity while preserving the nerve cells intact. The probe needle will be useful for creating a precise three-dimensional brain map, as well as providing electrical treatment for brain diseases such as dementia and Parkinson's disease.”
This research results were published online in the August 12, 2014 edition of ACS Nano.
2014.09.06 View 6453 -
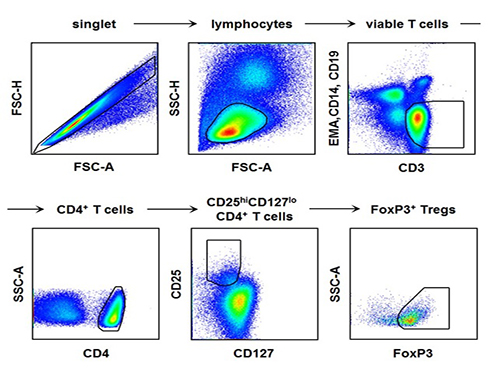 Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 9545
Regulatory T Cells Influence Liver Damage of Hepatitis A Patients
Liver damage becomes more severe with the decrease of regulatory T cells
“This research will aid the development of hepatitis A targeted drug,” said a KAIST researcher.
The KAIST Graduate School of Medical Science and Engineering’s Professor Eui-Cheol Shin and his research team have identified the mechanism, explaining how the regulatory T cells are responsible for the body’s immune system and how they have induced liver damage of hepatitis A patients.
The research results were published online in the July 9th edition of ‘Gut,’ the world’s most prominent journal in the field of gastroenterology.
Hepatitis A is an acute form of hepatitis caused by hepatitis A virus. The virus spreads through oral contact and enters the body via digestive organs.
Regulatory T cells play an important role in maintaining the homeostasis of the body’s immune system by inhibiting the activation of other immune cells. In the case of chronic viral infections, regulatory T cells are known to contribute to the duration of the infection, weakening the immune response to virus infections. However, there has been no information on what roles the regulatory T cells perform in the case of acute viral infections.
The research team used the fluorescence flow cytometry technique to determine the number and characteristics of a variety of immune cells, including regulatory T cells, in the blood of hepatitis A patients.
Consequently, the researchers confirmed that the decrease in the regulatory T cells immune inhibitory ability was consistent with a significant reduction in the number of regulatory T cells in the blood of hepatitis A patients. Furthermore, it was identified that the more noticeable decrease of regulatory T cells led to the occurrence of a more severe liver injury.
The analysis of hepatitis A patient’s blood proved that the cause of the decrease in the number and function of regulatory T cells was the increased expression of cell surface protein ‘Fas,’ which induces cell death.
Professor Shin said, “This study is the first case which proposes the mechanism for clinical aspects in not only hepatitis A, but also acute virus infection.” He added on the future prospect of the research that: “In the future, we can prevent tissue damage by inhibiting cell death of regulatory T cells for severe acute viral infections that do not have an effective treatment for the virus itself.”
[Picture]
The picture shows the process of fluorescence flow cytometry technique to study regulatory T cell in the blood of hepatitis A patients.
2014.08.11 View 9545 -
 The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 7604
The Journal of Clinical Investigation: Researchers Uncover the Secret Lymphatic Identity of the Schlemm's Canal
The Journal of Clinical Investigation (JCI), a peer-reviewed, top-tier medical journal published by the American Society for Clinical Investigation, carried a commentary entitled “Schlemm’s Canal: More Than Meets the Eye, Lymphatics in Disguise” in the July 25, 2014 issue.
In the commentary, the authors compared a research paper (“Lymphatic regular PROX1 determines Schlemm’s canal integrity and identity”) by Professor Gou-Young Koh of the Graduate School of Medical Science and Engineering at KAIST with research work from the University of Helsinki (article entitled “The Schlemm’s canal is a VEGF-C/VEGFR-3 responsive lymphatic-like vessel”).
The JCI released a press statement dated July 25, 2014 on its commentary. It mentioned that glaucoma, one of the leading causes of blindness worldwide, elevates eye pressure owing to poor drainage of aqueous humor. A specialized structure called “Schlemm’s canal” funnels aqueous humor from the eye back into circulation, which is critical to prevent pressure buildup in the eye. The article discussed the role of Schlemm’s canal in the context of lymphatic vascular characteristics by reviewing two research group’s papers back-to-back.
For the full text of the press release, please visit the link below:
Press Release from the Journal of Clinical Investigation, July 25, 2014
“Researchers uncover the secret lymphatic identity of the Schlemm’s canal”
http://www.eurekalert.org/pub_releases/2014-07/joci-rut072414.php
2014.07.28 View 7604 -
 Discovery of New Therapeutic Targets for Alzheimer's Disease
A Korean research team headed by Professor Dae-Soo Kim of Biological Sciences at KAIST and Dr. Chang-Jun Lee from the Korea Institute of Science and Technology (KIST) successfully identified that reactive astrocytes, commonly observed in brains affected by Alzheimer’s disease, produce abnormal amounts of inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) in reaction to the enzyme Monoamine oxidase B (Mao-B) and release GABA through the Bestrophin-1 channel to suppress the normal signal transmission of brain nerve cells.
By suppressing the GABA production or release from reactive astrocytes, the research team was able to restore the model mice's memory and learning impairment caused by Alzheimer’s disease. This discovery will allow the development of new drugs to treat Alzheimer’s and other related diseases.
The research result was published in the June 29, 2014 edition of Nature Medicine (Title: GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease).
For details, please read the article below:
Technology News, July 10, 2014
"Discovery of New Drug Targets for Memory Impairment in Alzheimer’s Disease"
http://technews.tmcnet.com/news/2014/07/10/7917811.htm
2014.07.16 View 8240
Discovery of New Therapeutic Targets for Alzheimer's Disease
A Korean research team headed by Professor Dae-Soo Kim of Biological Sciences at KAIST and Dr. Chang-Jun Lee from the Korea Institute of Science and Technology (KIST) successfully identified that reactive astrocytes, commonly observed in brains affected by Alzheimer’s disease, produce abnormal amounts of inhibitory neurotransmitter gamma-Aminobutyric acid (GABA) in reaction to the enzyme Monoamine oxidase B (Mao-B) and release GABA through the Bestrophin-1 channel to suppress the normal signal transmission of brain nerve cells.
By suppressing the GABA production or release from reactive astrocytes, the research team was able to restore the model mice's memory and learning impairment caused by Alzheimer’s disease. This discovery will allow the development of new drugs to treat Alzheimer’s and other related diseases.
The research result was published in the June 29, 2014 edition of Nature Medicine (Title: GABA from Reactive Astrocytes Impairs Memory in Mouse Models of Alzheimer’s Disease).
For details, please read the article below:
Technology News, July 10, 2014
"Discovery of New Drug Targets for Memory Impairment in Alzheimer’s Disease"
http://technews.tmcnet.com/news/2014/07/10/7917811.htm
2014.07.16 View 8240 -
 KAIST develops TransWall, a transparent touchable display wall
At a busy shopping mall, shoppers walk by store windows to find attractive items to purchase. Through the windows, shoppers can see the products displayed, but may have a hard time imagining doing something beyond just looking, such as touching the displayed items or communicating with sales assistants inside the store. With TransWall, however, window shopping could become more fun and real than ever before.
Woohun Lee, a professor of Industrial Design at KAIST, and his research team have recently developed TransWall, a two-sided, touchable, and transparent display wall that greatly enhances users' interpersonal experiences.
With an incorporated surface transducer, TransWall offers audio and vibrotactile feedback to the users. As a result, people can collaborate via a shared see-through display and communicate with one another by talking or even touching one another through the wall. A holographic screen film is inserted between the sheets of plexiglass, and beam projectors installed on each side of the wall project images that are reflected.
TransWall is touch-sensitive on both sides. Two users standing face-to-face on each side of the wall can touch the same spot at the same time without any physical interference. When this happens, TransWall provides the users with specific visual, acoustic, and vibrotactile experiences, allowing them to feel as if they are touching one another.
Professor Lee said, "TransWall concept enables people to see, hear, or even touch others through the wall while enjoying gaming and interpersonal communication. TransWall can be installed inside buildings, such as shopping centers, museums, and theme parks, for people to have an opportunity to collaborate even with strangers in a natural way."
He further added that "TransWall will be useful in places that require physical isolation for high security and safety, germ-free rooms in hospitals, for example." TransWall will allow patients to interact with family and friends without compromising medical safety.
TransWall was exhibited at the 2014 Conference on Computer-Human Interaction (CHI) held from April 26, 2014 to May 1, 2014 in Toronto, Canada.
YouTube Link:
http://www.youtube.com/watch?v=1QdYC_kOQ_w&list=PLXmuftxI6pTXuyjjrGFlcN5YFTKZinDhK
2014.07.15 View 7122
KAIST develops TransWall, a transparent touchable display wall
At a busy shopping mall, shoppers walk by store windows to find attractive items to purchase. Through the windows, shoppers can see the products displayed, but may have a hard time imagining doing something beyond just looking, such as touching the displayed items or communicating with sales assistants inside the store. With TransWall, however, window shopping could become more fun and real than ever before.
Woohun Lee, a professor of Industrial Design at KAIST, and his research team have recently developed TransWall, a two-sided, touchable, and transparent display wall that greatly enhances users' interpersonal experiences.
With an incorporated surface transducer, TransWall offers audio and vibrotactile feedback to the users. As a result, people can collaborate via a shared see-through display and communicate with one another by talking or even touching one another through the wall. A holographic screen film is inserted between the sheets of plexiglass, and beam projectors installed on each side of the wall project images that are reflected.
TransWall is touch-sensitive on both sides. Two users standing face-to-face on each side of the wall can touch the same spot at the same time without any physical interference. When this happens, TransWall provides the users with specific visual, acoustic, and vibrotactile experiences, allowing them to feel as if they are touching one another.
Professor Lee said, "TransWall concept enables people to see, hear, or even touch others through the wall while enjoying gaming and interpersonal communication. TransWall can be installed inside buildings, such as shopping centers, museums, and theme parks, for people to have an opportunity to collaborate even with strangers in a natural way."
He further added that "TransWall will be useful in places that require physical isolation for high security and safety, germ-free rooms in hospitals, for example." TransWall will allow patients to interact with family and friends without compromising medical safety.
TransWall was exhibited at the 2014 Conference on Computer-Human Interaction (CHI) held from April 26, 2014 to May 1, 2014 in Toronto, Canada.
YouTube Link:
http://www.youtube.com/watch?v=1QdYC_kOQ_w&list=PLXmuftxI6pTXuyjjrGFlcN5YFTKZinDhK
2014.07.15 View 7122 -
 Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 11986
Artificial Antibody-based Therapeutic Candidate for Lung Cancer Developed
Professor Hak-Sung Kim of Biological Sciences at KAIST publishes a cover article on artificial antibody in "Molecular Therapy".
Repebody-based lung cancer therapeutic drug candidate developed Repebody-based protein demonstrates the possibility of the development of a new drug
KAIST Biological Sciences Department’s Professor Hak-Sung Kim, in collaboration with Professor Eun-Kyung Cho from the College of Medicine at Chungnam National University, has successfully developed an artificial antibody-based, or repebody, cancer therapeutic candidate. These research results were published as a cover paper of the July edition of Molecular Therapy.
The repebody developed by Professor Kim and his team strongly binds to interleukin-6, a cancer-causing factor. It has also been confirmed that the repebody can significantly inhibit the proliferation of cancer cells in non-small-cell lung cancer animal model.
Numerous multinational pharmaceutical and biotechnology companies have invested astronomical amounts of money in research for the development of protein therapeutics with low side effects and high efficacy. More than 20 kinds of such therapeutics are currently under clinical trials, and over 100 drugs are under clinical demonstration. Among these, the majority is antibody-based therapeutics, and most of the investments are heavily concentrated in this field. However, antibody production cost is very high because it has large molecular weights and complex structural properties, and this makes it difficult to engineer. Consequently, the development costs a great deal of time and money.
In order to overcome the existing limitations of antibody-based therapeutics, Professor Kim and his team have developed a new artificial antibody, or repebody, which was published in Proceedings of the National Academy of Sciences (PNAS) in 2012. Based on this research, they have succeeded in developing a therapeutic candidate for treating non-small-cell lung cancer with a specifically strong cohesion to the cancer-causing factor, interleukin-6.
Interleukin-6 is a crucial substance within the body that is involved in immune and inflammatory-related signals. When abnormally expressed, it activates various carcinogenic pathways and promotes tumor growth and metastasis. Because of its importance, multinational pharmaceutical companies are heavily investing in developing therapeutics that can inhibit the signaling of interleukin-6.
In this study, Professor Kim and his team observed that a repebody consists of repeated modules, and they conceived a module-based affinity amplification technology that can effectively increase the binding affinity with the disease target. The developed therapeutic candidate has been confirmed in cell and animal experiments to show low immunogenicity, as well as to strongly inhibit the proliferation of non-small-cell lung cancer.
Furthermore, by investigating the complex structure of the repebody with interleukin-6, Professor Kim has identified its mechanism, which demonstrated the potential for therapeutic development. The researchers are currently carrying out pre-clinical trials for acquiring permission to perform clinical trials on animals with non-small-cell lung cancer. The repebody can be developed into a new protein drug after demonstrating its safety and efficacy.
Professor Hak-Sung Kim and his team have confirmed that the repebody can be utilized as a new protein drug, and this will be a significant contribution to Korea’s protein drugs and biotechnology industry development.
The research was supported by the Future Pioneer Industry project and sponsored by the Ministry of Science, ICT and Future Planning.
Figure 1. Professor Kim’s article published as the cover article of July edition of Molecular Therapy
Figure 2. Clinical proof of the repebody’s inhibition of cancer growth using animal models
2014.07.14 View 11986 -
 KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 8168
KAIST Researchers Develops Sensor That Reads Emotional States of Users
A piloerection monitoring sensor attached on the skin
The American Institute of Physics distributed a press release dated June 24, 2014 on a research paper written by a KAIST research team, which was published in its journal entitled Applied Physics Letters (APL). APL features concise, up-to-date reports in significant new findings in applied physics.
According to the release, “KAIST researchers have developed a flexible, wearable 20 mm x 20 mm polymer sensor that can directly measure the degree and occurrence on the skin of goose bumps, which is caused by sudden changes in body temperature or emotional states.”
The lead researcher was Professor Young-Ho Cho from the Department of Bio and Brain Engineering at KAIST.
If you would like to read the press release, please go to the link below:
American Institute of Physics, June 24, 2014
“New technology: The goose bump sensor”
http://www.eurekalert.org/pub_releases/2014-06/aiop-ntt062314.php
2014.06.26 View 8168 -
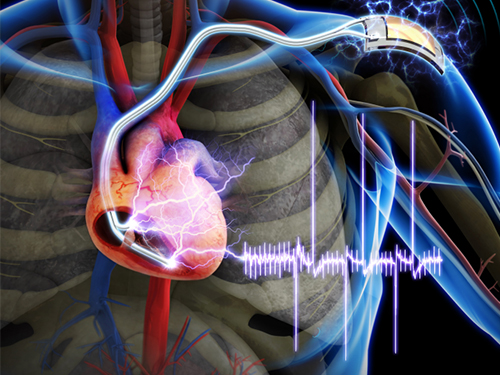 The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 15624
The First Demonstration of a Self-powered Cardiac Pacemaker
As the number of pacemakers implanted each year reaches into the millions worldwide, improving the lifespan of pacemaker batteries has been of great concern for developers and manufacturers. Currently, pacemaker batteries last seven years on average, requiring frequent replacements, which may pose patients to a potential risk involved in medical procedures.
A research team from the Korea Advanced Institute of Science and Technology (KAIST), headed by Professor Keon Jae Lee of the Department of Materials Science and Engineering at KAIST and Professor Boyoung Joung, M.D. of the Division of Cardiology at Severance Hospital of Yonsei University, has developed a self-powered artificial cardiac pacemaker that is operated semi-permanently by a flexible piezoelectric nanogenerator.
The artificial cardiac pacemaker is widely acknowledged as medical equipment that is integrated into the human body to regulate the heartbeats through electrical stimulation to contract the cardiac muscles of people who suffer from arrhythmia. However, repeated surgeries to replace pacemaker batteries have exposed elderly patients to health risks such as infections or severe bleeding during operations.
The team’s newly designed flexible piezoelectric nanogenerator directly stimulated a living rat’s heart using electrical energy converted from the small body movements of the rat. This technology could facilitate the use of self-powered flexible energy harvesters, not only prolonging the lifetime of cardiac pacemakers but also realizing real-time heart monitoring.
The research team fabricated high-performance flexible nanogenerators utilizing a bulk single-crystal PMN-PT thin film (iBULe Photonics). The harvested energy reached up to 8.2 V and 0.22 mA by bending and pushing motions, which were high enough values to directly stimulate the rat’s heart.
Professor Keon Jae Lee said:
“For clinical purposes, the current achievement will benefit the development of self-powered cardiac pacemakers as well as prevent heart attacks via the real-time diagnosis of heart arrhythmia. In addition, the flexible piezoelectric nanogenerator could also be utilized as an electrical source for various implantable medical devices.”
This research result was described in the April online issue of Advanced Materials (“Self-Powered Cardiac Pacemaker Enabled by Flexible Single Crystalline PMN-PT Piezoelectric Energy Harvester”: http://onlinelibrary.wiley.com/doi/10.1002/adma.201400562/abstract).
Youtube link: http://www.youtube.com/watch?v=ZWYT2cU_Mog&feature=youtu.be
Picture Caption: A self-powered cardiac pacemaker is enabled by a flexible piezoelectric energy harvester.
2014.06.25 View 15624 -
 Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 7475
Professor Won Do Heo on LED Light Technology for Controlling Proteins in Living Cells
With the newly developed LED technology, Professor Won Do Heo at the College of Life Science and Bioengineering, KAIST, was able to suppress cell migration and division when cells are exposed to LED light. This suggests a breakthrough to apply in future cancer cell research.
Professor Heo talked about the impact of his research in the following excerpt from a news article:
“We are already conducting research on the spread of cancer, as well as brain science in animal models with the Light-Activated Reversible Inhibition by Assembled Trap. I believe this technology will be a breakthrough in investigating cancer treatments and the function of neurons in a complex neural network, which existing technologies have not been able to do.”
From EE Times Europe, June 19, 2014
“LED Light Technology Controls Proteins in Living Cells”
http://www.ledlighting-eetimes.com/en/led-light-technology-controls-proteins-in-living-cells.html?cmp_id=7&news_id=222909336
2014.06.22 View 7475 -
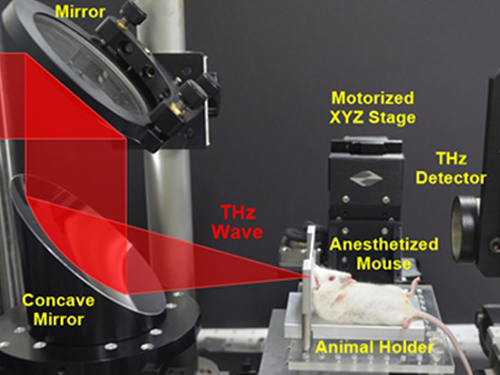 First Instance of Negative Effects from Terahertz-Range Electromagnetic Waves
Professor Philhan Kim
Electromagnetic waves (EM-wave) in the terahertz range were widely regarded as the “dream wavelength” due to its perceived neutrality. Its application was also wider than X-rays. However, KAIST scientists have discovered negative effects from terahertz EM-waves.
Professor Philhan Kim of KAIST’s Graduate School of Nanoscience and Technology and Dr. Young-wook Jeong of the Korea Atomic Energy Research Institute (KAERI) observed inflammation of animal skin tissue when exposed to terahertz EM-waves.
The results were published in the online edition of Optics Express (May 19, 20104).
Terahertz waves range from 0.1 to 10 terahertz and have a longer wavelength than visible or infrared light. Commonly used to see through objects like the X-ray, it was believed that the low energy of terahertz waves did not inflict any harm on the human body.
Despite being applied for security checks, next-generation wireless communications, and medical imaging technology, little research has been conducted in proving its safety and impact. Conventional research failed to predict the exact impact of terahertz waves on organic tissues as only artificially cultured cells were used.
The research team at KAERI developed a high power terahertz EM-wave generator that can be used on live organisms. A high power generator was necessary in applications such as biosensors and required up to 10 times greater power than currently used telecommunications EM-wave. Simultaneously, a KAIST research team developed a high speed, high resolution video-laser microscope that can distinguish cells within the organism.
The experiment exposed 30 minutes of terahertz EM-wave on genetically modified mice and found six times the normal number of inflammation cells in the skin tissue after six hours. It was the first instance where negative side effects of terahertz EM-wave were observed.
Professor Kim commented that “the research has set a standard for how we can use the terahertz EM-wave safely” and that “we will use this research to analyze and understand the effects of other EM-waves on organisms.”
2014.06.20 View 8666
First Instance of Negative Effects from Terahertz-Range Electromagnetic Waves
Professor Philhan Kim
Electromagnetic waves (EM-wave) in the terahertz range were widely regarded as the “dream wavelength” due to its perceived neutrality. Its application was also wider than X-rays. However, KAIST scientists have discovered negative effects from terahertz EM-waves.
Professor Philhan Kim of KAIST’s Graduate School of Nanoscience and Technology and Dr. Young-wook Jeong of the Korea Atomic Energy Research Institute (KAERI) observed inflammation of animal skin tissue when exposed to terahertz EM-waves.
The results were published in the online edition of Optics Express (May 19, 20104).
Terahertz waves range from 0.1 to 10 terahertz and have a longer wavelength than visible or infrared light. Commonly used to see through objects like the X-ray, it was believed that the low energy of terahertz waves did not inflict any harm on the human body.
Despite being applied for security checks, next-generation wireless communications, and medical imaging technology, little research has been conducted in proving its safety and impact. Conventional research failed to predict the exact impact of terahertz waves on organic tissues as only artificially cultured cells were used.
The research team at KAERI developed a high power terahertz EM-wave generator that can be used on live organisms. A high power generator was necessary in applications such as biosensors and required up to 10 times greater power than currently used telecommunications EM-wave. Simultaneously, a KAIST research team developed a high speed, high resolution video-laser microscope that can distinguish cells within the organism.
The experiment exposed 30 minutes of terahertz EM-wave on genetically modified mice and found six times the normal number of inflammation cells in the skin tissue after six hours. It was the first instance where negative side effects of terahertz EM-wave were observed.
Professor Kim commented that “the research has set a standard for how we can use the terahertz EM-wave safely” and that “we will use this research to analyze and understand the effects of other EM-waves on organisms.”
2014.06.20 View 8666 -
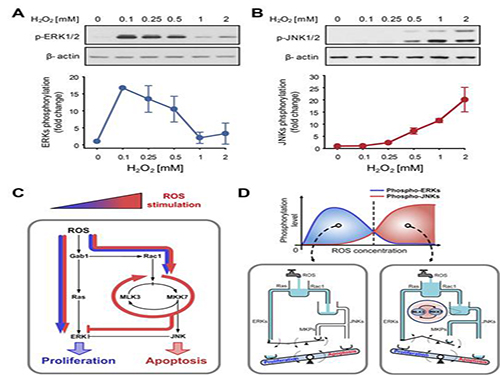 A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 8702
A mechanism for how reactive oxygen species cause cell responses studied
A research team led by Professor Kwang-Hyun Cho of the Department of Biology and Brain Engineering, KAIST, and Dr. Gi-Sun Kwon of the Korea Research Institute of Bioscience and Biotechnology succeeded in proving the mechanism behind the determination of cell life in relation to reactive oxygen species.
The results of the venture were published in the June 3rd edition of Science Signaling. The title of the research paper is “MLK3 is part of a feedback mechanism that regulates different cellular responses to reactive oxygen species.”
The research team discovered that the molecular switch that determines the division of apoptosis of a cell was based on MLK3 feedback mechanism. MLK stands for mixed-lineage kinase.
Under sufficient stress, the mechanism instructs the cell to undergo the division but in an overly stressful environment, the mechanism stops the cell division and instead, induces apoptosis. This discovery is expected to be a breakthrough in illnesses related to the concentration of the reactive oxygen species (ROS).
At low concentration of ROS, the protein associated with cell division, ERK (extracellular-signal-regulated kinase), is activated while as the ROS concentration increases, JNK (c-Jun N-terminal protein kinases), responsible for apoptosis, becomes activated. Furthermore, through computer simulation analysis and mathematical modeling, in tandem with molecular cell biology experiments, the MLK3 based feedback mechanism was the fundamental molecular switch that determines the balance between ERK and JNK, and ultimately the cell’s responses.
Professor Cho commented that “the contradicting cell responses to ROS had remained a mystery, but with the system biology, an approach in which information technology and biotechnology converge, such riddles can be resolved. We expect that the proven mechanism will be used to overcome aging or cancer growth as a result of ROS in the near future.”
Picture shows the process of identifying cell responses caused by reactive oxygen species.
2014.06.13 View 8702 -
 An Exploratory Study on Smartphone Abuse among College Students
Professor Uichin Lee
Professor Uichin Lee of the Department of Knowledge Service Engineering, KAIST, and his research team developed a system that automatically diagnoses the levels of smartphone addiction based on an analysis of smartphone use records.
Professor Lee investigated the usage patterns of 95 smartphone users (college students) by conducting surveys and interviews and collecting logged data. The research team divided participants into “risk” and “non-risk” groups based on a self-reported rating scale to evaluate their abuse of smartphones. As a result, 36 students were categorized as “high risk” and 59 were categorized as “low risk.”
The researchers collected over 50,000 hours of smartphone use encompassing power levels, screen, battery status, application use, internet use, calling, and texting.
The results showed that the “high risk” group used only 1~2 applications, focusing on mobile messengers (Kakotalk, etc.) and SNS (Facebook, etc.).
In addition, a relationship was found between alarm function and addiction levels. Users who set alarms for Kakaotalk messages and SNS comments used smartphones for an additional 38 minutes per day on average.
Results also showed that “high risk” students were on their smartphones for 4 hours and 13 minutes per day, 46 minutes longer than “low risk” students who used smartphones for 3 hours and 27 minutes. The difference was prevalent during 6 am and noon, and 6pm and midnight. In addition, “high risk” students accessed their smartphones 11.4 times more than “low risk” students.
Based on the collected data, Professor Lee developed an automatic system that distinguished users into “high risk” or “low risk” categories with 80% accuracy. The new system is expected to give an early diagnosis of addiction to smartphone users, thereby allowing for early treatment and intervention before the user becomes addicted.
Professor Lee commented that, "the conventional addiction analysis based on self-analysis surveys did not provide real-time data and were largely inaccurate. The new system overcomes these limitations through data science and personal big data analysis" and that he is "developing an application that monitors smartphone abuse."
Figure 1. Usage amount: overall and application-specific results
Figure 2. Usage frequency: overall and application-specific results
Figure 3. Overall diurnal usage time and frequency
2014.06.05 View 7053
An Exploratory Study on Smartphone Abuse among College Students
Professor Uichin Lee
Professor Uichin Lee of the Department of Knowledge Service Engineering, KAIST, and his research team developed a system that automatically diagnoses the levels of smartphone addiction based on an analysis of smartphone use records.
Professor Lee investigated the usage patterns of 95 smartphone users (college students) by conducting surveys and interviews and collecting logged data. The research team divided participants into “risk” and “non-risk” groups based on a self-reported rating scale to evaluate their abuse of smartphones. As a result, 36 students were categorized as “high risk” and 59 were categorized as “low risk.”
The researchers collected over 50,000 hours of smartphone use encompassing power levels, screen, battery status, application use, internet use, calling, and texting.
The results showed that the “high risk” group used only 1~2 applications, focusing on mobile messengers (Kakotalk, etc.) and SNS (Facebook, etc.).
In addition, a relationship was found between alarm function and addiction levels. Users who set alarms for Kakaotalk messages and SNS comments used smartphones for an additional 38 minutes per day on average.
Results also showed that “high risk” students were on their smartphones for 4 hours and 13 minutes per day, 46 minutes longer than “low risk” students who used smartphones for 3 hours and 27 minutes. The difference was prevalent during 6 am and noon, and 6pm and midnight. In addition, “high risk” students accessed their smartphones 11.4 times more than “low risk” students.
Based on the collected data, Professor Lee developed an automatic system that distinguished users into “high risk” or “low risk” categories with 80% accuracy. The new system is expected to give an early diagnosis of addiction to smartphone users, thereby allowing for early treatment and intervention before the user becomes addicted.
Professor Lee commented that, "the conventional addiction analysis based on self-analysis surveys did not provide real-time data and were largely inaccurate. The new system overcomes these limitations through data science and personal big data analysis" and that he is "developing an application that monitors smartphone abuse."
Figure 1. Usage amount: overall and application-specific results
Figure 2. Usage frequency: overall and application-specific results
Figure 3. Overall diurnal usage time and frequency
2014.06.05 View 7053